Abstract
In this study, mononuclear cells (MNCs) from granulocyte colony-stimulating factor (G-CSF)–mobilized blood stem cell (BSC) harvests from 104 healthy donors were analyzed for their immunological functions and compared with MNCs from 28 steady-state nonmobilized donors. The relationships between donor characteristics (age, gender, weight, and HLA type) and immune functions of the harvests were also analyzed. There was a significant (P < .01) decrease in natural killer and lymphokine-activated killer (LAK) cell–mediated cytotoxicity for G-CSF–mobilized effector cells compared with nonmobilized cells. Similarly, there was a significant (P < .005) decrease in both T-cell and B-cell mitogen response in G-CSF–mobilized cells compared with nonmobilized cells. There was dose-dependent inhibition of LAK cell–mediated cytotoxicity, but this effect was not seen with other immune function assays. Changes in immune function did not appear to be determined by frequency of cellular phenotypes or expression of effector function genes seen in a reverse-transcription polymerase chain reaction. There was a significant relationship between expression of certain HLA alleles (A1, A3, A24, B44, B62, DR15, DR17; allP < .01) and increased immune function, such as cytotoxicity and/or mitogen response. A decrease in immune function with the HLA-DR13 expression was also observed (P < .01). Since the G-CSF increases the number of MNCs, the increase in effector cells might compensate for decreased immune functions of these cells in vivo when transplanted into patients. These results suggest a decreased immune function in G-CSF–mobilized BSC harvests and warrant further studies to correlate these data with clinical outcome.
Introduction
Allogeneic blood stem cells mobilized with colony-stimulating factors as opposed to bone marrow are increasingly being used for transplantation in patients with hematological malignancies.1-4 Transplantation of allogeneic blood stem cells from HLA-identical donors has resulted in sustained hematopoietic recovery without a significant increase in acute graft-versus-host disease (GVHD) compared with bone marrow.5-10 Granulocyte colony-stimulating factor (G-CSF) is one of the commonly used cytokines for mobilizing stem/progenitor cells from bone marrow into peripheral blood while inducing differentiation of myeloid cells.11The G-CSF receptors are found on myeloid progenitors, mature granulocytes, monocytes, and some T- and B-lymphocytes.12In addition to hematopoietic properties, G-CSF is also known to exert pleotropic effects on different cell types of the host immune system.13-16 Studies in these areas have demonstrated that G-CSF alters the cytotoxic ability of natural killer (NK) cells,13 cytotoxic effectors activated by interleukin-2 (IL-2),14 and the alloantigen-presenting capability of immune accessory cells.15 Some of the down-regulatory effects of G-CSF could be due to a block in production of tumor necrosis factor α (TNF-α) by the effector cells.16However, detailed immunologic characteristics of G-CSF–mobilized blood stem cell harvests and their clinical relevance or implications for long-term safety have not been reported. Moreover, analyses of donor characteristics and their relationship to immunological functions and phenotypes of G-CSF–mobilized blood stem cells has not been reported. Therefore, a study was undertaken to investigate the immune functions and phenotypes of mononuclear cells (MNCs) isolated from G-CSF–mobilized blood stem cell harvests and compare them with those of steady-state nonmobilized apheresis MNCs from healthy donors. In addition, donor characteristics such as age, gender, weight, and HLA-type were correlated to their immune function levels.
Materials and methods
Donor characteristics
Donors included in this study were 104 healthy individuals who served as HLA-matched stem cell donors for their relatives undergoing allogeneic stem cell transplantation. They were participants in 1 of 2 institutional review board–approved, sequential studies of G-CSF mobilization. As a control, 28 normal-donor apheresis harvests without cytokine mobilization were used. Written informed consent was obtained from each donor. The age, gender, and weight of the donors are presented in Table 1.
Peripheral blood stem cell harvest
Peripheral blood was collected from each donor before mobilization for baseline studies. The donors received daily G-CSF (5 μg/kg/d or 10 μg/kg/d) until 2 apheresis collections were complete. Out of 104 donors, 35 received 5 μg/kg/d G-CSF and 69 received 10 μg/kg/d G-CSF for mobilization. Peripheral blood stem cells (PBSCs) were collected from the donors at the University of Nebraska Medical Center by means of the Cobe Spectra (Cobe, Boulder, CO) apheresis device.17 MNCs were isolated by density gradient centrifugation from an aliquot of each PBSC collection by means of lymphocyte separation medium (Accurate Chemical, Westbury, NY). The MNCs were washed once with RPMI 1640 medium and resuspended in RF10 medium, which consisted of RPMI 1640 medium supplemented with 10% fetal calf serum, L-glutamine (2 mM), penicillin (100 U/mL), and streptomycin (100 μg/mL).
Tumor cell line cultures and NK assays
The human chronic myeloid leukemic cell line K562 and the human B-cell lymphoma cell line Raji were grown in vitro in 25-cm2 tissue-culture flasks in RF10 medium. K562 and Raji cells were labeled with chromium-51 (Na251CrO4) and used as target cells for NK and lymphokine-activated killer (LAK)–cell cytotoxicity assays. K562 cells are NK sensitive, and Raji cells are NK resistant and LAK sensitive.
LAK cell assays
MNCs obtained from donor PBSC harvests were activated in vitro with IL-2 and assayed for cytotoxicity. We cultured 5 million cells in T-25 flasks containing 5 mL RF10 supplemented with 50 μM β-mercaptoethanol, and 1000 U/mL IL-2 for 72 hours at 37°C in 5% CO2 and 95% air. Control flasks contained MNCs in 5 mL of the above media without IL-2. Following the incubation period, the cells were harvested by means of a diSPo cell scraper and assayed for their cytotoxicity against 51Cr-labeled K562 or Raji target cells.
Cytotoxicity assay
MNCs obtained from donor PBSCs with or without IL-2 activation were tested for cytotoxic activity against 51Cr-labeled K562 and Raji tumor target cells before and after in vitro activation as we have previously described.18 The radioactivity was counted by means of a Beckman 5500 gamma counter (Beckman Coulter, Fullerton, CA). The percentage of target cells lysed was calculated with the following formula:
where the experimental counts per minute (CPM) = effector + target cells; spontaneous CPM = target cells + medium; maximum CPM (total release) = target cells + 1% Triton-X 100.
Mitogen assay
MNCs obtained from donor PBSC harvests were assayed for their response to concanavalin-A (Con-A) or phytohemagglutinin (PHA) (T-cell mitogen) and lypopolysaccharide (LPS) or pokeweed mitogen (PWM) (B-cell mitogens) as described previously.19 The cell concentration was adjusted to 105 cells per well per 200 μL containing the appropriate concentration of mitogens. The mitogen concentration was determined on the basis of titrations with MNCs from the blood of healthy volunteers. The cell suspension was incubated in a 96-well microtiter plate for 72 hours at 37°C in 5% CO2in air. A 1μCi-per-well aliquot of radiolabeled (3H) thymidine was added to the cultures 18 hours before termination of the culture. After the incubation period, the cells were harvested on glass-fiber filter paper disks by means of a PHD cell harvester (Cambridge Technologies, MA). The filter paper disks were dried and transferred to plastic tubes containing standard scintillation cocktail, and the radioactivity was counted by means of a liquid scintillation counter.
Immunophenotyping analysis
Cell-surface immunophenotyping of MNCs isolated from donor PBSC was carried out by flow cytometric analysis by means of the Coulter Profile II flow cytometer (Beckman Coulter), as described previously.20 We suspended 300 000 cells in 200 μL phosphate-buffered saline (PBS) containing 0.1% bovine serum albumin and 0.05% NaN3 (fluorescence buffer [FB]) in 12 × 75 mm diSPo culture tubes. Cells were incubated on ice for 30 minutes with 10 μL fluorescein isothiocyanate or 5 μL phycoerythrin-labeled monoclonal antibodies specific for the following cell surface markers: CD3, CD4, CD8, CD14, CD20, CD25, CD56, CD80, and CD83. After incubation, the cells were washed with 1 mL FB, centrifuged, and resuspended in 800 μL PBS for analysis.
Reverse transcription–polymerase chain reaction analysis
A reverse transcription–polymerase chain reaction (RT-PCR) technique was used to determine the expression of interferon γ (IFN-γ), perforin, Fas, Fas ligand, and TNF-α genes in activated MNCs, as we have described previously.21 Briefly, RNA was isolated from MNCs by means of TRIzol reagent obtained from Gibco BRL (Gaithersburg, MD). One microgram RNA was reverse-transcribed by means of 150 ng of random primers following standard procedures with the exception of the addition of dimethyl sulfoxide (DMSO) (1% final concentration) and was incubated at 44°C for 1 hour. PCR was then performed on 10 μL complementary DNA in 50 μL reaction medium including 20 picomoles of each primer, 2.5 mM MgCl2, 200 :M dNTPs, and 5% dimethyl sulfoxide (DMSO). The primers were as follows: for perforin, 5′(GTTGCATCTCACCCTCATGGGACCAGACTT) 3′ and 5′(TAAGCCCACCAG CAATGTGCATGTGTCTGT) 3′; for IFN-γ, 5′(AGG GGCAGGTTGTTGCCAGA)3′ and 5′(CACCCCAGTCCACCCCCT GA) 3′; for Fas, 5′(ATG CTGGGCATCTTGACCCTC CTA) 3′ and 5′(TCTGCACTTGGTATTCTGGGTCCG) 3′; for Fas ligand, 5′(CACCCCAGTCCACCCCCT GA) 3′ and 5′(AGGGGCAGGTTGTTGCAA GA) 3′; for TNF-α, 5′(CACCAGCTGGTTATCTCTCAG CTC) 3′ and 5′(CGGGACGTGGAGCTGGCCGAG GAG)3′. The temperature conditions were as follows: 5 minutes at 95°C followed by 2 minutes at 62°C followed by 25 cycles of 20 seconds at 72°C, 45 seconds at 94°C, and 20 seconds at 62°C. PCR products were visualized by electrophoresis in ethidium bromide–stained 2% agarose gels. As a control, α-tubulin gene expression was measured by means of the same procedure and appropriate primers: 5′(AAGAAATCCAAGCTGGAGTTC)3′ and 5′(GTTGGTCTGGAATTCTGTGAG)3′. All primer sets were designed to span introns to eliminate any potential signal from contaminating DNA in the RNA preparation.21 Following PCR, the extent of each gene expression was quantitated by densitometric analysis by means of ImagePro software (Media Cybernetics, Silver Spring, MD).
Correlative analyses of immune functions and donor characteristics
Donor characteristics such as age, gender, weight, and HLA type from the patient database were correlated with each of the above immune functions by means of the statistical methods described below.
Statistical analysis
For the statistical analysis, the 4 most common alleles at each locus (A, B, DR) were examined, and donor age and weight were dichotomized as being above or below the median value in this sample. Analysis of variance was used to examine the results of each experiment. Specifically, dilution (for cytotoxicity experiments) or concentration (for mitogen-response experiments) and each characteristic (eg, use of G-CSF, donor age, donor expression of a specific HLA allele) were used as factors in a 2-way analysis-of-variance model. To prevent inflating the type I error due to multiple testing, each dilution/concentration was separately examined only when the overall test in the analysis of variance was statistically significant.
Analyses of immune functions based on absolute numbers
The above analyses were based on cell-to-cell comparisons. Since the G-CSF mobilization increases the absolute number of white blood cells as well as MNCs, we also analyzed the immune function results on the basis of absolute numbers. For these analyses, the cytotoxicity results or mitogen-response results were converted as cytotoxicity per microliter of apheresis product and expressed as cytotoxicity units per microliter or counts per minute per microliter of apheresis product.
Results
Levels of NK cell–mediated cytotoxicity
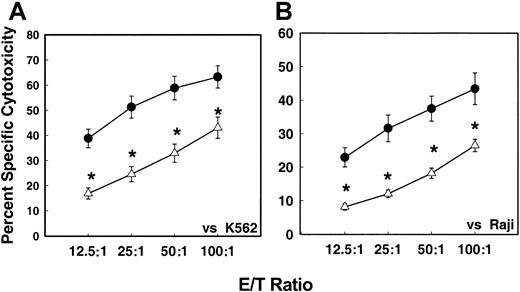
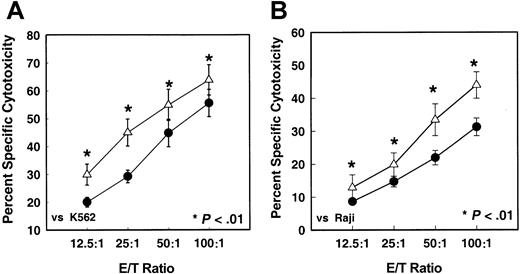
Figure 1 shows the levels of NK cell–mediated cytotoxicity of MNCs isolated from G-CSF–mobilized and nonmobilized apheresis harvests. There was a significant (P < .05) decrease in the NK cell–mediated cytotoxicity levels of MNCs from G-CSF–mobilized blood stem cell harvests compared with MNCs from steady-state nonmobilized stem cell harvests against both K562 (NK-sensitive) (Figure 1A) and Raji (NK-resistant/LAK-sensitive) (Figure 1B) target tumor cells. At an effector-to-target (E/T) ratio of 50:1, the cytotoxicity of G-CSF–mobilized effector cells against K562 was 6.1% ± 0.7% as compared with 12% ± 3.7% control nonmobilized apheresis product. A similar trend in decreased cytotoxicity of G-CSF–mobilized effector cells was seen with Raji target cells.
In vitro NK cell–mediated cytotoxicity levels of MNCs.
In vitro NK cell–mediated cytotoxicity levels of MNCs from G-CSF–mobilized (n = 98) or nonmobilized steady-state (n = 28) donor blood stem cell harvests against K562 (panel A) and Raji (panel B) tumor target cells by means of 51Cr release assay. Values represent mean percentages of specific cytotoxicity ± SEM from different samples. (For effector-to-target [E/T] ratios of 12.5:1, P = .005; 25:1, P = .0001; 50:1,P = .007; 100:1 P = .05.) ● indicates nonmobilized donors (n = 28); ▵, G-CSF mobilized donors (n = 104).
In vitro NK cell–mediated cytotoxicity levels of MNCs.
In vitro NK cell–mediated cytotoxicity levels of MNCs from G-CSF–mobilized (n = 98) or nonmobilized steady-state (n = 28) donor blood stem cell harvests against K562 (panel A) and Raji (panel B) tumor target cells by means of 51Cr release assay. Values represent mean percentages of specific cytotoxicity ± SEM from different samples. (For effector-to-target [E/T] ratios of 12.5:1, P = .005; 25:1, P = .0001; 50:1,P = .007; 100:1 P = .05.) ● indicates nonmobilized donors (n = 28); ▵, G-CSF mobilized donors (n = 104).
Levels of LAK cell–mediated cytotoxicity
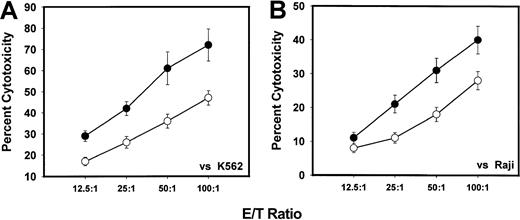
MNCs from both G-CSF–mobilized PBSC harvests and nonmobilized PBSC harvests were activated with IL-2 for 72 hours to generate LAK cells. Figure 2 demonstrates the cytotoxicity levels of these LAK cells against K562 and Raji cells in comparison with similar LAK cells generated from steady-state PBSCs. There was a significantly (P < .001) decreased level of cytotoxicity of LAK cells generated from G-CSF–mobilized PBSC harvests compared with their nonmobilized PBSC harvests against K562 (Figure 2A) and Raji (Figure 2B) target cells. The cytotoxicity of LAK cells generated from G-CSF–mobilized MNCs against Raji target was 18.2% ± 1.5% as compared with 43.4% ± 3.7% at an E/T ratio of 50:1. Similar decrease in cytotoxicty of G-CSF–mobilized LAK cells was seen at other E/T ratios. In addition to K562 and Raji tumor target cells, we also used Daudi cells in some experiments with LAK cell–mediated cytotoxicity. These results were similar to those with Raji cells (data not shown).
In vitro LAK cell–mediated cytotoxicity levels of MNCs.
In vitro LAK cell–mediated cytotoxicity levels of MNCs from G-CSF–mobilized (n = 75) or nonmobilized steady-state (n = 28) donor blood stem cell harvests against K562 (panel A) and Raji (panel B) tumor target cells by means of 51Cr release assay following in vitro activation with IL-2 for 72 hours. Values represent mean percentages of specific cytotoxicity ± SEM from different samples. (For E/T ratios of 12.5:1, P = .005; 25:1,P = .007; 50:1, P = .01; 100:1,P = .05.) ● indicates nonmobilized donors; ▵, G-CSF mobilized donors; *indicates the statistical significance of the value as compared to nonmobilized blood.
In vitro LAK cell–mediated cytotoxicity levels of MNCs.
In vitro LAK cell–mediated cytotoxicity levels of MNCs from G-CSF–mobilized (n = 75) or nonmobilized steady-state (n = 28) donor blood stem cell harvests against K562 (panel A) and Raji (panel B) tumor target cells by means of 51Cr release assay following in vitro activation with IL-2 for 72 hours. Values represent mean percentages of specific cytotoxicity ± SEM from different samples. (For E/T ratios of 12.5:1, P = .005; 25:1,P = .007; 50:1, P = .01; 100:1,P = .05.) ● indicates nonmobilized donors; ▵, G-CSF mobilized donors; *indicates the statistical significance of the value as compared to nonmobilized blood.
Since the frequencies of immune effector cells such as T, NK, and B cells in both nonmobilized and G-CSF–mobilized apheresis product were similar, we can rule out the possible dilution effect on effector-cell population. Furthermore, when we isolated CD56+ NK cells from both nonmobilized and G-CSF–mobilized PBSC harvest, NK cells from G-CSF–mobilized, but not from nonmobilized PBSC harvest, showed a decreased NK cell–mediated cytotoxicity (results not shown). These results further confirm that dilution effect is not the reason for defective immune functions in G-CSF–mobilized PBSC products.
We also analyzed LAK cell–mediated cytotoxicity results on the basis of the G-CSF dose used for mobilization. Figure3 demonstrates the LAK cell–mediated cytotoxicity of MNCs from donor-received 5 μg/kg or 10 μg/kg G-CSF for mobilization. There was a significant decrease in cytotoxicity with the 10 μg/kg G-CSF group compared with the 5 μg/kg group (P < .001).
In vitro LAK cell–mediated cytotoxicity levels of MNCs.
In vitro LAK cell–mediated cytotoxicity levels of MNCs from 2 different doses (5 μg/kg, n = 35; 10 μg/kg, n = 69) of G-CSF–mobilized donor stem cell harvests against K562 (panel A) and Raji (panel B) tumor target cells by means of 51Cr release assay following in vitro activation with IL-2 for 72 hours. Values represent mean percentages of specific cytotoxicty + SEM from different samples. (For all E/T ratios for K562, P = .001. For Raji, E/T ratios of 12.5:1 were not significant, while for E/T ratios of 25:1, P = .01; 50:1, P = .01; 100:1, P = .05). ● indicates 5 μg/kg G-CSF; ○, 10 μg/kg G-CSF.
In vitro LAK cell–mediated cytotoxicity levels of MNCs.
In vitro LAK cell–mediated cytotoxicity levels of MNCs from 2 different doses (5 μg/kg, n = 35; 10 μg/kg, n = 69) of G-CSF–mobilized donor stem cell harvests against K562 (panel A) and Raji (panel B) tumor target cells by means of 51Cr release assay following in vitro activation with IL-2 for 72 hours. Values represent mean percentages of specific cytotoxicty + SEM from different samples. (For all E/T ratios for K562, P = .001. For Raji, E/T ratios of 12.5:1 were not significant, while for E/T ratios of 25:1, P = .01; 50:1, P = .01; 100:1, P = .05). ● indicates 5 μg/kg G-CSF; ○, 10 μg/kg G-CSF.
T- and B-cell mitogen responses
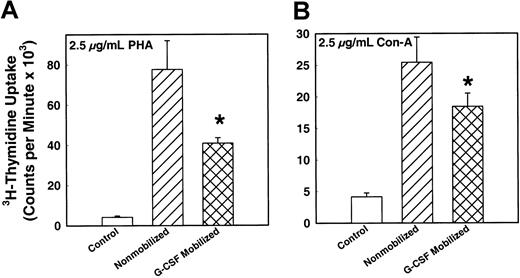
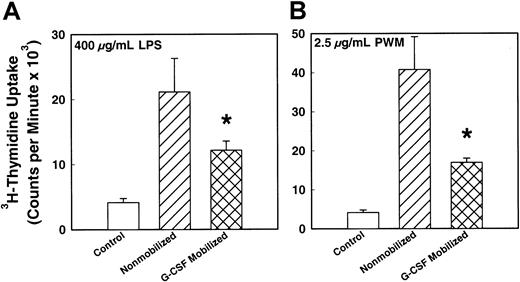
To determine the functional ability of T- and B-lymphocytes in the PBSC harvests, mitogen assay was performed by means of specific mitogens. Figure 4 demonstrates the T-lymphocyte response to 2 T-cell mitogens, PHA and Con-A. There was a significantly (P < .001) decreased T-cell mitogen response by MNCs from G-CSF–mobilized PBSC harvest compared with steady-state nonmobilized harvest (P < .005). The magnitude of decrease in the mitogen response of G-CSF–mobilized MNCs was 50% and 30% of the response of MNCs from nonmobilized donors for PHA and Con-A, respectively. Figure 5shows the B-lymphocyte response to mitogen LPS and PWM. As in the case of the T-lymphocytes' response to their mitogens, there was a significantly decreased response to LPS and PWM in MNCs from G-CSF–mobilized PBSC harvest compared with steady-state nonmobilized PBSC harvest. There was a 45% and 60% decrease in B-cell mitogen activity of G-CSF–mobilized MNCs compared with nonmobilized harvest for LPS and PWM, respectively. The G-CSF doses used for mobilization did not show any difference in mitogen response.
In vitro T-cell mitogen responses of MNCs from G-CSF–mobilized (n = 75) or nonmobilized steady-state (n = 28) donor blood stem cell harvests.
MNCs were cultured in vitro in the presence of 2.5 μg/mL mitogens and incubated for 72 hours. The proliferative response of the cells was measured by means of a 3H-thymidine uptake assay. (A) Response to mitogen Con-A (P < .001). (B) Response to mitogen PHA (P < .001). *indicates the statistical significance of the value as compared to nonmobilized blood.
In vitro T-cell mitogen responses of MNCs from G-CSF–mobilized (n = 75) or nonmobilized steady-state (n = 28) donor blood stem cell harvests.
MNCs were cultured in vitro in the presence of 2.5 μg/mL mitogens and incubated for 72 hours. The proliferative response of the cells was measured by means of a 3H-thymidine uptake assay. (A) Response to mitogen Con-A (P < .001). (B) Response to mitogen PHA (P < .001). *indicates the statistical significance of the value as compared to nonmobilized blood.
In vitro B-cell mitogen responses of MNCs from G-CSF–mobilized (n = 75) or nonmobilized steady-state (n = 28) donor blood stem cell harvests.
MNCs were cultured in vitro in the presence of mitogens and incubated for 72 hours. The proliferative response of the cells was measured by means of a 3H-thymidine uptake assay. (A) Response to mitogen LPS (P < .005). (B) Response to mitogen PWM (P < .005). *indicates the statistical significance of the value as compared to nonmobilized blood.
In vitro B-cell mitogen responses of MNCs from G-CSF–mobilized (n = 75) or nonmobilized steady-state (n = 28) donor blood stem cell harvests.
MNCs were cultured in vitro in the presence of mitogens and incubated for 72 hours. The proliferative response of the cells was measured by means of a 3H-thymidine uptake assay. (A) Response to mitogen LPS (P < .005). (B) Response to mitogen PWM (P < .005). *indicates the statistical significance of the value as compared to nonmobilized blood.
Immunophenotype analysis
Table 2 demonstrates the immunophenotypic analysis of MNCs from G-CSF–mobilized and steady-state nonmobilized PBSC harvests. Interestingly, there was no significant difference between
the 2 MNC populations with regard to their T cells, B cells, NK cells, and dendritic cells when their specific markers were used (Table 2). In addition, we compared the percentage of different immune cells from baseline blood samples collected before apheresis procedure to that of both apheresis samples, and all the baseline (Table 2) values were not different from either the nonmobilized or the G-CSF–mobilized apheresis products. The results of these phenotypic analyses also suggest that the decreased immune functions in G-CSF–mobilized PBSCs is not due to a difference in frequency of effector-cell content in the harvest.
Gene expression in G-CSF–mobilized PBSCs
To see whether the decrease in immune functions of G-CSF–mobilized MNCs is due to decrease in expression of some of the key cellular genes involved in effector functions, RT-PCR was carried out. The genes analyzed were IFN-γ, perforin, Fas, Fas ligand, and TNF-α and were quantitated by densitometric analysis. There was a significant decrease in expression of the TNF-α gene in MNCs from G-CSF–mobilized PBSCs as compared with MNCs from steady-state PBSCs (Figure 6A). However, there was no difference in expression of IFN-γ, perforin, Fas, and Fas ligand between MNCs from G-CSF–mobilized PBSCs and MNCs from nonmobilized steady-state PBSCs (Figure 6B-E), as demonstrated by the densitometric analysis of the results.
RT-PCR analyses of MNCs from G-CSF–mobilized (n = 6) and nonmobilized steady-state (n = 6) donor blood stem cell harvests.
RT-PCR analyses of MNCs from G-CSF–mobilized (n = 6) and nonmobilized steady-state (n = 6) donor blood stem cell harvest for the expression of perforin, Fas ligand, Fas, and TNF-α. (A) TFN-α. (B) IFN-γ. (C) Perforin. (D) Fas ligand. (E) Fas.
RT-PCR analyses of MNCs from G-CSF–mobilized (n = 6) and nonmobilized steady-state (n = 6) donor blood stem cell harvests.
RT-PCR analyses of MNCs from G-CSF–mobilized (n = 6) and nonmobilized steady-state (n = 6) donor blood stem cell harvest for the expression of perforin, Fas ligand, Fas, and TNF-α. (A) TFN-α. (B) IFN-γ. (C) Perforin. (D) Fas ligand. (E) Fas.
Correlation between donor characteristics and immune functions
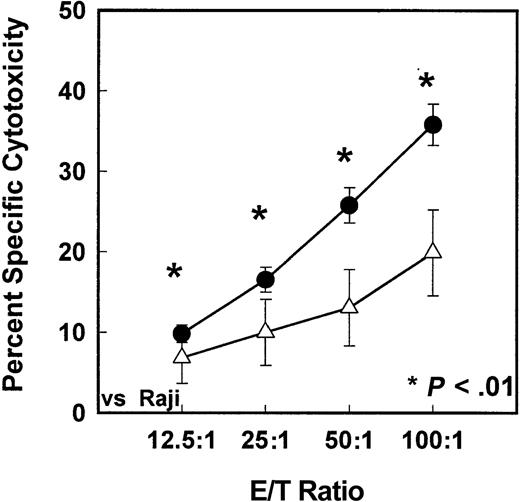
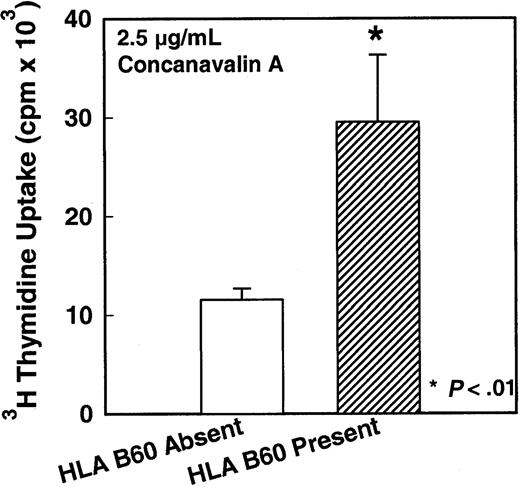
Since the immune response of MNCs from G-CSF–mobilized PBSC harvests was severely decreased compared with steady-state nonmobilized PBSC harvests, we analyzed the results of each of the immune assays with respect to G-CSF–mobilized donor characteristics. There was a significant association between expression of certain HLA alleles (A1, A3, A24, B44, B60, DR15, DR17; all P < .01) and increased immune function, such as cytotoxicity and/or mitogen response. A decrease in immune functions with the HLA-DR13 expression was also observed (P < .01). For the sake of brevity, a few examples of details of these results are given in the following figures. Figure 7A demonstrates the relationship between HLA-A3 expression and antitumor cytotoxic ability of MNCs from G-CSF–mobilized PBSC harvest. There was a significant increase in cytotoxicity against K562 tumor target cells in G-CSF–mobilized MNCs from donors who express HLA-A3 compared with donors who do not express HLA-A3 (P < .01). Similarly, Figure 7B demonstrates the relationship between HLA-DR17 expression and antitumor cytotoxic ability of MNCs from G-CSF–mobilized PBSC harvest. There was a significant increase in cytotoxicity against Raji tumor target cells in G-CSF–mobilized MNCs from donors who express HLA-DR17 compared with donors who do not express HLA-DR17 (P < .01). In contrast, lack of expression of another allele, HLA-DR13, correlated with increased cytotoxicity of IL-2–activated MNCs against Raji cells. There was a significant decrease in cytotoxicity against Raji tumor target cells in G-CSF–mobilized donors who express HLA-DR13 compared with donors who do not express HLA-DR13 (Figure 8) (P < .01). In addition to antitumor cytotoxicity, there was a correlation between expression of HLA-B60 and T-cell response to mitogen Con-A. Figure 9 demonstrates the relationship between HLA-B60 expression and mitogen response of MNCs from G-CSF–mobilized PBSC harvest. There was a significant increase in mitogen response in cells expressing HLA-B60 in G-CSF–mobilized donor MNCs compared with donors who do not express HLA-B60 (P < .01) (Figure 9). There was no significant correlation with the immune function and donor age or weight; however, there was correlation with donor gender and LAK cell–mediated cytotoxicity. MNCs from males had significantly lower response of LAK effector cells against Raji target cells compared with females (P < .01). There was no other statistically significant differences between males and females.
Cytotoxicity levels of HLA-A3– and HLA-DR17–expressing and HLA-A3– and HLA-DR17–nonexpressing MNCs.
(A) In vitro cytotoxicity levels of HLA-A3–expressing (n = 26) or HLA-A3–nonexpressing (n = 78) MNCs isolated from G-CSF–mobilized donor blood stem cell harvests against K562 tumor target cells (P < .01). ● indicates HLA-A3 absent; ▵, HLA-A3 present. (B) In vitro cytotoxicity levels of HLA-DR17–expressing (n = 19) or HLA-DR17–nonexpressing (n = 85) MNCs isolated from G-CSF–mobilized donor blood stem cell harvests against K562 tumor target cells (P < .01). ● indicates HLA-DR17 absent; ▵, HLA-DR17 present. * indicates the statistical significance of the value as compared to nonmobilized blood.
Cytotoxicity levels of HLA-A3– and HLA-DR17–expressing and HLA-A3– and HLA-DR17–nonexpressing MNCs.
(A) In vitro cytotoxicity levels of HLA-A3–expressing (n = 26) or HLA-A3–nonexpressing (n = 78) MNCs isolated from G-CSF–mobilized donor blood stem cell harvests against K562 tumor target cells (P < .01). ● indicates HLA-A3 absent; ▵, HLA-A3 present. (B) In vitro cytotoxicity levels of HLA-DR17–expressing (n = 19) or HLA-DR17–nonexpressing (n = 85) MNCs isolated from G-CSF–mobilized donor blood stem cell harvests against K562 tumor target cells (P < .01). ● indicates HLA-DR17 absent; ▵, HLA-DR17 present. * indicates the statistical significance of the value as compared to nonmobilized blood.
In vitro cytotoxicity levels of HLA-DR13–expressing or HLA-DR13–nonexpressing MNCs.
In vitro cytotoxicity levels of HLA-DR13–expressing (n = 17) or HLA-DR13–nonexpressing (n = 87) MNCs isolated from G-CSF–mobilized donors blood stem cell harvests against K562 tumor target cells (P < .01). ● indicates HLA-DR13 absent; ▵, HLA-DR13 present. *indicates the statistical significance of the value as compared to nonmobilized blood.
In vitro cytotoxicity levels of HLA-DR13–expressing or HLA-DR13–nonexpressing MNCs.
In vitro cytotoxicity levels of HLA-DR13–expressing (n = 17) or HLA-DR13–nonexpressing (n = 87) MNCs isolated from G-CSF–mobilized donors blood stem cell harvests against K562 tumor target cells (P < .01). ● indicates HLA-DR13 absent; ▵, HLA-DR13 present. *indicates the statistical significance of the value as compared to nonmobilized blood.
In vitro mitogen-response levels of HLA-B60–expressing or HLA-B60–nonexpressing MNCs.
In vitro mitogen-response levels of HLA-B60–expressing (n = 15) or HLA-B60–nonexpressing (n = 89) MNCs isolated from G-CSF–mobilized donors blood stem cell harvests (P < .01). *indicates the statistical significance of the value as compared to nonmobilized blood.
In vitro mitogen-response levels of HLA-B60–expressing or HLA-B60–nonexpressing MNCs.
In vitro mitogen-response levels of HLA-B60–expressing (n = 15) or HLA-B60–nonexpressing (n = 89) MNCs isolated from G-CSF–mobilized donors blood stem cell harvests (P < .01). *indicates the statistical significance of the value as compared to nonmobilized blood.
Expression of cytotoxicity results based on absolute number of MNCs
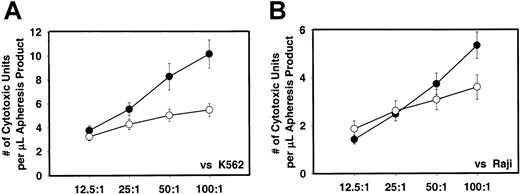
Since the G-CSF mobilization increases the total MNCs in the apheresis product, we also analyzed the results on the basis of absolute numbers of MNCs. Figure 10shows the results of such analyses. The cytotoxicity of IL-2–activated MNCs from G-CSF–mobilized apheresis products was significantly higher compared with nonmobilized apheresis product against K562 (P < .01) when expressed per microliter of product or absolute number of MNCs (Figure 10A). When similar analyses were done for the Raji cells as tumor targets, the results were not significantly different although the cytotoxicity with G-CSF–mobilized cells was higher (Figure 10B).
In vitro cytotoxicity of MNCs from G-CSF–mobilized and nonmobilized steady-state apheresis product.
In vitro cytotoxicity of MNCs from G-CSF–mobilized (n = 77) and nonmobilized steady-state apheresis product (n = 20) against K562 (panel A) and Raji (panel B) tumor target cells as expressed per absolute number of MNCs. ● indicates G-CSF mobilized donors; ○, nonmobilized donors.
In vitro cytotoxicity of MNCs from G-CSF–mobilized and nonmobilized steady-state apheresis product.
In vitro cytotoxicity of MNCs from G-CSF–mobilized (n = 77) and nonmobilized steady-state apheresis product (n = 20) against K562 (panel A) and Raji (panel B) tumor target cells as expressed per absolute number of MNCs. ● indicates G-CSF mobilized donors; ○, nonmobilized donors.
Expression of T- and B-lymphocyte mitogen response based on absolute number of MNCs
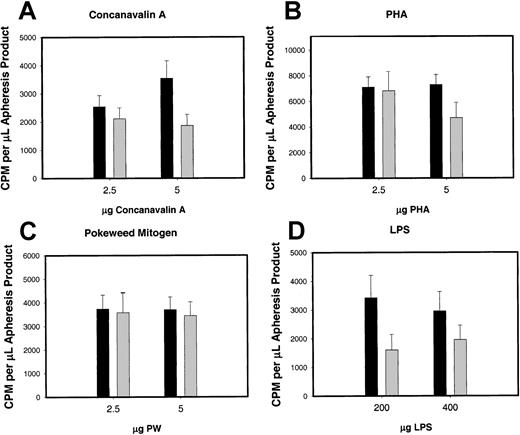
We also analyzed the results from mitogen responses of the MNCs from G-CSF–mobilized and nonmobilized apheresis product on the basis of absolute numbers. Figure 11shows these results. There was no statistically signficant difference between MNCs from G-CSF–mobilized and steady-state nonmobilized apheresis products for either T-lymphocyte mitogen response (PHA and Con A) (Figure 11A-B) or B-lymphocyte mitogen response (LPS and PWM) (Figure 11C-D).
In vitro T-cell and B-cell mitogen response of MNCs from G-CSF–mobilized or nonmobilized steady-state (n = 20) apheresis product.
In vitro T-cell and B-cell mitogen response of MNCs from G-CSF–mobilized (n = 77) or nonmobilized steady-state (n = 20) apheresis product as expressed per absolute number of MNCs. (A) T-lymphocyte mitogen response to PHA. (B) T-lymphocyte mitogen response to Con A. (C) B-lymphocyte mitogen response to LPS. (D) B-lymphocyte mitogen response to PWM. ▪ indicates G-CSF mobilized; ░, nonmobilized.
In vitro T-cell and B-cell mitogen response of MNCs from G-CSF–mobilized or nonmobilized steady-state (n = 20) apheresis product.
In vitro T-cell and B-cell mitogen response of MNCs from G-CSF–mobilized (n = 77) or nonmobilized steady-state (n = 20) apheresis product as expressed per absolute number of MNCs. (A) T-lymphocyte mitogen response to PHA. (B) T-lymphocyte mitogen response to Con A. (C) B-lymphocyte mitogen response to LPS. (D) B-lymphocyte mitogen response to PWM. ▪ indicates G-CSF mobilized; ░, nonmobilized.
Discussion
Transplantation of allogeneic PBSC harvests from healthy donors following mobilization with cytokines such as G-CSF has become an alternative to allogeneic bone marrow transplantation. Faster hematopoietic recovery and an increased number of hematopoietic stem/progenitor cells associated with G-CSF–mobilized peripheral blood apheresis harvests are added benefits of using this cytokine for mobilization. However, beyond hematopoietic reconstitution, long-term disease-free survival in these patients might depend on an antitumor effector-cell population to induce long-lasting graft-versus-tumor effects.9 In this regard, the results shown in this report indicate decreased immune effector functions in MNCs from G-CSF–mobilized PBSC harvests. Impairment in immune functions in NK-, LAK-, and T- and B-lymphocyte–mediated responses suggests a generalized immunosuppression.
The precise mechanisms of action of these immune dysfunctions observed in these studies is not yet known. They are not due to dilution of immune effector cells because the MNCs were purified from the apheresis product by means of density gradient separation. Furthermore, they are not due to decrease in the frequency of various immune effector cells since there are not significant changes between these 2 effector-cell populations (Table 2). As an additional effort to rule out the possibilities of differences in the frequency of the immune phenotypes, we also analyzed the immune function data on the basis of the absolute number of MNC population. The results were similar to those presented in this report. The cytomorphological examination of the MNC preparation showed the purity to be more than 90%. G-CSF mobilization might be altering the cytokine secretion from the MNCs; there might be an imbalance in type I and type II helper functions; or there might be a combination of both.22-25Our results show that there is a decreased expression of TNF-α gene in the G-CSF–mobilized MNCs. This decrease in TNF-α might have a role in at least some of the immune effector functions. Similar results have been reported by other investigators.16
Other investigators have reported the immune dysfunction associated with G-CSF–mobilized MNCs. Miller et al13 have reported functionally abnormal NK cells in G-CSF–mobilized blood stem cell harvests. Their results have shown that NK cells from G-CSF–mobilized PBSCs have defective functions. Kitabayashi et al16 have reported that G-CSF down-regulates allogeneic immune responses by posttranscriptional inhibition of TNF-α production by the MNCs. Taga et al26 have reported suppression of NK-cell activity by granulocytes in patients with aplastic anemia and have suggested that G-CSF might have a role in this inhibitory activity of NK cell–mediated cytotoxicity. Their study reports a significant depression of NK activity in patients who were not in remission and who received G-CSF administration. As in this study, granulocytes with natural suppressive activity might be one of the reasons for the decreased immune functions in G-CSF–mobilized MNCs. At present, we do not have any evidence for this phenomenon. Our study differs from the above-mentioned reports because of the large number of donors included in this study and the fact that results were compared with MNCs from steady-state nonmobilized donor apheresis harvests. Ageitos et al27 have reported monocyte-dependent inhibition of T-cell functions in blood stem cell harvests. When we separated the adherent monocyte populations from the MNC population in G-CSF–mobilized PBSCs, there was an increase in cytotoxicity of MNCs from G-CSF–mobilized harvests. However, all of these studies, including this report, warrant further studies to elucidate the precise mechanism of action for the decreased immune functions.
More interestingly, we have attempted to correlate the levels of immune functions and the donor characteristics, such as expression of certain HLA alleles (Figures 7-10) and donor weight, gender, and age. Several studies have reported an association between differences in HLA antigen expression and susceptibility to various diseases or immune effector functions of MNCs. In this regard, HLA-A3 is one of the widely studied antigens. Recently, Posthuma et al28 have demonstrated an association between HLA-A3 expression and decreased incidence of chronic myelogenous leukemia. A possible explanation is that HLA-A3–containing effector cells recognize the bcr-abl fusion protein, thus eliminating the tumor cells at an earlier stage. Several other studies also have implicated a role for an HLA allele in NK and T cell–mediated immune response.29-31 It is hoped that on the basis of our results, donor HLA type can be used as a predictor for donor immune function level as well as for donor selection. Alternatively, analysis of long-term follow-up for clinical outcome and the relationship between HLA expression and disease-free survival might demonstrate the significance of HLA expression and immune functions.
Although our results showed a significant decrease in NK, LAK, and T- and B-lymphocyte in vitro functions of G-CSF–mobilized MNCs, the clinical significance of these findings is not yet known. Because of the decreased immune functions of MNCs from G-CSF–mobilized donors, the severity or frequency of GVHD might be lower than for nonmobilized blood stem cell–transplanted patients. In fact, our clinical observation showed that patients who received G-CSF–mobilized blood stem cells had significantly less severe GVHD following allogeneic transplantation.32 In the current studies, we have not addressed the issue of mechanisms of action of G-CSF–induced immunosuppression. The decreased immune functions in G-CSF–mobilized MNCs might be due to secondary cytokines/monokines secreted by immune accessory cells. A detailed analyses of cytokine secretion pattern might shed light on the mechanism of action of decreased immune functions. However, Nawa et al33,34 and Mielcarek et al35 have reported that G-CSF administration suppresses T-cell responses to allogeneic stimulation by indirectly modulating monocyte function. Similarly, several other reports have indicated that certain monocytes in the G-CSF–mobilized PBSCs might be responsible for the immunodysfunctions.36-38 On the basis of our findings and other reports, the decreased immune functions of G-CSF–mobilized PBSCs might be beneficial for the patient at least in preventing occurrence of GVHD.
Initially, the pattern of immune reconstitution in the patients who received the G-CSF–mobilized blood stem cell harvests need to be analyzed for up to at least 1 year posttransplant. Furthermore, analyses of clinical outcome as measured by length of disease-free survival and/or time to relapse will be very important. These analyses will be of great interest in determining the significance of our findings. We have just begun the analyses addressing these issues.
Although on a cell-to-cell basis we have seen a significant decrease in cytotoxic ability and T- and B-cell mitogen response in MNCs from G-CSF–mobilized apheresis product in comparison with nonmobilized product, because of the higher number of MNCs in G-CSF–mobilized product, there would be no decrease in total immune response in vivo when these cells are transplanted into recipients. This may provide one possible explanation for not observing significance difference in the incidence of acute GVHD between patients who received marrow stem cells versus patients who received blood stem cells despite the fact that blood stem cell harvests contain a larger number of effector cells.
In summary, we have reported significantly decreased immune functions in MNCs from G-CSF–mobilized blood stem cell harvests as measured by NK, LAK, and T- and B-lymphocyte functions. In addition, relationship between donor characteristics, such as expression of certain HLA alleles, and immune-effector functions have been demonstrated. Future studies need to further investigate the immune functions and cytokine profiles of the MNCs from these harvests. These findings may have important clinical implications, especially in regard to acute GVHD and, potentially, a graft-versus-tumor effect observed with allogeneic stem cell transplantation. Furthermore, our results on the relationship between HLA-allele expression and certain immune functions in G-CSF–mobilized cells lay a foundation for further studies in this area. It is hoped that correlation of these and future trials with clinical outcome will lead to a further understanding of immune reconstitution and development of new treatment strategies in allogeneic stem cell transplants.
We thank Amgen for cytokines; Michelle Desler, Eileen Vu, Julie Sweeney, and Hemant Satpathy for their excellent technical assistance; and, especially, Professor David Crouse for critically evaluating the manuscript and Penni Davis for her excellent editorial assistance and for preparing this manuscript.
Partially supported by Amgen Research Funds.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Michael R. Bishop, Department of Experimental Transplantation and Immunology, National Cancer Institute, National Institutes of Health, Bldg 10, Rm 12N226, Bethesda, MD 20892.

![Fig. 1. In vitro NK cell–mediated cytotoxicity levels of MNCs. / In vitro NK cell–mediated cytotoxicity levels of MNCs from G-CSF–mobilized (n = 98) or nonmobilized steady-state (n = 28) donor blood stem cell harvests against K562 (panel A) and Raji (panel B) tumor target cells by means of 51Cr release assay. Values represent mean percentages of specific cytotoxicity ± SEM from different samples. (For effector-to-target [E/T] ratios of 12.5:1, P = .005; 25:1, P = .0001; 50:1,P = .007; 100:1 P = .05.) ● indicates nonmobilized donors (n = 28); ▵, G-CSF mobilized donors (n = 104).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/98/6/10.1182_blood.v98.6.1963/7/m_h81811531001.jpeg?Expires=1768901270&Signature=5B0GgTtjHc63c6ZM21BRtOr99tvqPcCOiW9~PINBWEaehcllFnD1mFGcmiAsb0z0LZC5pjajjLeXg61LZXfHeng~0YQomzipObIN-b6PBBzzgG0MomT~nyuADxJwz36TozAd-a1V54ir1dlBa9d-CunfJxNMm3MXT2ZBel7BjXHP6kOdIYo4z0ZgGIBh8T~30mS0VmbGMZ1gy~X2FyAgP~CbRsu4LTGMJC32Pw7caChxcIqHJ1PconxTlzQqSvPLsiX6qZy~D2rjAv17xdK1boQSs71HQxu-8CwT9ZxFm3W7yqG9YLW-eoZq93teRIAIVf~TiC6ZH6JhwTe6w8YWpQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)