Abstract
Unusual Epstein-Barr virus (EBV) infection into T or natural killer cells plays a pivotal role in the pathogenesis of acute EBV-associated hemophagocytic lymphohistiocytosis (EBV-HLH) and chronic active EBV infection (CAEBV). The precise frequency and localization of EBV genome in lymphocyte subpopulations especially within T-cell subpopulations are unclear in these EBV-related disorders. This study analyzed the frequency of EBV-infected cells in circulating lymphocyte subpopulations from 4 patients with acute EBV-HLH and 4 with CAEBV. EBV- encoded small RNA-1 in situ hybridization examination of peripheral blood lymphocytes showed a significantly higher frequency of EBV-infected cells of 1.0% to 13.4% in EBV-HLH and 1.6% to 25.6% in CAEBV, respectively. The patterns of EBV infection in lymphocyte subpopulations were quite different between acute EBV-HLH and CAEBV. EBV infection was predominant in CD8+ T cells in all EBV-HLH patients, whereas the dominant EBV-infected cell populations were non-CD8+ lymphocyte subpopulations in CAEBV patients. Phenotypical analysis revealed that EBV-infected cell populations from both EBV-HLH and CAEBV were activated. There was no predominance of any EBV substrain of latent membrane protein-1, EBV-associated nuclear antigen (EBNA)-1, and EBNA-2 genes between the 2 abnormal EBV-associated disorders, and self-limited acute infectious mononucleosis. These results showing differential virus-cell interactions between acute EBV-HLH and CAEBV indicated different pathogenic mechanisms against EBV infection between the 2 EBV-associated diseases, which accounts for the difference in clinical manifestations between the 2 diseases.
Introduction
The primary infection of Epstein-Barr virus (EBV) is usually asymptomatic, and EBV has been shown to be latent in B cells for life in the normal host after primary infection.1 Symptomatic acute EBV infection is clinically recognized as infectious mononucleosis (IM) characterized by fever, hepatosplenomegaly, lymphoadenopathy, and increase of activated CD8+ T lymphocytes in peripheral blood.1,2These manifestations seen in IM are the consequence of the major histocompatibility class-restricted cytotoxic T lymphocyte (CTL) response against polyclonal proliferation of EBV-infected B cells.3 Although IM usually follows a self-limited course without severe complications, uncommon acute EBV infection with fulminant manifestations such as persistent fever, severe hepatosplenomegaly, severe cytopenia, coagulopathy, central nervous system (CNS) abnormality, and vascular dysfunction has been demonstrated, and histiocytic erythrophagocytosis has been found in bone marrow and secondary lymphoid organs from most of these patients.4-6 These cases are clinically recognized as EBV-associated hemophagocytic lymphohistiocytosis (EBV-HLH) or EBV-associated hemophagocytic syndrome, and the systemic release of several cytokines and mediators produced by activated T cells and macrophages due to imbalanced immunoregulation by CTLs has been considered to account for the severe systemic tissue damage mainly to the vascular system in these patients.4-6 Two distinct pathways, Fas (CD95)/Fas ligand and perforin/granzyme, have been shown to mediate mainly the cytotoxicity of CTLs and natural killer (NK) cells in the regulation of viral infections.7,8 The gene knockout mice model has demonstrated the functional significance of perforin in experimental animals.9 Perforin has been shown to be a candidate for familial hemophagocytic lymphohistiocytosis in humans.10 Furthermore, X-linked lymphoproliferation, a fatal disease developing in response to acute EBV infection, has been linked to mutations in the SAP/SH2D1A gene.11These findings indicate that negative regulation of immune effector cells by the induction of apoptosis and activation of CTLs play significant roles in the immune regulation against infections with viruses, in particular EBV. Although the precise pathogenesis of acute fulminant EBV infection with hemophagocytosis is not fully clarified, several reports have suggested that activated T cells are the main cellular target of EBV infection in acute EBV-HLH.12-14
A direct contribution of EBV infection has been demonstrated in the pathogenesis of chronic and severe manifestations that persist for months to years, which have been recognized as chronic active EBV infection (CAEBV).15-17 Although latent EBV infection is demonstrated exclusively in B cells and nasopharyngeal epithelial cells from self-limited IM and EBV seropositive normal individuals,1 an ectopic EBV infection in T- or NK-cell populations has been shown in patients with CAEBV.18-24Both acute EBV-HLH and CAEBV are directly related to EBV infection and possess pathologic similarities, but they also show marked clinical differences. The acute type of EBV-HLH shows more fulminant and severe manifestations, including systemic organ failure with vascular damage, than CAEBV and is sometimes fatal,4-6 unless adequate immunosuppressive therapy is used. On the other hand, acute death is rarely seen in CAEBV. However, CAEBV that is occasionally associated with acute episodes of hemophagocytic syndrome has been found during the course as an early manifestation of EBV-related lymphoid malignancy.24 Recent advances in the control of acute EBV-HLH have shown that the majority of patients with EBV-HLH are successfully cured by immunochemotherapy without relapse,25 whereas it is difficult to control CAEBV following malignant transformation except for treatment with stem cell transplantation.26 The precise mechanisms of EBV infection into T or NK cells in both acute EBV-HLH and CAEBV are still unknown. The clonal proliferation of EBV-infected T or NK cells or pauciclonality of isolated EBV suggests that an ectopic EBV infection in the non–B-cell population plays a pivotal role in both abnormal EBV-associated diseases.12-14 18-24 In the present study, we analyzed the cellular targets of EBV infection to clarify the different pathogeneses of these 2 EBV-associated diseases.
Patients, materials, and methods
Patients
Four previously healthy infants showed sudden onset of fever, hepatosplenomegaly, lymphoadenopathy, jaundice with hepatic dysfunction, and severe cytopenia. Based on the presence of hemophagocytes in bone marrow specimens, they were diagnosed as having hemophagocytic syndrome. Clinical and laboratory features of the 4 patients are listed in Table 1. All patients showed a significantly high serum level of ferritin and lactic dehydrogenase (LDH), 3 showed coagulopathy, and 3 revealed abnormal CNS symptoms. EBV genome was easily detected from blood and bone marrow specimens by the EBV-specific polymerase chain reaction (PCR) method. All received exchange transfusion or plasma exchange for the purpose of reducing hypercytokinemia and immunosuppressive therapy such as methylprednisolone pulse therapy, cyclosporin A, and γ-immunoglobulin infusion. Etoposide (VP-16) was used in 2 patients (EBV-HLH1 and EBV-HLH4) because of prolonged fever and sustained liver dysfunction. Three patients were cured without any manifestations seen in CAEBV, and the EBV genome was not detected in peripheral blood at 6 to 16 months after discontinuation of therapy. One patient (EBV-HLH2) died of uncontrollable coagulopathy and multiple organ failure at 27 days after onset.
Four other patients were diagnosed with CAEBV based on clinical findings such as hepatomegaly, splenomegaly, lymphoadenopathy, persistent hepatic dysfunction, and unusually high antibody titer to EBV antigens (Table 2) including patients CAEBV1 and CAEBV2 who were reported previously.20 22 One patient (CAEBV4) showed hypersensitivity to mosquito bites and an intermittent exacerbation of skin ulcer and fever by mosquito bites for 8 years. Rearrangement of T-cell receptor β chain was detected in 3 lymph node specimens from patient CAEBV1 and blood lymphocytes from patient CAEBV3. Both patients developed T-cell lymphoma during the course and patient CAEBV3 died 2 months later after onset of the lymphoma. T-cell lymphoma seen in another patient (CAEBV1) was refractory to conventional chemotherapy with the combination of cyclophosphamide, daunorubicin, vincrinstine, and prednisolone and the infusion of CD3-activated and interleukin-2 (IL-2)–expanded autologous T cells. She subsequently underwent bone marrow transplantation from an HLA-matched unrelated donor with no recurrence of the clinical manifestations of CAEBV and no increase of EBV genome in peripheral blood.
Flow cytometric analysis and separation of lymphocyte subpopulation
Peripheral blood samples were drawn from patients after informed consent of the patients or their parents. Peripheral blood mononuclear cells (PBMNCs) were separated from heparinized blood by the Ficoll-Hypaque method. PBMNCs were also obtained in the acute phase of typical IM diagnosed by clinical manifestations and EBV serologic analysis. Lymphocytes were stained with phycoerythrin (PE)– or fluorescein isothiocyanate (FITC)–conjugated monoclonal antibodies (MoAbs) against CD3, CD4, CD8, CD16, CD20, CD45RO, or HLA-DR (Pharmingen, San Diego, CA). The expression of HLA-DR or CD45RO in CD4+ or CD8+ T cells was evaluated by 2-color fluorescence flow cytometry using Cytoron Absolute (Ortho Diagnostic, Tokyo, Japan). Lymphocytes were also immunostained with a combination of 2 MoAbs against PE- or FITC-conjugated CD4, CD8, CD16, or CD20 and sorted into CD4+ T cells, CD8+ T cells, CD16+ NK cells, or CD20+ B cells using EPICS ELITE (Coulter Electronics, Hialeah, FL). The efficiency of separation was above 98% determined by flow cytometry.
Detection of EBV genome
The presence of EBV was estimated by EBV-encoded small RNA1 (EBER-1) messenger RNA (mRNA) by in situ hybridization (ISH) as described.20 The cells (5 × 104 to 1 × 105) were cytocentrifuged onto silanized slides (Dako, Kyoto, Japan), and the presence of EBER-1 mRNA was determined by ISH using the alkaline phosphatase-conjugated antisense probe (AGCAGAGTCTGGGAAGACAACCACAGACACCGTCCTCACC) or sense probe to EBER-1. Three independent persons enumerated the frequency of EBER-1+ cells under a microscope by counting 50 000 to 100 000 cells.
Analysis of EBV-related gene
DNA was extracted from peripheral blood lymphocytes or sorted lymphocyte subpopulations from patients with acute IM, EBV-HLH, and CAEBV. PCR was amplified with specific primer sets for carboxyl-terminal (C-terminal) of latent membrane protein (LMP)-1, U2 region of EBV-related nuclear antigen (EBNA)-2, and C-terminal region of EBNA-1 as described.20 PCR products were run on 2% agarose gels and visualized by ethidium bromide staining. Deletion of C-terminal region of LMP-1 and type I (A) or II (B) assessed by U2 region of EBNA-1 was clearly identified by the difference in PCR product size. Mutations of EBNA-2 C-terminal region were evaluated by sequencing of PCR products with the same primers. The nucleotide sequence was determined by the dideoxy chain termination method with the DyeDeoxy Terminator Cycle Sequence Kit (PerkinElmer Applied Biosystems, Norwalk, CT) according to the manufacturer's recommendations with a DNA sequencer 371A (PerkinElmer). The sequence was confirmed 2 or 3 times by examination with forward and reverse reactions.
Results
Frequency of EBV-infected cells in EBV-associated diseases
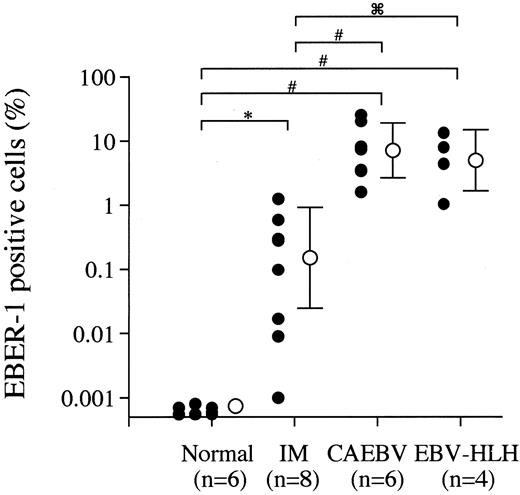
The frequency of EBV-infected cells in peripheral blood from different EBV-associated diseases was quantitatively estimated by the EBER-1 ISH method because of the high sensitivity of detection of EBV-infected cells and reliability of this method. EBER-1+cells were detected at a frequency of 0.0016% to 1.26% within PBMNCs from patients with acute phase IM, but not in 105 PBMNCs from the EBV-seropositive normal adults. PBMNCs from CAEBV showed higher percentages of EBV-infected cells (1.6%-25.6%) than those from IM (Figure 1). EBER-1+ cells were also found with high frequency (1.0%-13.4%) in PBMNCs from the patients with the early phase of EBV-HLH. The percentage of EBV-infected cells declined with time with immunosuppressive therapy in all cases (data not shown), but a reincrease of EBER-1+cells was detected in the one deceased case (EBV-HLH2) with multiple organ failure and uncontrolled coagulopathy. No EBV-infected cells were detected by EBV ISH in the 3 cured patients with EBV-HLH at 6 to 18 months after immunotherapy was discontinued.
Frequency of EBV-infected cells in EBV-seropositive normal individuals and patients with acute IM, EBV-HLH, and CAEBV.
The frequency of EBV-infected cells in PBMNCs from EBV-seropositive normal subjects and patients with acute phase of IM, CAEBV, and EBV-HLH were estimated by the EBER-1 ISH as described. The closed circles indicate the data of each sample and the open circles and vertical bars represent the logarithmic mean and SD of each disease category. *P < .001, #P < .005, and P < .05 represent the significance estimated by the Mann-WhitneyU test.
P < .05 represent the significance estimated by the Mann-WhitneyU test.
Frequency of EBV-infected cells in EBV-seropositive normal individuals and patients with acute IM, EBV-HLH, and CAEBV.
The frequency of EBV-infected cells in PBMNCs from EBV-seropositive normal subjects and patients with acute phase of IM, CAEBV, and EBV-HLH were estimated by the EBER-1 ISH as described. The closed circles indicate the data of each sample and the open circles and vertical bars represent the logarithmic mean and SD of each disease category. *P < .001, #P < .005, and P < .05 represent the significance estimated by the Mann-WhitneyU test.
P < .05 represent the significance estimated by the Mann-WhitneyU test.
Differential pattern of EBV infection in lymphocyte subpopulations between EBV-HLH and CAEBV
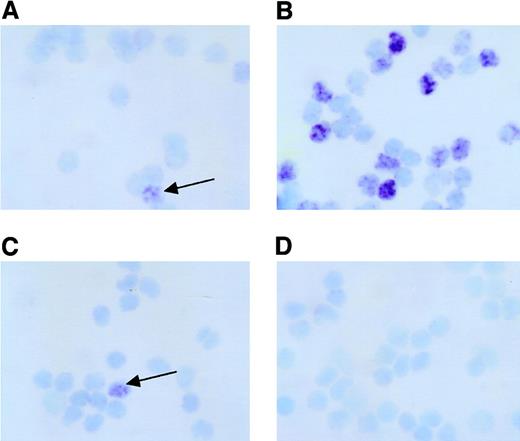
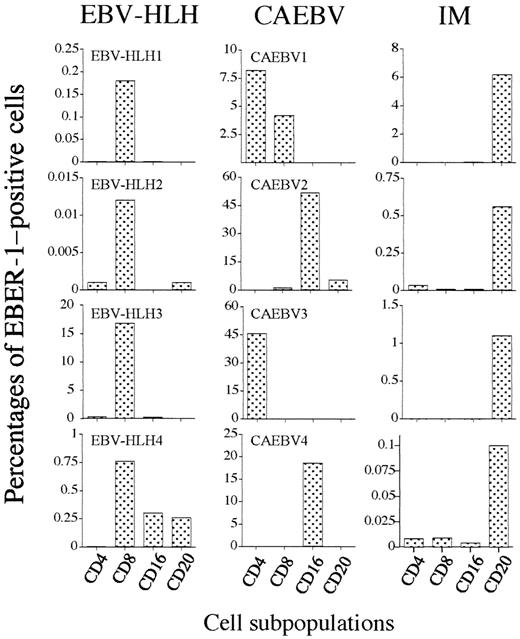
To determine the precise localization of EBV infection in lymphocyte subpopulations, CD4+ T cells, CD8+ T cells, CD16+ NK cells, and CD20+ B cells from EBV-HLH, CAEBV, and acute IM were electronically sorted, and the presence of EBV was estimated in each lymphocyte subpopulation. Figure2 shows a representative microscopic photograph of EBER-1 ISH of 4 sorted populations from patient EBV-HLH2 at 8 days after onset. Figure 2 shows a clear difference of EBER-1+ and EBER-1− cells. The analysis of EBV-infected cells in each lymphocyte subpopulation showed clear differences in the infectious pattern between 3 EBV-associated diseases (Figure 3). The EBV-infected cell population in acute IM was found mainly in CD20+ B cells, but fewer EBER-1+ cells were detected in CD4+ T cells, CD8+T cells, and CD16+ NK cells in 2 cases. In contrast to IM, the infection of EBV into lymphocytes other than B cells was prominent in EBV-HLH and CAEBV. The dominant population of EBV-infected cells was CD8+ T cells in all EBV-HLH patients, although EBV-infected cells were found in NK cells and B cells with lower frequency in patient EBV-HLH4. The absolute frequency of EBV-infected CD8+ T cells was different in each case, which might be related to the time elapsed after disease onset. In contrast to EBV-HLH, the pattern of mainly EBV-infected populations was different in each CAEBV case, CD4+ T cells alone in case CAEBV3, mainly CD16+ NK cells in CAEBV2 and CAEBV4, and both CD4+ and CD8+ T cells in case CAEBV1.
Detection of EBER-1+ cells in lymphocyte subpopulations in patients with EBV-HLH.
PBMNCs obtained from patient EBV-HLH2 at 8 days after onset were separated into CD4+ T cells (A), CD8+ T cells (B), CD16+ NK cells (C), or CD20+ B cells (D) by electronic sorting using EPICS-Elite as described. The presence of EBER-1 mRNA was detected by ISH in each cytocentrifuged cell subpopulation. The arrows indicate EBER-1+cells. Original magnification × 400.
Detection of EBER-1+ cells in lymphocyte subpopulations in patients with EBV-HLH.
PBMNCs obtained from patient EBV-HLH2 at 8 days after onset were separated into CD4+ T cells (A), CD8+ T cells (B), CD16+ NK cells (C), or CD20+ B cells (D) by electronic sorting using EPICS-Elite as described. The presence of EBER-1 mRNA was detected by ISH in each cytocentrifuged cell subpopulation. The arrows indicate EBER-1+cells. Original magnification × 400.
Differential infection of EBV into lymphocyte subpopulations between EBV-related disorders.
PBMNCs from patients with EBV-HLH (left column), CAEBV (center column), or acute phase IM (right column) were separated into CD4+ T cells, CD8+ T cells, CD16+ NK cells, and CD20+ B cells the same as in Figure 2. Frequency of each lymphocyte subpopulation was estimated by EBER-1 ISH.
Differential infection of EBV into lymphocyte subpopulations between EBV-related disorders.
PBMNCs from patients with EBV-HLH (left column), CAEBV (center column), or acute phase IM (right column) were separated into CD4+ T cells, CD8+ T cells, CD16+ NK cells, and CD20+ B cells the same as in Figure 2. Frequency of each lymphocyte subpopulation was estimated by EBER-1 ISH.
EBV-infected cells exist in an activated lymphocyte population in both acute EBV-HLH and CAEBV
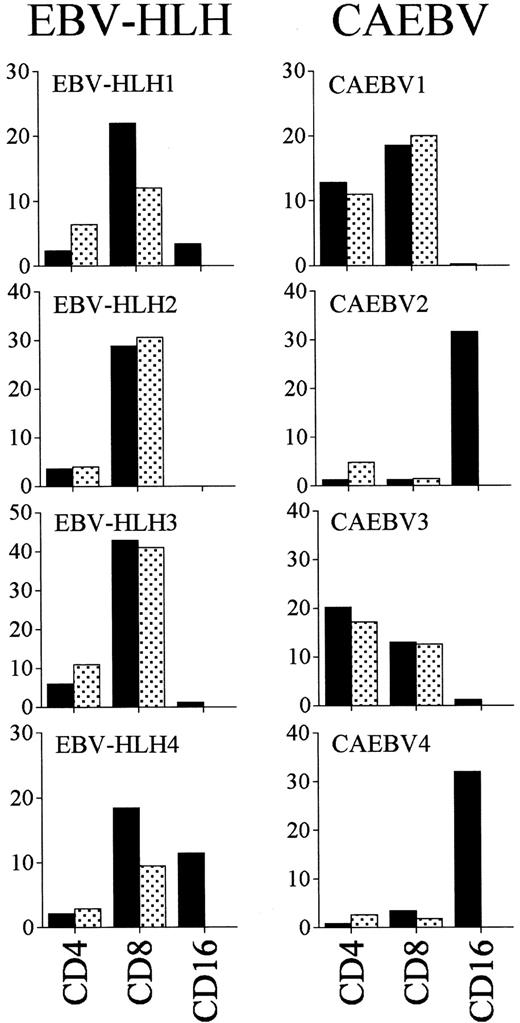
The phenotypic analysis of lymphocytes from EBV-HLH showed a relative decrease of CD4+ T cells and relative increase of CD8+ T cells, but the absolute number of CD8+ T cells was decreased (data not shown). Expression of activation markers such as CD45RO and HLA-DR antigen on T cells was markedly up-regulated in the CD8+ T-cell population from EBV-HLH, which was the main EBV-infected population (Figure 4). Interestingly, almost half of CD16+ NK cells from patient EBV-HLH4, in which EBV infection was detected, expressed HLA-DR. In CAEBV, activation of the cell population in which EBER-1+cells existed, CD4+ and CD8+ T cells in case CAEBV1 and case CAEBV3, and CD16+ NK cells in case CAEBV2 and case CAEBV4, was also detected.
Lymphocyte phenotype of EBV-HLH and CAEBV.
Peripheral lymphocytes from EBV-HLH and CAEBV patients were stained with PE-conjugated anti-CD4, CD8, MoAbs in combination with FITC-conjugated anti-CD45RO or HLA-DR MoAbs and analyzed with flow cytometry. PE-conjugated anti-CD16 MoAb was used only with FITC-conjugated anti–HLA-DR MoAb. ▪ indicates the percentages of HLA-DR+ and indicates the percentages of CD45RO+ cells in CD4+ T cells, CD8+ T cells, and CD16+ NK cells. Increase of activated cells was noted in subpopulations in which EBV infection was detected.
indicates the percentages of CD45RO+ cells in CD4+ T cells, CD8+ T cells, and CD16+ NK cells. Increase of activated cells was noted in subpopulations in which EBV infection was detected.
Lymphocyte phenotype of EBV-HLH and CAEBV.
Peripheral lymphocytes from EBV-HLH and CAEBV patients were stained with PE-conjugated anti-CD4, CD8, MoAbs in combination with FITC-conjugated anti-CD45RO or HLA-DR MoAbs and analyzed with flow cytometry. PE-conjugated anti-CD16 MoAb was used only with FITC-conjugated anti–HLA-DR MoAb. ▪ indicates the percentages of HLA-DR+ and indicates the percentages of CD45RO+ cells in CD4+ T cells, CD8+ T cells, and CD16+ NK cells. Increase of activated cells was noted in subpopulations in which EBV infection was detected.
indicates the percentages of CD45RO+ cells in CD4+ T cells, CD8+ T cells, and CD16+ NK cells. Increase of activated cells was noted in subpopulations in which EBV infection was detected.
Subtype of EBV isolated from EBV-HLH and CAEBV
Mutations of EBV-encoded genes such as LMP1,EBNA1, and EBNA2 have been suggested to play pivotal roles in the pathogenesis of EBV-associated diseases.27 28 We evaluated these EBV genes in isolates from EBV-HLH and CAEBV to clarify the virus-cell interaction in these EBV-associated diseases (Table 3). Deletion of 30 base pairs (bp) in the C-terminal region ofLMP1 was detected in the majority of cases with EBV-HLH and CAEBV except for CAEBV4, which showed the prototype of B95.8. All of isolated EBV from the patients with the 2 EBV-related diseases was type A (I) determined by EBNA2 gene analysis. Analysis ofEBNA1 showed that valiant-valine (V-Val; 3-letter amino acid code) subtype was present in all cases except for CAEBV4 from whom prototype-alanine (P-Ala) was isolated. Interestingly, dual infection with 2 types of EBV, P-Ala and V-Val was detected, in some cell subpopulations in 3 of the 4 EBV-HLH patients, whereas only one type was detected in EBV isolated from the patients with CAEBV. Because ethnic differences in EBV subtype have been shown, we analyzed 30 EBV isolates from IM in Japanese patients. EBV subtypes with a 30-bp deletion of LMP-1, type A, and V-Val subtype of EBNA-1 showed the highest frequencies with 78%, 92%, and 81%, respectively.
Discussion
Although EBV infection into T or NK cells has been implicated in the pathogenesis of EBV-HLH12-14 and CAEBV,17-24 the difference in the cellular target of EBV infection between these 2 abnormal EBV-associated diseases has been unknown. In this report, we clearly showed the difference of the cellular targets of EBV infection between acute EBV-HLH and CAEBV. EBV-infected cells in the acute phase of EBV-HLH were detected mainly in the activated CD8+ T-cell population, whereas the main EBV-infected populations in CAEBV were populations other than CD8+ T cells. Several other reports of single cases with acute fulminant EBV infection and hemophagocytosis showed that EBV-infected cells were found mainly in the CD8+ T-cell population,29-31 consistent with our results. Recently, Quinatallia-Martinaz and colleagues14 could not find any clear T-cell subset predominance by double-staining analysis of EBER-1 and CD4, CD8 in specimens from 5 deceased patients with acute fulminant EBV infection. The difference in subset predominance may arise from differences in the time of analysis after onset because they used pathologic samples obtained from autopsy, whereas we used peripheral blood samples obtained in the early phase of EBV-HLH. EBV-infected subset predominance might be a dynamic phenomenon because transition from acute EBV-HLH to CAEBV has been found. Furthermore, we have experienced a case (CAEBV1) in which CD4+ T cells were infected exclusively with EBV in the early stage. However, both CD4+ and CD8+ T cells became infected with the virus as the disease progressed and malignant lymphoma developed (Y. K. et al, unpublished observation, July 1997). A recent analysis of CAEBV2 found EBV infection into CD8+ T cells, in which EBV infection has not been detected in the early stage of disease.
Similar to previously reported results, at least 2 distinct types of CAEBV existed in our patient population. In one group of patients, EBV was found within T cells, in particular CD4+ T cells.17-21 In another group of patients, NK cells were the target of EBV infection,21-23 which is frequently associated with mosquito hypersensitivity in the Orient. Further analysis of the cellular target of EBV infection in CAEBV or T- or NK-lymphoproliferative disorders may help to distinguish 2 different clinical entities of chronic abnormal EBV infection.
The relationship between EBV infection into CD8+ T cells and the development of EBV-HLH, especially regarding the severity and high mortality associated with this disorder, is still unclear. Infection of EBV into CD4+ T-cell line in vitro has been shown to up-regulate the production of tumor necrosis factor-α and to subsequently activate macrophages.32 A similar phenomenon could be speculated to occur in the case of CD8+ T cells. An experimental approach using gene transfer or EBV infection into CD8+ T cells with transfection of EBV receptor in vitro may clarify the functional role of EBV infection in CD8+ T cells. Considering the effector functions of CD8+ T cells such as FasL-mediated and perforin/granzyme-mediated cytotoxicity, prolonged proliferation of EBV-infected CD8+ T cells should be harmful in vivo. In self-limited acute IM, we could easily find spontaneous apoptosis of activated CD8+ T cells,33 which plays the central role in CTL responses against EBV-infected B cells, and we could also detect the down-regulation of Bcl-2 protein, one of the antiapoptotic oncogene family proteins, in these cell populations.34 From the fact that EBV gene possesses Bcl-2 homologue BHRF1, with the antiapoptotic activity of this viral oncogene demonstrated in a transfection experiment,35 we speculate that viral–Bcl-2 inhibits the apoptosis of EBV-infected CD8+ T cells in EBV-HLH, resulting in sustained cell survival and proliferation with strong undesired cytotoxic activity. We could not examine the Bcl-2 protein expression in specimens of EBV-infected CD8+ T cells from EBV-HLH patients because of the limited number of slide preparations available. Further analysis of antiapoptotic Bcl-2 family proteins might be required in pathologic specimens from this disorder to confirm this hypothesis.
Several approaches to determine the relationship between individual EBV strains and EBV-related diseases such as malignant lymphoma,27 nasopharyngeal carcinoma,28Burkitt lymphoma,29 and gastric carcinoma30have been reported. The significance of type B in the immunocompromised host in Western countries27 and the variants of EBNA-1 C-terminal region have been suggested.36,37Ethnic differences in EBV type have been demonstrated, and especially in Asian populations type A EBV has been found to predominate in some EBV-related diseases. The relationship between the deletion of 30 bp in C-terminal region of LMP-1 and malignant transformation of EBV+ tumors has been suggested.38 However, we could not find predominance of any EBV subtype of LMP1 andEBNA1 genes between acute IM and 2 abnormal disorders with EBV infection. From these results, we cannot confirm the pathologic significance of type A EBV or subtype with 30-bp deletion type LMP-1 in the 2 EBV-associated diseases.
The intriguing finding in this EBV gene analysis was the simultaneous presence of 2 different subtypes of EBNA-1 in 3 of 4 patients in the acute phase of EBV-HLH. Dual infection with 2 types of EBV strains was not detected in CAEBV or acute IM, except for 2 cases of IM with V-Val and V-Leu from 30 analyzed IM patients (data not shown). The pathologic significance of the variant strain with V-Val inEBNA1 gene has been demonstrated from its predominance in other EBV-related malignant diseases,36 but we could not find any difference in the frequency of the V-Val type of EBNA-1 between 3 EBV-related diseases, as in a previous report.37The significance of dual infection with 2 types of EBV strains in the pathogenesis of EBV-HLH was obscure. Dual infection of these 2 types of EBV strains into EBV-receptor–transfected CD8+ T-cell lines may clarify the cellular activation and message up-regulation of cytokine and cell activation and apoptosis.
Although it has been suggested that T cells express EBV receptor CD21 on the cell surface,39 the precise mechanism of ectopic EBV infection into T cells or NK cells is still unknown. Our preliminary data showed that EBV occasionally infects non–B-cell lymphocyte populations, although at a very low frequency, during the very early stages of acute IM. Under normal immune regulation by CTLs, the early and early lytic EBV antigen expressed on B cells evokes strong immune responses.1-3 In the absence of EBV infection into B cells, the EBV-specific CTL response may be significantly delayed or defective. Absence or decrease of EBV infection in B cells seen in patients with EBV-HLH and CAEBV may be related to the persistence of EBV genome in T- or NK-cell populations. Escape from immune surveillance by diminished expression of EBV antigen has been suggested in other EBV+malignancies.1,39 Although EBV latency in EBV-infected CD8+ T cells in EBV-HLH was not determined, several EBV+ T- or NK-cell lines and malignant lymphomas of T- or NK-cell origin have been shown to be in latency II,1which may escape from host immunoregulation. Loss of expression of early lytic antigen of EBV in T cells may augment cellular viral load and the expansion of EBV-infected T-cell proliferation, although the latency status of EBV infection in EBV-HLH was not determined in this analysis. To clarify the mechanism of EBV infection into non–B-cell lymphocytes, molecular analysis of EBV latency might be required to better understand the pathogenesis of these EBV-related hematologic diseases.
We appreciate the excellent technical assistance of H. Matsukawa and M. Kitakata.
Supported by a Grant-in-Aid for Scientific Research from the Ministry of Education, Science, and Culture of Japan; a grant from the Ministry of Welfare and Health of Japan; and a grant from the Japan Rheumatic Foundation.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Yoshihito Kasahara, Department of Pediatrics, Angiogenesis and Vascular Development, Graduate School of Medical Science, Kanazawa University, 13-1, Takaramachi, Kanazawa, Ishikawa 920-8641, Japan; e-mail:ykasa@ped.m.kanazawa-u.ac.jp.