Interleukin-10 (IL-10) is a multifunctional cytokine that can exert suppressive and stimulatory effects on T cells. It was investigated whether IL-10 could serve as an immunostimulant for specific CD8+ cytotoxic T cell (CTL) in vivo after vaccination and, if so, under what conditions. In tumor prevention models, administration of IL-10 before, or soon after, peptide-pulsed primary dendritic cell immunization resulted in immune suppression and enhanced tumor progression. Injection of IL-10, however, just after a booster vaccine significantly enhanced antitumor immunity and vaccine efficacy. Analysis of spleen cells derived from these latter animals 3 weeks after IL-10 treatment revealed that the number of CD8+CD44hi CD122+ T cells had increased and that antigen-specific proliferation in vitro was enhanced. Although cytotoxicity assays did not support differences between the various treatment groups, 2 more sensitive assays measuring antigen-specific interferon-γ production at the single-cell level demonstrated increases in the number of antigen-specific responder T cells in animals in the vaccine/IL-10 treatment group. Thus, IL-10 may maintain the number of antitumor CD8+ T cells. In adoptive transfer studies, the ability of IL-10 to maintain CTL function could be enhanced by the depletion of CD4+ T cells. This suggests that IL-10 mediates contrasting effects on both CD4+ and CD8+ T cells that result in either immune dampening or immune potentiation in situ, respectively. Appreciation of this dichotomy in IL-10 immunobiology may allow for the design of more effective cancer vaccines designed to activate and maintain specific CD8+ T-cell effector function in situ.
Introduction
Dendritic cells (DCs) play a key role in the initiation of CD8+ cytotoxic T cell (CTL)–mediated immune responses and have been used successfully in cancer vaccines.1,2 We have previously reported that CD8+ CTLs can be generated from naive precursors using DC preloaded with leukemia antigens as stimulators.3-5Leukemic cell-derived DCs have been used to successfully treat patients with chronic myelogenous leukemia.6 To more effectively treat patients with cancer, vaccines must not only amplify antitumor CTL responses, they must maintain them to preclude disease recurrence. The main predictor of a strong response versus a weak CTL appears to be the size of a proliferative burst.7,8 However, for the promotion of long-term effector CTL activity, the participation of T-cell growth and antiapoptotic factors, such as interleukin 2 (IL-2), IL-10, IL-12, and IL-15 appear important.9-14 In this study, we have evaluated whether IL-10 plays a dominant role in supporting the maintenance of effector CD8+ T-cell function after initial priming in situ.
IL-10, which is produced by a variety of cells including T lymphocytes, B lymphocytes, and monocytes, has been identified as a key immunomodulatory cytokine15,16 capable of mediating both immunosuppressive and immunostimulant effects.17 In vitro studies showed that IL-10 inhibits antigen-specific activation and proliferation of human CD4+ T cells, at least in part by down-regulating the cytokine production (ie, IL-2 and tumor necrosis factor-α).18,19 IL-10 also reduces the expression of CD54 (ICAM-1), CD80, CD86, and major histocompatibility complex (MHC) class II on monocytes and DCs, resulting in incomplete T-cell signaling and the induction of long-lasting, antigen-specific anergy.20,21 In contrast, IL-10 enhances the proliferative responses of murine IL-2– and IL-4–activated CD8+ T cells and can rescue T cells from apoptotic cell death.22,23Injection of high doses of IL-10 in mice with graft-versus-host disease results in the exacerbation of this T-cell–mediated disease.24,25 Groux et al26 showed that in an IL-10 transgenic model, IL-10 enhances the promotion of antitumor CD8+ T cells, leading to the reduced growth of immunogenic tumors. In addition, our group and others27-29 have previously shown that at high doses (40 μg/day for 7 days), IL-10 induces the rejection of certain tumors, delaying tumor outgrowth or resulting in complete cure. These findings suggest IL-10 may mediate either immunosuppressive or immunostimulatory effects on antitumor activities for CD8+ T cells in vivo, though the mechanism for its activation remains poorly delineated. In this study, we have evaluated the impact of IL-10 administration on specific CD8+ T effector cell priming and so-called memory in a series of murine tumor models.
Materials and methods
Mice and cell lines
Female C57BL/6 mice, 6 to 10 weeks of age, were purchased from The Jackson Laboratory (Bar Harbor, ME), and OT-1 mice were the kind gifts of Dr Lou Falo (University of Pittsburgh, PA). Mice were maintained and treated in the Central Animal Facility, University of Pittsburgh, according to institutional animal care committee guidelines. EL-4 (H-2b) lymphoma cells, the derivative ovalbumin (OVA)–expressing EG.7-OVA cells, were obtained from American Type Culture Collection (Rockville, MD). EG.7-OVA transfectants carrying the neo marker were maintained in the presence of geneticin (400 μg/mL) (Life Technologies, Gaithersburg, MD). MC38 colorectal adenocarcinoma was generously provided by Dr S. A. Rosenberg (National Cancer Institute, Bethesda, MD). A CTL clone reactive against MC38 was previously established in our group.30 These cell lines were grown in complete medium (RPMI 1640 containing 10% fetal bovine serum, 2 mM L-glutamine, 100 U/mL penicillin, and 100 μg/mL streptomycin [all from Life Technologies]). The CTL clone was restimulated with irradiated B7-1–expressing MC38 in the presence of recombinant human (rh) IL-2 (60 U/mL) on a weekly basis, as described.30
Cytokines and peptide
rhIL-10, recombinant murine (rm) granulocyte macrophage–colony-stimulating factor (mGM-CSF), and murine IL-4 (mIL-4) were provided by Schering-Plough (Kenilworth, NJ), and human IL-2 (hIL-2) was generously donated by the Chiron (Emeryville, CA). rhIL-15 was purchased from Genzyme (Cambridge, MA). High-performance liquid chromatography-purified peptide (more than 95% pure) of OVA epitope (amino acids 257-264, SIINFEKL, H-2Kb restricted) and irrelevant peptide (SIINFKEL) were provided by a peptide synthesis facility (University of Pittsburgh), dissolved in sterile dimethyl sulfoxide at 10 mg/mL, and stored at −80°C until use.
Preparation of dendritic cells
Dendritic cells were prepared from bone marrow cultures as previously described.31 32 Briefly, bone marrow was flushed from femurs and tibias and subsequently depleted of erythrocytes with ammonium chloride. Bone marrow cells were depleted of T cells and B cells using a mixture of monoclonal antibodies (anti-CD4/rat immunoglobulin [Ig] G2b, GK1.5; anti-CD8/rat IgG2b, 53-6.7; anti-B220/rat IgG2b obtained from ATCC) and rabbit complement (Cedarlane Laboratories, Ontario, Canada). Cells were cultured in complete medium containing 20% fetal bovine serum supplemented with mGM-CSF (1000 U/mL) and mIL-4 (1000 U/mL). At day 7, nonadherent cells were collected and further purified by first blocking with mouse serum (Sigma, St Louis, MO) and then positively selecting with hamster anti–mouse CD11c magnetic beads (Miltenyi Biotec, Auburn, CA). Cells were washed 3 times with phosphate-buffered saline (PBS; Life Technologies) and were used for subsequent experiments.
Tumor protection model: vaccination with peptide-pulsed dendritic cells or cytotoxic T cell adoptive transfer
In the preliminary tests, we determined the optimal concentration of OVA peptide as 1 μM peptide for use in our study by comparing vaccines consisting of DCs that had been preincubated with varying concentrations of OVA peptide (1 nM-100 μM) for 2 hours. For our studies, 2 × 106 tumor cells (EG.7-OVA) were selected as a standard because this was the maximal dose that could be effectively protected against by prior vaccination with 1 × 106 DCs. In DC vaccination models, mice were randomized and injected intravenously with 1 × 106peptide-pulsed DC. One week later, syngeneic tumor cells (2 × 106 for EG.7-OVA) suspended in 0.1 mL Hanks balanced salt solution (HBSS; Life Technologies) were injected subcutaneously on one flank. For studies involving IL-10 administration, 0.2 mL rhIL-10 (40 μg/mouse per day) in HBSS was injected intraperitoneally once daily for 4 consecutive days after DC vaccination models, or for 7 days after CTL adoptive transfer. In CTL adoptive transfer studies (EG.7-OVA and MC38), mice were injected intravenously with activated CTLs (1 × 106 reactive against EG.7-OVA and 1 × 105 reactive against MC38). One week later, syngeneic tumor cells (2 × 106 for EG.7-OVA or 3 × 105 for MC38) suspended in 0.1 mL HBSS were injected subcutaneously in one flank. In both vaccination models, serial micro-caliper measurements of perpendicular tumor diameters were obtained in a masked fashion. For the evaluation of in vivo immune response, the phenotype and the total number of each CD8+T-cell subpopulation, such as CD8+ CD44+ CD122 (IL-2Rβ)+ T cells or CD8+ CD44+IFN-γ-expressing T cells, in the spleen were evaluated. Tumor size was measured every 2 to 4 days, and tumor volumes were calculated using the formula: longest diameter × shortest diameter. Tumor volumes were depicted as the mean tumor area in graphic representation of tumor progression–regression. All experiments for protection models were performed using 5 mice per group, and experiments for the analysis model used 3 mice per group. Each experiment was confirmed at least once, as indicated.
Proliferation assay in the time course in the vaccination models
Cell proliferation was measured by [3H]-thymidine incorporation in 96-well round-bottom plates (Costar, Cambridge, MA) by using 1 × 106 spleen cells/well, pulsed with and without peptide, or added hIL-2 (100 U/mL), hIL-15 (100 U/mL), and PMA (25 ng/mL) plus ionomycin (0.5 μM) (Sigma) as indicated. After 48 hours, 0.037 MBq/well (1 μCi/well) [3H]-thymidine (NENTH Life Science Products, Boston, MA) was added, and cells were harvested 16 hours later.
Flow cytometric and tetramer staining
Single-cell suspensions of spleen cells were washed in PBS containing 0.1% bovine serum albumin and 0.02% sodium azide and were incubated for 20 minutes on ice with purified anti–mouse CD16/CD32 (Fcγ III/II receptor) monoclonal antibody (mAb; 2.4G2; PharMingen, San Diego, CA) to block Fc receptors. For detecting the activated–memory CD8+ T cells (CD8+CD44hi CD122+ T cell), phycoerythrin (PE)–conjugated anti-CD44 (Pgp-1, Ly24) and fluorescein isothiocyanate (FITC)–conjugated anti-CD122 (IL-2Rβ) (TM-β1) expression in CD8+ T cells were performed by gating peridinin chlorophyll protein (Per CP)–conjugated anti-CD8+ (53-6.7) T cells from spleen cells. Total CD8+ CD44hi CD122+ cells were designated as activated–memory CD8+ T cells during time course experiments. In cases of tetramer staining, anti–mouse CD8a mAb (Caltag Laboratories, Burlingame, CA) was used for blocking nonspecific binding. These cells were then stained using FITC-conjugated Vβ5.1, 5.2 mAb (MR9-4), and PE-conjugated MHC class I (H-2Kb) tetramer complexed with OVA257-264 peptide, which was provided by the NIAID tetramer facility (Rockville, MD). For detecting OVA-specific activated CD8+ T cells (CD8+CD44hi tetramer+), FITC-conjugated anti-CD44 and PE tetramer were evaluated in CD8+ T cells by gating Per CP-CD8+ T cells. Other antibodies used in phenotype analysis included FITC-conjugated anti-CD4 (GK1.5), anti-CD8a (53-6.7), anti-CD25 (7D4), anti-CD69 (H1.2F3), anti-CD62L (MEL-14), anti-Ly6C (AL-21), anti-CD45R/B220 (RA3-6B2), and PE-conjugated anti-CD8a, anti-NK1.1 (PK136); all were purchased from BD Pharmingen (San Jose, CA). Samples were acquired on a FACScan flow cytometer, and the data were analyzed using Cellquest software (Becton Dickinson).
Intracellular cytokine staining and flow cytometry
Spleen cells from DC-vaccinated mice or naive mice were cultured at 37°C in 5% CO2 for 24 hours in the presence or absence of 1 × 10−7 M peptide (OVA257-264) in complete medium. Brefeldin A (Golgistop; PharMingen) was added at a final concentration of 1 μl/mL during the last 5 hours of the culture period. After culture, the cells were harvested, washed once in FACS buffer, and surface stained in FACS buffer with CD8a-FITC for CTL or with CD8a-Per CP and CD44-FITC for spleen cells. After washing the unbound antibody, cells were subjected to intracellular cytokine stain using the Cytofix/Cytoperm kit according to the manufacturer's instructions (PharMingen). For intracellular interferon γ (IFN-γ), IL-2, and IL-4 staining, we used PE-conjugated monoclonal rat anti–mouse IFN-γ antibody (XMG 1.2), anti–mouse IL-2 antibody (S4B6), and anti–mouse IL-4 antibody (BVD4-1D11) and its isotype control antibody (rat IgG) (PharMingen). Samples were resuspended in PBS containing 2% formaldehyde and acquired on either a FACScan (50 000 gated events acquired per sample) and analyzed using Cellquest software (Becton Dickinson). Total numbers of IFN-γ–producing cells in the spleen were evaluated in kinetic time course experiments.
Single-cell enzyme-linked immunospot assay for interferon-γ–secreting cells
For detecting tumor-specific CD8+ T cells, enzyme-linked immunospot (ELISPOT) assays were performed. For preparation of the first antibody-coated plates, 96-well filtration plates (Millipore, Bedford, MA) were coated with rat anti–mouse IFN-γ antibody (20 μg/mL) (clone R4-6A2; PharMingen) for 16 hours. Spleen cells (5 × 106 cells/well) from each group were incubated for 36 hours either with or without peptide stimulation (1 × 10−7 M OVA peptide) or were incubated for 24 hours with EG.7-OVA or EL4 (1.25 × 105 cells/well) at an effector–target ratio of 40:1. After culture, the plates were washed and then incubated with biotinylated anti–mouse IFN-γ antibody (4 μg/mL) (clone XMG 1.2; PharMingen). Spots were developed using avidin-peroxidase-complex (Vectastain Elite Kit; Vector Laboratories, Burlingame, CA) and fresh, prepared substrate buffer (0.4 mg/mL 3-amino-9-ethyl-carbazole in 2.5 mL N, N-dimethyl formamide [Sigma] and 0.015% H2O2 in 0.2 M sodium acetate and 0.2 N acetic acid). The frequency of peptide-specific CD8+T cells was calculated based on the percentage of CD8+ T cells in the responding population. Spots were counted microscopically.
Transfer study of purified naive or activated CD8+ T cells into the syngeneic mice
For OVA-specific T-cell transfer studies, magnetically purified naive and activated CD8+ OT-1 (OT-1) T cells were used. After removing adherent cells by 2-hour culture, cells pretreated with mouse serum (Sigma) and then incubated with anti-CD8a mAb labeled microbeads (Miltenyi Biotec) by using 2 beads/cell, and they were isolated on a SuperMACS cell sorter (Miltenyi Biotec). Purity of the eluted cells was more than 97%. This procedure did not activate naive or activated cells (T-cell activation; ie, neither cytokine secretion nor proliferation in vitro was shown). For the generation of CTL (effector cell) from OT-1 transgenic mice, 1 × 106spleen cells per milliliter from OT-1 mice were stimulated with irradiated (30 Gy), 1 × 10−6 M OVA peptide-pulsed syngeneic C57BL/6 spleen cells (1 × 106 cells/mL) in 24-well plates (Costar). After 36 hours, OT-1 T cells were isolated using anti-CD8a mAb-labeled microbeads, investigated for the phenotype of activation marker, and tested for cytotoxicity for the use of transfer studies.
In the OT-1 T-cell transfer studies, we adoptively transferred naive or activated OT-1 T cells into syngenic C57BL/6 mice on day 0, which was followed by IL-10 injection. Seven days later, we analyzed the frequency of specific T cells in spleen using tetramer and anti-Vβ5.1 and 5.2 mAb. In some experiments, natural killer (NK) cells, CD4+ T cells, or CD8+ T cells were depleted by the administration of anti-asialo GM1 mAb (Wako Chemicals USA, Richmond, VA), anti-CD4 mAb (GK1.5), and anti-CD8 mAb (53-6.7) (ATCC) 2 days before injection of OT-1 T cells. In the CTL clone transfer studies, we investigated the therapeutic effects of adoptive transfer of 1 × 106 CTL specific for OVA or 1 × 105 CTL specific for MC38 into syngeneic mice, followed by challenge with the relevant tumor cells (2 × 106 EG.7-OVA or 3 × 105 MC38).
Statistical analysis
Statistical analysis in our experiments was performed using standard Student t tests.
Results
Analysis of the efficacy of vaccines consisting of peptide-pulsed dendritic cells, combined with IL-10 treatment
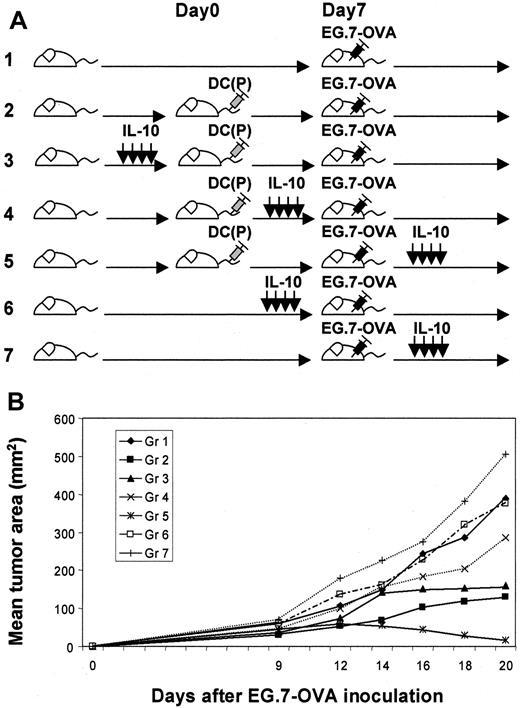
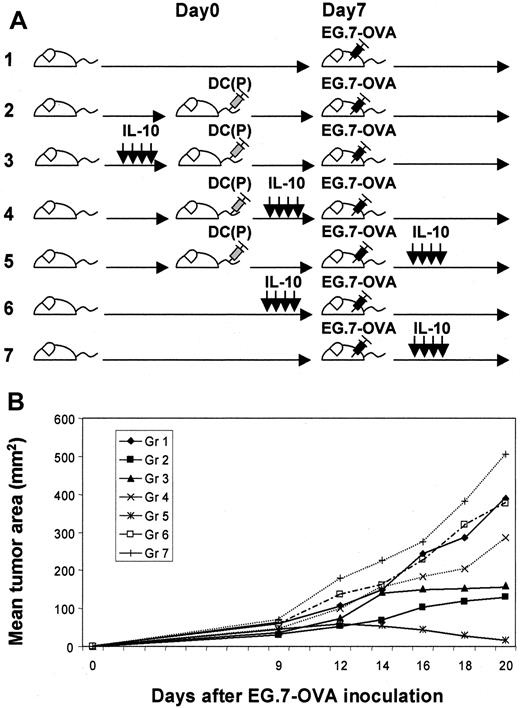
For investigation of the optimal phase for the efficacy of IL-10, we modulated the time of administration of IL-10 in the tumor protection models after OVA peptide-pulsed DC vaccination (Figure1A). Immunized mice in group 5 (IL-10 after EG.7-OVA challenge) exhibited transient tumor growth that regressed after 18 to 20 days; however, mice in other groups, such as groups 2 (no IL-10) and 3 (IL-10 before DC vaccination), displayed gradually progressive tumor growth (Figure 1B). This suggests that vaccination by peptide-pulsed DCs could be enhanced with IL-10 administration in vivo under certain conditions. To further analyze the impact of immunization by DCs and IL-10 in group 5, we characterized the responder splenocytes harvested from this group in comparison to other groups (Figure 2). Proliferation assays with and without IL-10, antigen, cytokine (IL-2 or IL-15), or PMA–ionomycin were assessed using splenocytes harvested from mice 14, 21, and 28 days after DC vaccination. Splenocytes from mice administered IL-10 exhibited good proliferative responses to peptide, especially at later time points (more than 21 days later) (group 1 vs group 4; P < 0.05 on day 14, P < 0.01 on days 21 and 28). Moreover, such splenocytes responded to IL-2/IL-15 (P < 0.01) but not to PMA–ionomycin, suggesting that they might represent memory T cells (Figure3). In these groups, no statistical phenotypic differences were noted in the fractions of CD4+T cells, CD8+ T cells, B cells, and NK cells (data not shown). We performed comparative phenotypic analyses of lymphocyte subpopulations by gating for CD8+ T cells, and the numbers of CD8+ CD44hi CD122 (IL-2Rβ)+ T cells (activated–memory T cells) were determined to be elevated in groups 3 and 4 on day 21 (groups 1, 2 vs group 3,P < .05; groups 1, 2 vs group 4, P < .05; group 3 vs group 4, P > .05 on day 21; butP > .01 for all on days 14 and 28), suggesting that activated CD8+ T cells could be increased by systemic IL-10 administration (Figure 4). In group 3, the progressive decline of size after day 21 may support the therapeutic efficacy of this enhanced cell population. As depicted in Figure 5, effector CD8+CD44hi T cells expressing IFN-γ on day 21 were detected by intracellular analysis in group 3 (groups 1, 4 vs group 3,P < .05; group 2 vs group 3, P > .05; on day 28, groups 1, 4 vs group 3, P < .0001; group 2 vs group 3, P = .005). In preliminary tests in which activated OT-1 T cells were assayed for both cytotoxicity and IFN-γ production against EG.7-OVA target cells, we confirmed that CTL function correlated well with IFN-γ production deduced by ELISPOT assays (data not shown). In addition, specific CD8+ T-cell reactivation was also documented in Figure6 using ELISPOT assays and specific stimuli (ie, OVA peptide and EG.7-OVA) in groups. On day 21, statistical differences were noted: groups 1, 2 vs group 3,P < .05; group 3 vs group 4, P < .01 for EG.7-OVA; group 1 vs group 3, P < .01; groups 2, 4 vs group 3, P > .05 for OVA peptide; on day 28, groups 1, 2, 4 vs group 3, P < .01 for EG.7-OVA,P < .0001 for OVA peptide. This suggests antigen-specific CD8+ effector cells producing IFN-γ can be rapidly activated and can mediate therapeutic functions relevant to the clinical benefit associated with animals in group 3.
Evaluation of various schemas for the administration of IL-10 in tumor protection models involving vaccination with peptide-pulsed DCs.
(A) Fundamentally, we evaluated several tumor challenge models involving DC vaccination. We compared the various indicated application schedules for IL-10 administration in such models. (B) EG.7-OVA tumor growth in vaccinated mice was evaluated for each of these treatment schemas. In group 5, tumors grew transiently but decreased afterward, whereas in other groups tumors grew progressively. In groups 2 and 3, tumors grew progressively after 30 days. These data represent the mean tumor area for 5 mice per group. Standard deviations were less than 15%. This experiment was performed 3 times, with comparable data obtained in each experiment.
Evaluation of various schemas for the administration of IL-10 in tumor protection models involving vaccination with peptide-pulsed DCs.
(A) Fundamentally, we evaluated several tumor challenge models involving DC vaccination. We compared the various indicated application schedules for IL-10 administration in such models. (B) EG.7-OVA tumor growth in vaccinated mice was evaluated for each of these treatment schemas. In group 5, tumors grew transiently but decreased afterward, whereas in other groups tumors grew progressively. In groups 2 and 3, tumors grew progressively after 30 days. These data represent the mean tumor area for 5 mice per group. Standard deviations were less than 15%. This experiment was performed 3 times, with comparable data obtained in each experiment.
Models for analysis of vaccinated mice treated with IL-10.
Vaccinated mice were compared with several controls groups either with or without tumor challenge or with or without IL-10 administration.
Models for analysis of vaccinated mice treated with IL-10.
Vaccinated mice were compared with several controls groups either with or without tumor challenge or with or without IL-10 administration.
Cell proliferation over time with and without IL-10 in the vaccination models.
Proliferation assays were performed in the presence and absence of antigen, cytokines (100 U/mL of IL-2 and IL-15, respectively) or PMA–ionomycin (PMA, 25 ng/mL; ionomycin, 0.5 μM) using splenocytes (1 × 106 cells/well) harvested from mice 14, 21, and 28 days after DC vaccination in each group depicted in Figure 2. After 48-hour culture, 0.037 MBq (1 μCi) [3H]-thymidine was added, and cells were harvested 16 hours later. Statistically significant differences relative to DC-vaccinated mice that did not receive IL-10 are indicated. Control mice were immunized with DCs in the absence of peptide. Each bar represents the mean of 3 mice evaluated.
Cell proliferation over time with and without IL-10 in the vaccination models.
Proliferation assays were performed in the presence and absence of antigen, cytokines (100 U/mL of IL-2 and IL-15, respectively) or PMA–ionomycin (PMA, 25 ng/mL; ionomycin, 0.5 μM) using splenocytes (1 × 106 cells/well) harvested from mice 14, 21, and 28 days after DC vaccination in each group depicted in Figure 2. After 48-hour culture, 0.037 MBq (1 μCi) [3H]-thymidine was added, and cells were harvested 16 hours later. Statistically significant differences relative to DC-vaccinated mice that did not receive IL-10 are indicated. Control mice were immunized with DCs in the absence of peptide. Each bar represents the mean of 3 mice evaluated.
Assessment of memory type CD8+ T cells in vaccinated mice.
After vaccination, the number of CD8+ T cells expressing activated–memory phenotype (CD44hi CD122+) were estimated by flow cytometry. On day 21, cells expressing these phenotypes increased in IL-10–treated mice (groups 3 and 4) (left), and the absolute number in spleen increased until day 28 (right). (On day 21, group 3 vs group 1, [P < .05]; group 3 vs group 2 [P < .01]; group 3 vs group 4 [P > .05]). The absolute number of such T cells in spleen was thus calculated as (percentage × spleen cell number). Mean values are shown for 3 mice evaluated at each time point.
Assessment of memory type CD8+ T cells in vaccinated mice.
After vaccination, the number of CD8+ T cells expressing activated–memory phenotype (CD44hi CD122+) were estimated by flow cytometry. On day 21, cells expressing these phenotypes increased in IL-10–treated mice (groups 3 and 4) (left), and the absolute number in spleen increased until day 28 (right). (On day 21, group 3 vs group 1, [P < .05]; group 3 vs group 2 [P < .01]; group 3 vs group 4 [P > .05]). The absolute number of such T cells in spleen was thus calculated as (percentage × spleen cell number). Mean values are shown for 3 mice evaluated at each time point.
IFN-γ production by CD8+CD44hi cells in vaccinated mice.
Using flow cytometry-bound assessment of cytokine production on day 21, phenotypically high expression of IFN-γ was demonstrated in either IL-10–administered or antigen-challenged groups (groups 2-4) (left). However, the absolute number of IFN-γ–producing CD8+CD44hi cells in spleen persisted in groups 2 and 3 through day 28. (On day 21, group 3 vs group 1 [P < .05]; group 3 vs group 2 [P > .05]; group 3 vs group 4 [P < .05]; on day 28, groups 1, 4 vs group 3 [P < .0001]; group 2 vs group 3 [P < .01]). The absolute number of IFN-γ–secreting T cells in spleen was calculated as (percentage × spleen cell number). Mean values are shown for 3 mice at each time point.
IFN-γ production by CD8+CD44hi cells in vaccinated mice.
Using flow cytometry-bound assessment of cytokine production on day 21, phenotypically high expression of IFN-γ was demonstrated in either IL-10–administered or antigen-challenged groups (groups 2-4) (left). However, the absolute number of IFN-γ–producing CD8+CD44hi cells in spleen persisted in groups 2 and 3 through day 28. (On day 21, group 3 vs group 1 [P < .05]; group 3 vs group 2 [P > .05]; group 3 vs group 4 [P < .05]; on day 28, groups 1, 4 vs group 3 [P < .0001]; group 2 vs group 3 [P < .01]). The absolute number of IFN-γ–secreting T cells in spleen was calculated as (percentage × spleen cell number). Mean values are shown for 3 mice at each time point.
Tumor antigen-specific IFN-γ production by responding splenocytes harvested from vaccinated mice (ELISPOT).
(A) Splenocyte IFN- γ production specific for tumor cells (EG.7-OVA) was shown in group 3 mice by ELISPOT assay. Spleen cells (5 × 106 cells/well) from each group were incubated for 24 hours with EG7-OVA or irrelevant tumor cell (EL4). (On day 21, group 3 vs group 2 [P < .05]; and groups 1, 4 vs group 3 [P < .01]; on day 28, group 3 vs group 1 [P < .01]; group 3 vs group 2 [P < .01]; group 3 vs group 4 [P < .005]). (B) These specific cells were also OVA peptide-specific CD8+ T cells. Spleen cells (5 × 106 cells/well) from each group were incubated for 36 hours with OVA peptide (SIINFEKL) or irrelevant peptide (SIINFKEL). These peptide-specific spots in each group were similar to the responding spots for EG7-OVA in Figure 6A. (On day 21, group 3 vs groups 2, 4 [P > .05]; group 1 vs group 3 [P < .01]; on day 28, group 3 vs group 1 [P < .0001]; group 3 vs group 2 [P < .0001]; group 3 vs group 4 [P < .0001]). Mean values are shown for 3 mice at each time point.
Tumor antigen-specific IFN-γ production by responding splenocytes harvested from vaccinated mice (ELISPOT).
(A) Splenocyte IFN- γ production specific for tumor cells (EG.7-OVA) was shown in group 3 mice by ELISPOT assay. Spleen cells (5 × 106 cells/well) from each group were incubated for 24 hours with EG7-OVA or irrelevant tumor cell (EL4). (On day 21, group 3 vs group 2 [P < .05]; and groups 1, 4 vs group 3 [P < .01]; on day 28, group 3 vs group 1 [P < .01]; group 3 vs group 2 [P < .01]; group 3 vs group 4 [P < .005]). (B) These specific cells were also OVA peptide-specific CD8+ T cells. Spleen cells (5 × 106 cells/well) from each group were incubated for 36 hours with OVA peptide (SIINFEKL) or irrelevant peptide (SIINFKEL). These peptide-specific spots in each group were similar to the responding spots for EG7-OVA in Figure 6A. (On day 21, group 3 vs groups 2, 4 [P > .05]; group 1 vs group 3 [P < .01]; on day 28, group 3 vs group 1 [P < .0001]; group 3 vs group 2 [P < .0001]; group 3 vs group 4 [P < .0001]). Mean values are shown for 3 mice at each time point.
Enhancement of antigen-specific CD8+ T cell function by interleukin-10
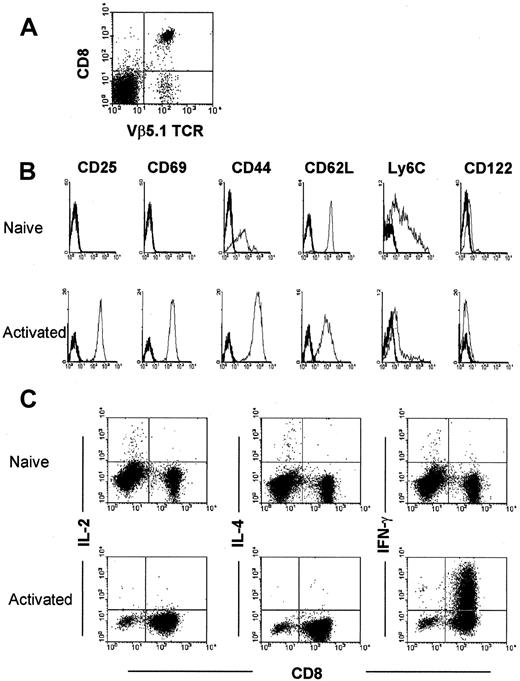
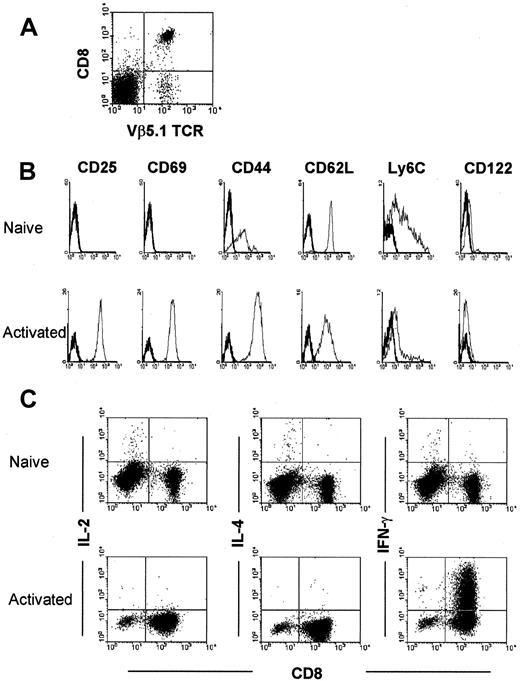
To study the interaction between rapidly activated CD8+ T cell and IL-10, we established that activated T cells express CD25, CD69, CD44hi, and CD62Llowphenotypes and produce IFN-γ (45.3%) on short-term (36 hours) culturing of OT-1 T cells with peptide (Figure7).
Characterization of naive and activated OVA-specific OT-1 T cells from spleen cells of OT-1 mice.
(A) Isolated CD8+ T cells from spleen cells of OT-1 mice were analyzed by 2-color flow cytometric analysis using anti-CD8 and anti-Vβ 5.1 and 5.2 mAbs. (B) After 36 hours culture with 1 × 10−6 M OVA peptide-pulsed syngeneic C57BL/b spleen cells, these T cells expressed an activated phenotype—ie, they expressed CD25, CD69, and CD44 antigen. (C) They also produced IFN-γ but not IL-2 and IL-4.
Characterization of naive and activated OVA-specific OT-1 T cells from spleen cells of OT-1 mice.
(A) Isolated CD8+ T cells from spleen cells of OT-1 mice were analyzed by 2-color flow cytometric analysis using anti-CD8 and anti-Vβ 5.1 and 5.2 mAbs. (B) After 36 hours culture with 1 × 10−6 M OVA peptide-pulsed syngeneic C57BL/b spleen cells, these T cells expressed an activated phenotype—ie, they expressed CD25, CD69, and CD44 antigen. (C) They also produced IFN-γ but not IL-2 and IL-4.
To study whether the number and function of these activated T cells was affected by IL-10 in vivo, we transferred naive or activated OT-1 T cells into syngeneic C57BL/6 mice. Using a double-staining assay with FITC-conjugated anti-Vβ 5.1 mAb and the PE-tetramer, we determined that IL-10 did not expand the number of naive OT-1 T cells in transferred mice but that it augmented the number of activated OT-1 T cells (Figure 8A). To compare the functional properties of naive and activated OT-1 T cells, ELISPOT assays were performed. Enhancement of cytotoxicity was shown for immunized mice provided activated OT-1 T cells and IL-10 (naive group with vs without IL-10, P > .01; activated group with vs without IL-10, P < .01) (Figure 8B). In addition, the activated CTL did not show significantly different ability to mediate the lysis of EG.7-OVA in 2 groups with or without IL-10 in in vitro studies (data not shown). To assess the indirect influences of other types of cells in vivo, we depleted each immune cell type by administering specific depleting antibodies 2 days before the administration of activated OT-1 T cells. Preadministration of antibodies, such as anti-asialo GM1 mAb, anti-CD8 mAb, and anti-CD4 mAb did not influence the number of in vivo activated T cells deduced by tetramer staining (Figure 9A). However, in functional activity assays using ELISPOT, the preadministration of anti-CD4 mAb increased the number of responder CD8+ T cells (control groups and control with IL-10 vs anti-CD4 mAb-treated group,P < .005; control with IL-10 group vs groups with other antibodies, P > .05), suggesting that IL-10–associated suppression of CD8+ T-cell reactivity may involve CD4+ T cells (Figure 9B). In the vaccination models, IL-10 administered for 7 days after CTL transfer enhanced the duration of CD8+ T-cell function in situ. Activated CTLs reactive against EG.7-OVA tumor cells appeared more effective in mice treated afterward with IL-10 than in mice pretreated or not treated with IL-10 (Figure 10). In studies involving the adoptive transfer of a CTL clone into tumor (MC38)–bearing mice, we compared the differences between groups treated afterward with IL-10 and groups pretreated or not treated with IL-10. The MC38-specific CTL clone that produced IFN-γ, but not IL-2 and IL-4 (Figure11A), also did not show IL-10–dependent effects on the ability to mediate the lysis of MC38 in vitro (data not shown). Adoptively transferred MC38-reactive CTL clones did, however, appear more efficacious in mice treated afterward (vs pretreated or not treated) with IL-10 (Figure 11B). These findings indicated that in vivo administration of IL-10 promotes the expansion and the augmented function of these antitumor CTLs (CTL–IL-10–MC38 vs others; P < .05).
In vivo effects of IL-10 on transferred OVA-specific CD8+ OT-1 T cells.
Isolated OT-1 T cells, as in Figure 7, were adoptively transferred into syngeneic C57BL/6 mice followed by no treatment or by the administration of IL-10 for 7 days. (A) IL-10 did not modulate the frequency of adoptively transferred naive OT-1 T cells, but IL-10 increased the number of activated OT-1 T cells. (B) Cytotoxicity mediated by transferred, activated OT-1 T cells was also augmented by IL-10. In contrast, specific CTL activity was not observed in animals receiving naive OT-1 T cells with or without IL-10. Mice receiving activated OT-1 T cells and IL-10 displayed statistically increased frequencies of specific spleen effector cells against EG.7-OVA in the ELISPOT assay. (In activated transfer groups, with IL-10 vs without IL-10 [P < .005].) (C) They also displayed statistically increased frequencies of OVA peptide-specific spleen effector cells in the ELISPOT assay. (In activated T-cell transfer groups, with IL-10 vs without IL-10 [P < .001].)
In vivo effects of IL-10 on transferred OVA-specific CD8+ OT-1 T cells.
Isolated OT-1 T cells, as in Figure 7, were adoptively transferred into syngeneic C57BL/6 mice followed by no treatment or by the administration of IL-10 for 7 days. (A) IL-10 did not modulate the frequency of adoptively transferred naive OT-1 T cells, but IL-10 increased the number of activated OT-1 T cells. (B) Cytotoxicity mediated by transferred, activated OT-1 T cells was also augmented by IL-10. In contrast, specific CTL activity was not observed in animals receiving naive OT-1 T cells with or without IL-10. Mice receiving activated OT-1 T cells and IL-10 displayed statistically increased frequencies of specific spleen effector cells against EG.7-OVA in the ELISPOT assay. (In activated transfer groups, with IL-10 vs without IL-10 [P < .005].) (C) They also displayed statistically increased frequencies of OVA peptide-specific spleen effector cells in the ELISPOT assay. (In activated T-cell transfer groups, with IL-10 vs without IL-10 [P < .001].)
Recipient CD4+ T cells, CD8+ T cells, and NK cell-independent proliferation of activated donor T cells by IL-10.
C57BL/6 mice were injected intravenously with 300 μg rat IgG (control IgG), anti-CD4, anti-CD8, or anti-asialo–GM1 mAbs 2 day before they received activated OT-1 T cells, followed by IL-10 administration. Mice were killed 1 week later. Complete depletion of the relevant lymphoid subset was confirmed by CD4, CD8, and NK cell staining monitored by flow cytometry (data not shown). (A) Efficacy of T- or NK-cell depletion was evaluated by anti-CD8 mAb (FITC) and OVA-specific tetramer (PE) staining of spleen cells from treated and healthy mice. The number indicates the percentage of OVA-specific activated T cells (CD8+ CD44hi population). OVA-specific tetramer-staining showed that prior antibody-mediated depletion of CD4+ and CD8+ T cells did not affect the ability of IL-10 to trigger the increase in numbers of transferred activated OT-1 T cells. (B, C) Functional efficacy (cytotoxicity) by depletion of CD8+ T cells or NK cells did not show significant differences; however, depletion of CD4+ T cells increased the number of spots for EG.7-OVA (B) and OVA-peptide (C). (Mice with control IgG vs mice with control IgG and IL-10 [P < .0001], and mice with control IgG and IL-10 vs mice with anti-CD4 and IL-10 [P < .005]), determined in ELISPOT assays.
Recipient CD4+ T cells, CD8+ T cells, and NK cell-independent proliferation of activated donor T cells by IL-10.
C57BL/6 mice were injected intravenously with 300 μg rat IgG (control IgG), anti-CD4, anti-CD8, or anti-asialo–GM1 mAbs 2 day before they received activated OT-1 T cells, followed by IL-10 administration. Mice were killed 1 week later. Complete depletion of the relevant lymphoid subset was confirmed by CD4, CD8, and NK cell staining monitored by flow cytometry (data not shown). (A) Efficacy of T- or NK-cell depletion was evaluated by anti-CD8 mAb (FITC) and OVA-specific tetramer (PE) staining of spleen cells from treated and healthy mice. The number indicates the percentage of OVA-specific activated T cells (CD8+ CD44hi population). OVA-specific tetramer-staining showed that prior antibody-mediated depletion of CD4+ and CD8+ T cells did not affect the ability of IL-10 to trigger the increase in numbers of transferred activated OT-1 T cells. (B, C) Functional efficacy (cytotoxicity) by depletion of CD8+ T cells or NK cells did not show significant differences; however, depletion of CD4+ T cells increased the number of spots for EG.7-OVA (B) and OVA-peptide (C). (Mice with control IgG vs mice with control IgG and IL-10 [P < .0001], and mice with control IgG and IL-10 vs mice with anti-CD4 and IL-10 [P < .005]), determined in ELISPOT assays.
IL-10 potentiates the efficacy of adoptively transferred activated OT-1 T cells (CTLs).
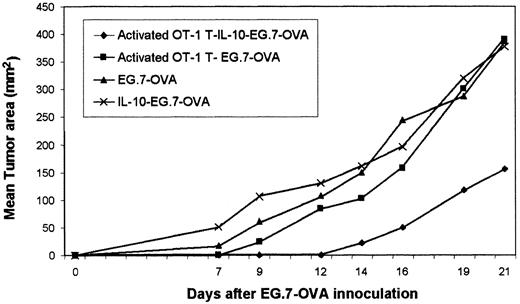
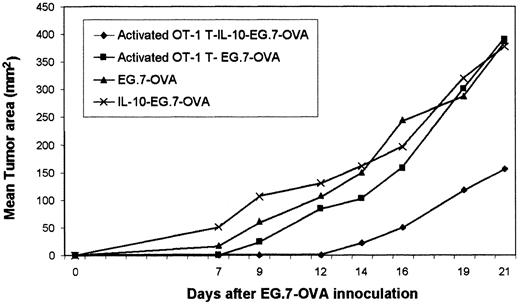
Activated OT-1 T cells were generated from OT-1 spleen by culturing with peptide for 36 hours before adoptive transfer into syngeneic mice. In some groups, systemic IL-10 was then provided. IL-10 administration provided after adoptive transfer of activated OT-1 T cells significantly restricted tumor (EG.7-OVA) growth, suggesting that they showed CTL activity. These data represented the mean of 3 mice. Standard deviations were less than 10%. (OVA-specific CTLs were described as activated T cells. Activated T–IL-10–EG7 indicates the sequence of injected events.)
IL-10 potentiates the efficacy of adoptively transferred activated OT-1 T cells (CTLs).
Activated OT-1 T cells were generated from OT-1 spleen by culturing with peptide for 36 hours before adoptive transfer into syngeneic mice. In some groups, systemic IL-10 was then provided. IL-10 administration provided after adoptive transfer of activated OT-1 T cells significantly restricted tumor (EG.7-OVA) growth, suggesting that they showed CTL activity. These data represented the mean of 3 mice. Standard deviations were less than 10%. (OVA-specific CTLs were described as activated T cells. Activated T–IL-10–EG7 indicates the sequence of injected events.)
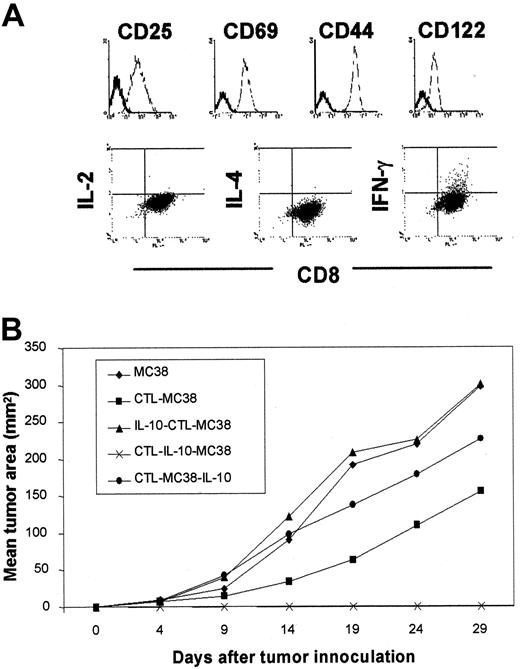
Prophylactic activity of tumor-specific CTL clone is enhanced by IL-10 in MC38 models.
(A) An MC38-reactive CTL clone expressing CD25+, CD69+, CD122+, and CD44hi phenotype and capable of secreting IFN-γ, but not IL-2 and IL-4, in a stable state was used in adoptive-protection experiments. (B) Injection of the MC38-reactive CTL clone, followed by IL-10, suppressed the growth of subsequently transplanted MC38 tumors. As in the schedule of the experiment in Figure 2, 1 × 105 cells of the CTL clone were adoptively transferred into syngeneic B6 mice. One week later, 3 × 105 MC38 were inoculated as a tumor challenge. Scheduling IL-10 injections (40 μg per mouse for 7 consecutive days) was different in each treatment group and was allowed for the discrimination of IL-10 effects when provided before CTL transfer (IL–10-CTL–MC38) versus after CTL transfer (CTL–IL-10–MC38) or after tumor challenge (CTL–MC38–IL-10).
Prophylactic activity of tumor-specific CTL clone is enhanced by IL-10 in MC38 models.
(A) An MC38-reactive CTL clone expressing CD25+, CD69+, CD122+, and CD44hi phenotype and capable of secreting IFN-γ, but not IL-2 and IL-4, in a stable state was used in adoptive-protection experiments. (B) Injection of the MC38-reactive CTL clone, followed by IL-10, suppressed the growth of subsequently transplanted MC38 tumors. As in the schedule of the experiment in Figure 2, 1 × 105 cells of the CTL clone were adoptively transferred into syngeneic B6 mice. One week later, 3 × 105 MC38 were inoculated as a tumor challenge. Scheduling IL-10 injections (40 μg per mouse for 7 consecutive days) was different in each treatment group and was allowed for the discrimination of IL-10 effects when provided before CTL transfer (IL–10-CTL–MC38) versus after CTL transfer (CTL–IL-10–MC38) or after tumor challenge (CTL–MC38–IL-10).
Discussion
This study provides evidence that effector CD8+ T cells capable of rapid activation (ie, memory) can be maintained by the administration of IL-10 in vivo. To discern the impact of IL-10 on vaccine-induced antitumor T-cell numbers and function, we initially had to establish optimal conditions for our vaccine–tumor challenge models. Using this schema, IL-10 was observed to promote optimal vaccine-associated inhibition of tumor growth when applied just after tumor challenge (ie, antigen boosting) (Figure 1). Minimal differences were shown in the numbers of immune subsets, such as B cells, macrophages, DC, CD4+, and CD8+ T cells in the spleens of treated mice on days 14, 21, and 28 in our investigated groups (data not shown).18-22,33,34 Although recent reports suggest that IL-10 can augment NK function in vivo, we observed no increase in NK cell numbers.35 Subsequent analysis focused on the antitumor activity associated with effector CD8+ T cells.
In particular, we compared CD8+ T-cell numbers and function in the spleens of mice in group 3 (group 5 in Figure 1A is equal to group 3 in Figure 2) with those of the other control groups in antigen-specific proliferation assays after initial immunization by DC peptide (Figure 2). Three weeks after DC vaccination, spleen cells from mice administered IL-10 exhibited antigen-specific proliferation (Figure 3). We also noted that the activity of antigen-specific T cells in mice treated with IL-10 could be enhanced by antigen, IL-2, and IL-15. Because IL-2 and IL-15 have profound effects on CD8+T-cell proliferation,9,10 it was suggested that antigen-specific activated–memory type CD8+ T cells might be induced to proliferate by IL-10.15,16 After 14, 21, and 28 days, spleen cells from immunized mice were cultured with OVA peptide and assayed for specific lytic activity and their phenotypes. IL-10 did not promote discernible differences in the phenotypes of CD4+ or CD8+ T cells (data not shown). However, splenocytes obtained from mice 21 days after initial vaccination with DC (+ OVA peptide) contained the activated–memory type CD8+ T-cell populations (CD8+CD44hi CD122+ population). This effector T-cell population was significantly increased in group 3–treated animals (Figure 4).12-14,36,37 On day 6, the day before tumor challenge, the mean number of this T-cell population in vaccinated mice was 2.3 × 106 (control, 9.3 × 105). However, we could not demonstrate any differences in cytotoxicity between groups after in vitro restimulation with antigen for 5 days (data not shown). Therefore, by application of 2 sensitive ex vivo assays to our studies, we characterized low-frequency, antigen-specific splenic effector CD8+ T cells (ie, cells capable of producing IFN-γ) within 24 hours of antigen restimulation in vitro. On day 6, the mean number of IFN-γ–producing cells in group 3 mice was 2.7 × 105, whereas control mice contained 1.7 × 104 of such cells reactive against EG.7-OVA. The observation in group 3 that IFN-γ release (within 24 hours) was triggered by exposure to cognate antigen (peptide or EG.7-OVA) in both intracellular cytokine analysis and the ELISPOT assay in the absence of exogenous cytokines on day 21 (Figures 5, 6) suggested that IL-10 treatment preserves the rapid activation protocol of memory effector CD8+ T cells. In preliminary analysis, we confirmed that the number of spots using OVA peptide in IFN-γ ELISPOT assays was directly correlated with the degree of cytotoxic function mediated by CTLs derived from OT-1 mice in an OVA peptide-specific manner (data not shown). These T cells can respond to antigen in vitro with rapid kinetics, similar to that of CTLs recently stimulated with antigen.38-42 Circulating tumor antigen-specific CD8+ T cells capable of such rapidly activated effector function would provide protective immunologic memory against evolving progressive disease. This population of CD8+ T cells does differ from conventional resting memory T cells that require culture for several days, antigenic restimulation, and proliferation for the recovery of effector function.39-41
The addition of IL-10 does not appear to have any direct cytopathic effects on activated CTLs in vitro (data not shown). To address the possibility that preactivated antigen-specific CD8+ T cells can survive in the absence of antigen on the provision of IL-10 in vivo, we adoptively transferred OT-1 T cells expressing T-cell activation phenotype and IFN-γ (OVA-specific CD8+ CTL) (Figure 7), followed by the injection of IL-10 into syngeneic recipients. To evaluate activated OT-1 T cells in transferred mice directly, double staining was performed using OVA-specific tetramers and antibody reactive to T-cell receptor (TCR) Vβ 5.1(OVA-specific TCR). With the administration of IL-10, antigen-specific T cells capable of being bound by both tetramers and anti-Vβ 5.1 mAb were increased (Figure 8A), and their effector function was enhanced in the ELISPOT assay (Figure 8B) but not in naive OT-1 T cells. In control mice treated with HBSS, we could not detect these types of CD8+ T cells at all, presumably because of the lack of OVA stimulation. We conclude that IL-10 not only plays a role in maintenance, it also promotes the proliferation of T cells exhibiting rapid effector function after reactivation by TCR–antigen–MHC class I stimulation.38-47
It has been noted that IL-10 can synergize with IL-2 or IL-4 to promote CD8+ T cell proliferation.34,35,43 However, as shown here in our studies, cultured OVA-specific cultured CD8+ T cells from mice primed with OVA peptide did not produce either IL-2 or IL-4 (Figure 7C); therefore, the mechanism of this effect was different from the previously cited IL-2– or IL-4–driven systems. These findings also indicated that IL-10 could selectively drive the proliferation of the activated CD8+ T cells while maintaining effector function. Neither unprimed nor activated OT-1 T cells proliferated in response to low concentrations of IL-10 or in the absence of antigen (data not shown). This may suggest overall that OVA-specific cultured CD8+ T cells may present cross-reacting peptides to themselves, resulting in apoptosis (activation-induced cell death) in vitro,22,44,47 unless cytokines such as IL-10 rescue them from death. This may indicate that the blockade of activation-induced, caspase-dependent T-cell death by IL-10 also rescues the function of “undead” T cells. However, it was unclear whether these “maintained” T cells would respond or be anergic to subsequent antigen restimulation. Therefore, T-cell proliferation in vivo after antigen stimulation may support the functional responsiveness to antigen. It has been recently shown that resistance to activation-induced cell death can be attributed to high levels of intracellular FLIP (IL-1β-converting enzymelike, proteaselike inhibitory protein), which interacts with the adaptor protein FADD (Fas-associated death domain protein) and the protease FLICE (CD95-associated IL-1β-converting enzymelike protease) to block CD95 signaling.48,49 The mechanism involved in maintaining activated antigen-specific CD8+ CTL by antigen restimulation followed by IL-10 treatment may involve the activation of FLIP expression in memory-type T cells, resulting in resistance to activation-induced cell death. Alternatively, our findings may support a mechanism involving IL-10 inducers of endogenous nitric oxide that acts a mediator in antagonizing the activation-induced cell death of activated CD8+ CTLs.50
Thus, our in vivo observations indicate that specific CD8+T-cell proliferation can be modulated by IL-10. This does not preclude additional indirect effects mediated by alternative IL-10 responsive cells, such as CD4+ T cells and NK cells. To investigate the potential involvement of these alternative cell types in our IL-10–dependent system, we performed depletion analyses by administration (intravenous) of specific mAbs (such as rat IgG, anti-CD4 Ab, anti-CD8 Ab, and anti-asialo GM1 mAb) 2 days before transferring purified naive or activated OT-1 T cells (Figure 9A). Seven days after transfer, the number of antigen-specific CD8+ CD44hi T cells (OVA-tetramer positive activated T cells) was observed to increase to the extent observed for control mice (ie, rat IgG-treated hosts) and in all other mAb-treated mice (Figure 9A). The injection of mAbs, such as anti-CD8 mAb, anti-CD4 mAb, or asialo-GM1 mAb before activated OT-1 T-cell transfer, did not change the results obtained in the ELISPOT assays. In the ELISPOT assay, however, the injection of anti-CD4 mAb followed by the administration of IL-10 increased the observed number of spots in response to OVA peptide (P < .005) (Figure 9B), suggesting that high-dose IL-10 may promote a suppressive CD4+ T-cell population, in part composed of CD4 regulatory T cells.51,52 Hence, IL-10 may act as an immune suppressor of CD4+ T cells and monocytes–DCs, and conversely it may act as an immunostimulant for an activated population of CD8+ T cells. These opposing effects of IL-10 may serve as a regulator for immune responsiveness.17,25 53
Adoptively transferred OVA-primed OT-1 T cells (CTLs) were augmented in their antitumor efficacy after the injection of mice with IL-10 (Figure 10). In the additional tumor models, IL-10 administration potentiated the antitumor effectiveness of an adoptively transferred CTL clone specific for the MC38 colorectal adenocarcinoma (Figure 11). Thus, the administration of in vitro activated CTLs, followed by the injection of mice with IL-10, may result in the extended durability of effector CD8+ T cells associated with the observed augmentation in antitumor efficacy. Our findings, along with the previous data, demonstrate that despite the suppressive effects of certain CD4+ T cells, IL-10 stimulates activated CD8+ T cells to proliferate, and it supports memory-type CD8+ T-cell effectors capable of rapidly activation in an antigen-specific manner in situ. Enhanced survival and function of CTLs mediated by concurrent IL-10 administration may boost clinical effectiveness or may be useful after adoptive immunotherapy approaches. Furthermore, when applied in a vaccination strategy, we anticipate that tumor vaccination followed by the systemic administration of rIL-10 may result in enhanced antitumor T-cell efficacy.
We thank Drs K. Murali-Krishna, J. Altman (Emory Vaccine Center, Emory University, Atlanta, GA), T. Takayama, and H. Kanaya (University of Pittsburgh, PA) for providing technical advice, and we thank Dr W. Storkus for peer-reviewing the manuscript.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Shin-ichiro Fujii, Laboratory of Cellular Physiology and Immunology, The Rockefeller University, 1230 York Ave, New York, NY, 10021-6399; e-mail: fujiis@mail.rockefeller.edu.


![Fig. 3. Cell proliferation over time with and without IL-10 in the vaccination models. / Proliferation assays were performed in the presence and absence of antigen, cytokines (100 U/mL of IL-2 and IL-15, respectively) or PMA–ionomycin (PMA, 25 ng/mL; ionomycin, 0.5 μM) using splenocytes (1 × 106 cells/well) harvested from mice 14, 21, and 28 days after DC vaccination in each group depicted in Figure 2. After 48-hour culture, 0.037 MBq (1 μCi) [3H]-thymidine was added, and cells were harvested 16 hours later. Statistically significant differences relative to DC-vaccinated mice that did not receive IL-10 are indicated. Control mice were immunized with DCs in the absence of peptide. Each bar represents the mean of 3 mice evaluated.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/98/7/10.1182_blood.v98.7.2143/5/m_h81911575003.jpeg?Expires=1769313634&Signature=BwsVvY6KR6Otkk26NDJ0oJGz1VxCyNbE0YZOLQNpVcSG-jX~mW-AegkPEfWDQRvQf4ErnZzRjmJ0ZjW8pE-O3iAilecBJLG2DzhP9XFVEpZYeiC94G~ejQw6KBLrJi65aniqg9oKGx53n~WzAnsvyqHhzjfoRozBl8ztmLM0I48JCefm6k8Bn1nJgUFLSMsKfhVQEFHFh0DsVpb7SGanITqGCUz1sp0mmlNOEPp7NON8DmsS-punWYmIsYE9CpnFfQkvlJJmc03aOV9QbOnxuFzNa5r9tHudxX0faI~yyCsdkMTLPXRUzYYB-zWGjQQlQyOW7jQhIUhek57CKUCX8A__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 4. Assessment of memory type CD8+ T cells in vaccinated mice. / After vaccination, the number of CD8+ T cells expressing activated–memory phenotype (CD44hi CD122+) were estimated by flow cytometry. On day 21, cells expressing these phenotypes increased in IL-10–treated mice (groups 3 and 4) (left), and the absolute number in spleen increased until day 28 (right). (On day 21, group 3 vs group 1, [P < .05]; group 3 vs group 2 [P < .01]; group 3 vs group 4 [P > .05]). The absolute number of such T cells in spleen was thus calculated as (percentage × spleen cell number). Mean values are shown for 3 mice evaluated at each time point.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/98/7/10.1182_blood.v98.7.2143/5/m_h81911575004.jpeg?Expires=1769313634&Signature=o8AN4nlYe1PrR07Pi63Oc-w~qZ17T8avVkjzaiJnKMUInSsBFqnweQobuVOhw5cJIyMaH12Lu6KcO3DumiFItVP9d7v3I04dxU7pdYAExlyGP4i6QcIgSkNdfqPvpqCUmJHxXk6RAFdFLFXkRzb6WrcZqfBrMDcz9TqBL0y04C04tbDkyUyYuG2OlLOGOZ8W-DQGQ5dYljcgK~qugLv8Zlf3omPd8JdWjF46Y1jFEV2J0Wjz6HCkPAYKYx~8mnaDbiAryVZpXFPY~MuM21aGfNZ69R~qdbeGfFylnErRJvVF5d-i0a4uZgJEmUNni7F9Y3cdMr-2OAajGsxXlKbWlA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 5. IFN-γ production by CD8+CD44hi cells in vaccinated mice. / Using flow cytometry-bound assessment of cytokine production on day 21, phenotypically high expression of IFN-γ was demonstrated in either IL-10–administered or antigen-challenged groups (groups 2-4) (left). However, the absolute number of IFN-γ–producing CD8+CD44hi cells in spleen persisted in groups 2 and 3 through day 28. (On day 21, group 3 vs group 1 [P < .05]; group 3 vs group 2 [P > .05]; group 3 vs group 4 [P < .05]; on day 28, groups 1, 4 vs group 3 [P < .0001]; group 2 vs group 3 [P < .01]). The absolute number of IFN-γ–secreting T cells in spleen was calculated as (percentage × spleen cell number). Mean values are shown for 3 mice at each time point.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/98/7/10.1182_blood.v98.7.2143/5/m_h81911575005.jpeg?Expires=1769313634&Signature=GWqXdFMhQ3Xwgd9LQ~nrRbXRuF9~u4sRHPlxWyFDmv-pcROCRu2q1lgO~A81zzlc2X3xEbnr2pRM6goTNOGqCHyfbmhU25~vq1sHbYG5a0XgWvEMX5TQXjoCRPuI~GwvLYRFPuZVBX4MITi2cCAyr-JBKJwyK-GB1MRzjYIhWIqqoNuuOam2FEqaZgicm1bFJbZs8XXiBs9cIs1w0iVt7cxuh-T8D9L3lCB3vAEJAEvqXmpbIGijVVjmGdIrJgcKLTImQRSbZPsuHWD68Ujaycip3Fp52lvtACI2uqx4DO30MyQbl2OrcrjWbJlokhdtniWCPhbpqah1XWSdExHghA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 6. Tumor antigen-specific IFN-γ production by responding splenocytes harvested from vaccinated mice (ELISPOT). / (A) Splenocyte IFN- γ production specific for tumor cells (EG.7-OVA) was shown in group 3 mice by ELISPOT assay. Spleen cells (5 × 106 cells/well) from each group were incubated for 24 hours with EG7-OVA or irrelevant tumor cell (EL4). (On day 21, group 3 vs group 2 [P < .05]; and groups 1, 4 vs group 3 [P < .01]; on day 28, group 3 vs group 1 [P < .01]; group 3 vs group 2 [P < .01]; group 3 vs group 4 [P < .005]). (B) These specific cells were also OVA peptide-specific CD8+ T cells. Spleen cells (5 × 106 cells/well) from each group were incubated for 36 hours with OVA peptide (SIINFEKL) or irrelevant peptide (SIINFKEL). These peptide-specific spots in each group were similar to the responding spots for EG7-OVA in Figure 6A. (On day 21, group 3 vs groups 2, 4 [P > .05]; group 1 vs group 3 [P < .01]; on day 28, group 3 vs group 1 [P < .0001]; group 3 vs group 2 [P < .0001]; group 3 vs group 4 [P < .0001]). Mean values are shown for 3 mice at each time point.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/98/7/10.1182_blood.v98.7.2143/5/m_h81911575006.jpeg?Expires=1769313634&Signature=I1UkkmJ7xLcMLvHWzHOcmYSWDBhtoU5sCBSOs2Ix3MNUz~kwcPgvuLeybFcYIDhMc~C0Oc4xpGDsWyXYxutZXwZ~BEdsLydhKsefSumICmhk1id4twX-E2u7dgyuosw2P2OQ2IjSRq0PLWxeTHmbYRcIu3jbweX5j9-w2BDdZWKizgclV7zJWM3tA5eSRl4MCzDZu6NdLF5C7~LurtpSEWUS80Z2mi1SWAB7-2dISkDKPvZYt5fwr8itA8vQnidQfY7d5l0rZ8ht0KZVkJklFX89xBLM-QRfNKjPOK4AfJDV15xmDRErRKuu6vBa0auMKzxQVgZmA4O~O66eI4gEaQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 8. In vivo effects of IL-10 on transferred OVA-specific CD8+ OT-1 T cells. / Isolated OT-1 T cells, as in Figure 7, were adoptively transferred into syngeneic C57BL/6 mice followed by no treatment or by the administration of IL-10 for 7 days. (A) IL-10 did not modulate the frequency of adoptively transferred naive OT-1 T cells, but IL-10 increased the number of activated OT-1 T cells. (B) Cytotoxicity mediated by transferred, activated OT-1 T cells was also augmented by IL-10. In contrast, specific CTL activity was not observed in animals receiving naive OT-1 T cells with or without IL-10. Mice receiving activated OT-1 T cells and IL-10 displayed statistically increased frequencies of specific spleen effector cells against EG.7-OVA in the ELISPOT assay. (In activated transfer groups, with IL-10 vs without IL-10 [P < .005].) (C) They also displayed statistically increased frequencies of OVA peptide-specific spleen effector cells in the ELISPOT assay. (In activated T-cell transfer groups, with IL-10 vs without IL-10 [P < .001].)](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/98/7/10.1182_blood.v98.7.2143/5/m_h81911575008.jpeg?Expires=1769313634&Signature=kNrEMJVfLoszJnMp5r2kOyzTOaiitB6LrohZAjUqSsbtVbVCsQhczJWP0TytLrW572t3awS2V9uY4IVO8-ymwyTte2mKyjEZIzdpTolUNMCqn3~vjHpmwVLkGauhc9WU1lm~aC-GwCoziJb4LRFaNckRN1FxmG5B~8hl5GEiR24OelK3wuewYqhE5ea~nPma6IOohyCYjbsZnIuTy7GXqNJ~hfAQrHh79U2kqt0DyNYDh45LNfDBw12meiFEzQztzgjCetEvDBKiX06jzPE39HXJ8uMwXk3O1gGxuG-Gkp6AP1-mGjZYcISQej89nhXJQ8mAfYeGAvbcnwsI7ElMdg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 9. Recipient CD4+ T cells, CD8+ T cells, and NK cell-independent proliferation of activated donor T cells by IL-10. / C57BL/6 mice were injected intravenously with 300 μg rat IgG (control IgG), anti-CD4, anti-CD8, or anti-asialo–GM1 mAbs 2 day before they received activated OT-1 T cells, followed by IL-10 administration. Mice were killed 1 week later. Complete depletion of the relevant lymphoid subset was confirmed by CD4, CD8, and NK cell staining monitored by flow cytometry (data not shown). (A) Efficacy of T- or NK-cell depletion was evaluated by anti-CD8 mAb (FITC) and OVA-specific tetramer (PE) staining of spleen cells from treated and healthy mice. The number indicates the percentage of OVA-specific activated T cells (CD8+ CD44hi population). OVA-specific tetramer-staining showed that prior antibody-mediated depletion of CD4+ and CD8+ T cells did not affect the ability of IL-10 to trigger the increase in numbers of transferred activated OT-1 T cells. (B, C) Functional efficacy (cytotoxicity) by depletion of CD8+ T cells or NK cells did not show significant differences; however, depletion of CD4+ T cells increased the number of spots for EG.7-OVA (B) and OVA-peptide (C). (Mice with control IgG vs mice with control IgG and IL-10 [P < .0001], and mice with control IgG and IL-10 vs mice with anti-CD4 and IL-10 [P < .005]), determined in ELISPOT assays.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/98/7/10.1182_blood.v98.7.2143/5/m_h81911575009.jpeg?Expires=1769313634&Signature=i9Mb0w8NHwT-3XhhoEfhKmhsh1vX-ALqvCsR1ncM9dRuG3gxSUIv8VwX0L6n10xmXTph263iCltMq6g50Ub4ia1MCaG0ZFCHVBULUkawDUzfaL9N6nKkq7vPZ2W5Qofo59esGAWrdr0DU7cq4CJDFQwmaQyy36-CCNBA8L6sCGGpFxcST-oZc3c21UGpVQ~CDI20HFbSB3mA7W126h9l~whnOIWuOpo6OBy5zdZqgc3QOBZSLbuTz6-81kVcmyx91Xf149cGQ-L2A8L~yj7Rq-gIlKXhTA~QKwmxYMYarMFixTpZJap2AaoQRgvNyiLsVb2pgfC0eUNrF5n0jfh49A__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

![Fig. 3. Cell proliferation over time with and without IL-10 in the vaccination models. / Proliferation assays were performed in the presence and absence of antigen, cytokines (100 U/mL of IL-2 and IL-15, respectively) or PMA–ionomycin (PMA, 25 ng/mL; ionomycin, 0.5 μM) using splenocytes (1 × 106 cells/well) harvested from mice 14, 21, and 28 days after DC vaccination in each group depicted in Figure 2. After 48-hour culture, 0.037 MBq (1 μCi) [3H]-thymidine was added, and cells were harvested 16 hours later. Statistically significant differences relative to DC-vaccinated mice that did not receive IL-10 are indicated. Control mice were immunized with DCs in the absence of peptide. Each bar represents the mean of 3 mice evaluated.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/98/7/10.1182_blood.v98.7.2143/5/m_h81911575003.jpeg?Expires=1769313635&Signature=oKfifqeKXFHi-AMKJstMoFd4XdHl8kF8p7Jc9hE4kmqVkLYHEozoI9uyGvhqIx-WOkMTHKmaix~BB5~xe~tcoIo6GySZIbe64Nxe4epcJfsUnNJltzeryJiMmy~4moYTX06Sv0A1nvRcEsu42SOcCayulzM1HsBCiXMYRYXHeBzEIcch0hLgxn4g6bWMSg5HWk4PBuVmi3tZX0gjZIVPoAqzVBQ8sd8V~JWArV6ByL~DHWR4bHmF0FMM5ziDgZjbrfvW~jph-Ps0HyohVasq0PnnqmNuScPM4sFWajsIFz1lQxcD235gThtS-ujF7V5JLcgyz32xdb3zEkM2ECbUiw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 4. Assessment of memory type CD8+ T cells in vaccinated mice. / After vaccination, the number of CD8+ T cells expressing activated–memory phenotype (CD44hi CD122+) were estimated by flow cytometry. On day 21, cells expressing these phenotypes increased in IL-10–treated mice (groups 3 and 4) (left), and the absolute number in spleen increased until day 28 (right). (On day 21, group 3 vs group 1, [P < .05]; group 3 vs group 2 [P < .01]; group 3 vs group 4 [P > .05]). The absolute number of such T cells in spleen was thus calculated as (percentage × spleen cell number). Mean values are shown for 3 mice evaluated at each time point.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/98/7/10.1182_blood.v98.7.2143/5/m_h81911575004.jpeg?Expires=1769313635&Signature=rLmeSlXk-npFbqWvXqNy9Sp3ZgyLhtRcf9yVQLS6C7zmaembE2bFVmv46pAPT4pm5qgjMOzymSkQn4RYuBGiq5jSfFf2rj4WuVfXInW~1ny0n4VlAe5ZOreazIrNc5gTTg4gNIycg7bhBwtd0RC9gtpxDM2~eJUFqcMGCGD9N8hyqbdoFqglrbrVzwn6oqvS1X82wSmZBDG7i7Tma4YNrMhRM5HJmvT1rbQZ5qLt9xyanvFdjnUonmmL28Vp75qQLhafkqrhjadyGbau-avhkkndFGnaErLD09WUAT~zcFjzU82z-NuESNp8dDAddYtZs9j8SlhUZfYuLlFn2sOCtQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 5. IFN-γ production by CD8+CD44hi cells in vaccinated mice. / Using flow cytometry-bound assessment of cytokine production on day 21, phenotypically high expression of IFN-γ was demonstrated in either IL-10–administered or antigen-challenged groups (groups 2-4) (left). However, the absolute number of IFN-γ–producing CD8+CD44hi cells in spleen persisted in groups 2 and 3 through day 28. (On day 21, group 3 vs group 1 [P < .05]; group 3 vs group 2 [P > .05]; group 3 vs group 4 [P < .05]; on day 28, groups 1, 4 vs group 3 [P < .0001]; group 2 vs group 3 [P < .01]). The absolute number of IFN-γ–secreting T cells in spleen was calculated as (percentage × spleen cell number). Mean values are shown for 3 mice at each time point.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/98/7/10.1182_blood.v98.7.2143/5/m_h81911575005.jpeg?Expires=1769313635&Signature=0FdcT1XjGUiIFwZi3kqw8DPgaA35L0W0ChEbgaLyPS4ubAWAHHtUTLiEbKB~LDQ9G2B8571Cegvf1qiGPvrf0E~l718EjAcSrOjiLzOn1sokrNhn7cLen2H8dhsGwUrWyqu2fZtUOMQyS7u4k6it3KD8J-vDMP7~QPpmx7o~wK~6BRY8S~IJlWUavc3z6KVG9bOfB0xAu5DvmpoAasjYbEXSztVK6C51GOXOU5g3elqowvSg5bbDAAtO0j-uOjPm9dr94YHP6s9NiU3~CeEpSMm37IoLkTQ33dVKDnjgr2oNOon53j~srsRWFeY7HGKqjht75-zGAmEQ4MlIItRDlA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 6. Tumor antigen-specific IFN-γ production by responding splenocytes harvested from vaccinated mice (ELISPOT). / (A) Splenocyte IFN- γ production specific for tumor cells (EG.7-OVA) was shown in group 3 mice by ELISPOT assay. Spleen cells (5 × 106 cells/well) from each group were incubated for 24 hours with EG7-OVA or irrelevant tumor cell (EL4). (On day 21, group 3 vs group 2 [P < .05]; and groups 1, 4 vs group 3 [P < .01]; on day 28, group 3 vs group 1 [P < .01]; group 3 vs group 2 [P < .01]; group 3 vs group 4 [P < .005]). (B) These specific cells were also OVA peptide-specific CD8+ T cells. Spleen cells (5 × 106 cells/well) from each group were incubated for 36 hours with OVA peptide (SIINFEKL) or irrelevant peptide (SIINFKEL). These peptide-specific spots in each group were similar to the responding spots for EG7-OVA in Figure 6A. (On day 21, group 3 vs groups 2, 4 [P > .05]; group 1 vs group 3 [P < .01]; on day 28, group 3 vs group 1 [P < .0001]; group 3 vs group 2 [P < .0001]; group 3 vs group 4 [P < .0001]). Mean values are shown for 3 mice at each time point.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/98/7/10.1182_blood.v98.7.2143/5/m_h81911575006.jpeg?Expires=1769313635&Signature=0w6bcPSh-X1YcVK5q9aX85vx7kvcM4NcGJWrS2mc43IPwuLe6D8CUTJGA2RtDbq~K4-RRF9tWuc7KiyQX3F-sVrweBe9iWXoeuf1AWr9AvClKWOjcq~3135pXxb3eiv4mJL97AyL33~8fdzENoS6sWDXMR5l5rIIec7z96-NNPLC7EaGqsAwD4-HgXIQMv0TF2vncWOSBF4s1yYC2GOc7QhRsTz68anuJ90s28BaxjcN5AVui6TCtQAt0-2T4RSNY8VX7anQEnBot6EwlkbvRCrW9v~1TA57uhD38SoY9iWs2vmMMs75MHCG8i5enO7hf0X2p6yQLZIBjkUE3jTgBA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 8. In vivo effects of IL-10 on transferred OVA-specific CD8+ OT-1 T cells. / Isolated OT-1 T cells, as in Figure 7, were adoptively transferred into syngeneic C57BL/6 mice followed by no treatment or by the administration of IL-10 for 7 days. (A) IL-10 did not modulate the frequency of adoptively transferred naive OT-1 T cells, but IL-10 increased the number of activated OT-1 T cells. (B) Cytotoxicity mediated by transferred, activated OT-1 T cells was also augmented by IL-10. In contrast, specific CTL activity was not observed in animals receiving naive OT-1 T cells with or without IL-10. Mice receiving activated OT-1 T cells and IL-10 displayed statistically increased frequencies of specific spleen effector cells against EG.7-OVA in the ELISPOT assay. (In activated transfer groups, with IL-10 vs without IL-10 [P < .005].) (C) They also displayed statistically increased frequencies of OVA peptide-specific spleen effector cells in the ELISPOT assay. (In activated T-cell transfer groups, with IL-10 vs without IL-10 [P < .001].)](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/98/7/10.1182_blood.v98.7.2143/5/m_h81911575008.jpeg?Expires=1769313635&Signature=T~up4H4FgHFKgzam8gGCosniZ3krfZg1spsibgrbvyqPModHZdjqDJYYt5Ep7vPBenLTWg~1H1Oo1UBYDA9~gtG9V9FA9cnjY0HhhDVb1bIQ5a9aQnGrhO6dEDYK5FMXGfwxKmUEQvtpdbckXIeXaZja2Hd36Ce6X5f0iuRdcidh5zEA~xd5HhMhMs-usBrGGRtUFMx0ydjPmUNPJeYe61pPIuQuuqUKmuTMJlGsD3AsY4ysHzAWojawjCUw8uBOLYfWbW9~rQlD7Jmf6lcOlkbmQiS2Zq-EBA32yP9tj9UvDdVZdjoF6EouH0SxUJDa1qyiNoDOnHlM1SLakbNetw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 9. Recipient CD4+ T cells, CD8+ T cells, and NK cell-independent proliferation of activated donor T cells by IL-10. / C57BL/6 mice were injected intravenously with 300 μg rat IgG (control IgG), anti-CD4, anti-CD8, or anti-asialo–GM1 mAbs 2 day before they received activated OT-1 T cells, followed by IL-10 administration. Mice were killed 1 week later. Complete depletion of the relevant lymphoid subset was confirmed by CD4, CD8, and NK cell staining monitored by flow cytometry (data not shown). (A) Efficacy of T- or NK-cell depletion was evaluated by anti-CD8 mAb (FITC) and OVA-specific tetramer (PE) staining of spleen cells from treated and healthy mice. The number indicates the percentage of OVA-specific activated T cells (CD8+ CD44hi population). OVA-specific tetramer-staining showed that prior antibody-mediated depletion of CD4+ and CD8+ T cells did not affect the ability of IL-10 to trigger the increase in numbers of transferred activated OT-1 T cells. (B, C) Functional efficacy (cytotoxicity) by depletion of CD8+ T cells or NK cells did not show significant differences; however, depletion of CD4+ T cells increased the number of spots for EG.7-OVA (B) and OVA-peptide (C). (Mice with control IgG vs mice with control IgG and IL-10 [P < .0001], and mice with control IgG and IL-10 vs mice with anti-CD4 and IL-10 [P < .005]), determined in ELISPOT assays.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/98/7/10.1182_blood.v98.7.2143/5/m_h81911575009.jpeg?Expires=1769313635&Signature=ZhEqZUHxN4ZDa~hs5oKW~HCJ8RBiYqRjLiUcth0MiRXiElLeBjrrlarLZArVu7kpUKZdl4HAKIj78RaU1TS~alQDYd3dJW41MLkXwrfBarOzQBpV2DT1~jauGH85-PQIYNoeT4s1s5F-JVSvoz~vOLRfYG~SeufullK6M-po7QZeMvYfh907jOkls78NIf45eICDlmu7sIk1hZgOKAFgSuo9utAvbfagqZwUtvU1RO6ogpMijcIDmEznR8bQWHbA60uVwAwsua~dEh5zc3FkOGH0sY7wsbofbR1QkXZU8QfBdIiF0x0aXUhxfVmbKxJy9iyw4Rj3lIAtxCtBbgomDA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)