Dendritic cells (DC) are highly specialized antigen-presenting cells that on activation by inflammatory stimuli (eg, tumor necrosis factor α [TNF-α] and interleukin-1β [IL-1β]) or infectious agents (eg, lipopolysaccharide [LPS]), mature and migrate into lymphoid organs. During maturation, DC acquire the capacity to prime and polarize resting naive T lymphocytes. Maturation of monocyte-derived DC (MDDC) is inhibited by the p38 mitogen-activated protein kinase (MAPK) inhibitor SB203580. This study found that in the presence of the mitogen-activated protein kinase kinase 1–extracellular signal-regulated kinase (ERK) inhibitors PD98059 or U0126, TNF-α– and LPS-induced phenotypic and functional maturation is enhanced. ERK pathway inhibitors increased expression of major histocompatibility complex and costimulatory molecules; loss of mannose-receptor–mediated endocytic activity; nuclear factor-κB DNA-binding activity; release of IL-12 p40; and allogeneic T-cell proliferation induced by LPS or TNF-α. Moreover, PD98059 and U0126 enhanced LPS-triggered production of IL-12 p70. In agreement with the effect of ERK inhibitors, maturation of MDDC was delayed in the presence of serum, an effect that was reversed by U0126. These results indicate that the ERK and p38 MAPK signaling pathways differentially regulate maturation of MDDC and suggest that their relative levels of activation might modulate the initial commitment of naive T-helper (Th) cells toward Th1 or Th2 subsets. The findings also suggest that maturation of MDDC might be pharmacologically modified by altering the relative levels of activation of both intracellular signaling routes.
Introduction
Dendritic cells (DC) are professional antigen-presenting cells that are critically involved in the initiation of T cell–dependent immune responses as a consequence of their high expression of major histocompatibility complex (MHC) and costimulatory molecules.1 DC are sparsely distributed throughout the body and, in most tissues, are present in an immature state, showing a high capacity for antigen uptake and processing but unable to stimulate T cells.1,2 Once activated by inflammatory stimuli or infectious agents, DC undergo a maturation process whose hallmarks are up-regulated expression of costimulatory (CD40, CD80, and CD86) and adhesion (CD54 and CD58) molecules, migration into lymphoid organs, and subsequent acquisition of the capacity to activate quiescent, naı̈ve, and memory lymphocytes.1-4 In vitro, DC can be derived from either precursor cells or peripheral blood monocytes5-8 when the appropriate cytokine signals are provided. Immature monocyte-derived DC (MDDC) can be obtained from peripheral blood monocytes in the presence of granulocyte-macrophage colony-stimulating factor (GM-CSF) and interleukin-4 (IL-4). Addition of lipopolysaccharide (LPS) or tumor necrosis factor α (TNF-α) leads to the appearance of MDDC with all the morphologic, phenotypic, and functional characteristics of mature DC,5,6 including de novo expression of CD83,9 up-regulated expression of adhesion and costimulatory molecules,1-4,10 loss of mannose-receptor–mediated endocytosis, synthesis and release of IL-12, and enhanced antigen presentation capacity.11 Thus, in vitro maturation of MDDC represents a useful system for analyzing the molecular and functional changes that take place during acquisition of optimal T-cell–stimulating activity by DC.
At least 3 distinct mitogen-activated protein kinase (MAPK) signaling cascades exist in mammals, including the extracellular signal-regulated kinase (ERK), the c-Jun N-terminal kinase (JNK), and the p38 MAPK pathways.12 These kinases are activated by phosphorylation by distinct upstream MAPK kinases. The ERK signaling cascade regulates cell proliferation and differentiation in response to mitogens and growth factors, whereas the JNK and p38 MAPK pathways are preferentially activated by stress-inducing agents.12 The availability of specific inhibitors for the ERK and p38 MAPK pathways allows evaluation of their respective involvement in cellular responses to extracellular stimuli. The ERK pathway inhibitors PD9805913 and U012614 prevent activation of mitogen-activated protein kinase kinase (MEK) 1/2,15upstream activators of ERK 1/2, whereas the pyridinyl imidazole SB203580 inhibits p38 MAPK activity.15 16
The intracellular signaling pathways implicated in maturation of MDDC are just beginning to be explored. TNF-α stimulation of immature MDDC initiates activation of several MAPKs, including ERK 2, stress-activated protein kinase-JNK, and p38 MAPK.10,17,18Several reports have suggested that p38 MAPK is involved in the functional maturation of DC, since DC from Mkk3−/− mice have defective IL-12 production19; p38 MAPK appears to be constitutively activated in mature murine DC20; and CpG-DNA–specific and CD40-specific activation of DC is strongly blocked by the p38 MAPK inhibitor SB203580.21,22 We and others previously reported that SB203580 prevents maturation-dependent induction of CD83,10,23 CD49d,10 and other maturation factors.18
In this study, we found that the ERK signaling pathway regulates maturation of MDDC, since acquisition of phenotypic and functional maturation markers was enhanced in the presence of the MEK 1/2 inhibitors PD98059 and U0126. Together, our results indicate that the ERK and p38 MAPK signaling pathways exert opposite effects on the maturation of DC, thereby suggesting that maturation of MDDC can be pharmacologically modified by altering the relative levels of activation of both intracellular signaling routes.
Materials and methods
Cytokines, enzyme-linked immunosorbent assay, and reagents
GM-CSF (Leucomax) was purchased from Schering-Plough (Kenilworth, NJ) and used at a concentration of 1000 U/mL. IL-4 and TNF-α were obtained from PreProtech (Rocky Hill, NJ) and used at concentrations of 1000 U/mL and 20 ng/mL, respectively. The level of IL-12 p70 released by MDDC under each culture condition was determined with the IL-12 Eli-pair system (capture monoclonal antibody [mAb] B-T21 and detection biotinylated antibody B-P24; Diaclone Research, Besançon, France) used according to the manufacturer's recommendations (Diaclone Research). Levels of IL-12 p40 were measured by using an IL-12 p40 set (OptEIA; BD Pharmingen, San Diego, CA) and the manufacturer's protocol. LPS from Escherichia coli055:B5 was obtained from Sigma (Barcelona, Spain) and used at a concentration of 10 ng/mL. SB203580, U0126, and PD98059 were purchased from Calbiochem (San Diego, CA), resuspended in dimethyl sulfoxide (DMSO), and used at 13 μM (SB203580), 2.5 μM (U0126), or 40 μM (PD98059).
Cells
Human peripheral blood mononuclear cells (PBMC) from healthy donors were isolated from buffy coats over a Lymphoprep gradient (Nycomed, Norway) by using standard procedures. Monocytes were purified from PBMC by means of a 1-hour adherence step at 37°C in complete medium. Nonadherent cells were washed off by extensive washing with phosphate-buffered saline (PBS), and the remaining adherent monocytes were immediately subjected to the DC differentiation protocol described previously.5,6 10 Briefly, monocytes were resuspended at a concentration of 0.5 to 1 × 106 cells/mL and cultured in RPMI supplemented with 10% fetal calf serum, 25 mM HEPES, and 2 mM glutamine (complete medium) containing 1000 U/mL GM-CSF and 1000 U/mL IL-4. Cells were cultured for 5 to 7 days, with cytokines added every second day, to obtain a population of immature MDDC. For maturation, immature MDDC were treated with TNF-α (20 ng/mL) or LPS (10 ng/mL). In experiments using SB203580, U0126, or PD98059, cells were incubated for 1 hour in the presence of each inhibitor before LPS or TNF-α was added. Control cells were treated with an identical amount of DMSO. The differentiation and maturation protocol for MDDC was always done in serum-containing RPMI medium, except for the serum-free experiments, which were carried out in macrophage serum-free medium (Gibco BRL, Grand Island, NY).
Flow cytometry and antibodies
Cellular phenotypic analysis was done by using indirect immunofluorescence. The mAbs used for cell-surface staining included T3b (anti-CD3), TS1/2 (anti–MHC class II), HP2/1, and ALC1/6.3 (anti-CD49d) (kindly provided by Dr F. Sánchez-Madrid, Hospital Universitario de La Princesa, Madrid, Spain); HB1/5 (anti-CD83; Immunotech, Marseille, France); B-T7 (anti-CD86; Diaclone Research); and B-B20 (anti-CD40; Diaclone Research). All incubations were done in the presence of 50 μg/mL human IgG to prevent binding through the Fc portion of the antibodies. The supernatant from the myeloma P3 (X63) cell line was always included as a negative control. Flow cytometry analysis was done with an EPICS-CS instrument (Coulter Cientı́fica, Madrid, Spain) using log amplifiers. Where indicated, results are expressed as an expression index calculated as the percentage of marker-positive cells multiplied by their mean fluorescence intensity (MFI).
Northern blotting
After extensive washing in PBS, cells were harvested, and total cellular RNA was isolated by using RNeasy columns (Qiagen, Hilden, Germany) according to the manufacturer's recommendations. RNA integrity was initially confirmed in agarose gels containing formaldehyde. Denatured RNA (10 μg) was size fractionated on formaldehyde-containing 1% agarose gels in the presence of ethidium bromide. After electrophoresis, RNA was transferred overnight to nitrocellulose membranes with 20 × standard saline citrate (SSC). Prehybridization was done overnight at 42°C in 50% formamide, 5 × SSC, 5 × Denhardt solution, 50 mM sodium phosphate (pH 6.5), and 250 μg/mL denatured salmon-sperm DNA. Membranes were hybridized for 16 hours at 42°C in the same solution but containing 106 cpm/mL oligo-labeled probe. Blots were washed sequentially in 2 × SSC, 0.5% sodium dodecyl sulfate (SDS) at room temperature and then in 0.3 × SSC, 0.5% SDS at 65°C and exposed to x-ray film at −70°C. Detection of CD49d messenger RNA (mRNA) was accomplished with a 1.8-kilobase-pair EcoRI fragment of the CD49d complementary DNA (cDNA).24 For detection of CD83 mRNA, full-length CD83 cDNA was obtained by polymerase chain reaction using oligonucleotides 5′-GGGAATTCGCGCTCCAGCCATGTCGCGC-3′ and 5′-CCCCAAGCTTCCTGCAGAAATCCTGCTCATACC-3′ as primers. For this procedure, 2 μg total RNA from mature MDDC was reverse transcribed in a total volume of 20 μL amplification buffer (50 mM Tris-hydrochloric acid [HCl] [pH 8.2], 5 mM magnesium chloride, 10 mM dithiothreitol [DTT], 50 mM potassium chloride, 1 mM of each deoxynucleotide, and 0.5 μM random hexamers) including RNAsin and avian myeloblastosis virus reverse transcriptase at a concentration of 1 U/μL. The mixture was incubated at 42°C for 60 minutes and then at 52°C for 30 minutes, and the final volume was increased to 100 μL with water. Amplification of the full-length CD83 mRNA was done on 5 μL of each cDNA synthesis reaction in 50 μL of a solution containing 0.2 mM of each deoxynucleotide, 1 μM of each oligonucleotide primer, and 2.5 UPfu DNA polymerase (Stratagene, La Jolla, CA). Using a similar approach, we employed oligonucleotides 5′-GATGTGTCACCAGCAGTTGG-3′ and 5′-CTAACTGCAGGGCACAGATG-3′ to amplify the whole coding sequence of IL-12 p40 from total RNA of mature MDDC.
Measurement of phagocytic activity of MDDC
Mannose-receptor–mediated endocytosis was measured after 48 hours of maturation. Mature MDDC (5 × 105 cells/mL) were incubated in a 200-μL solution buffered with 25 mM HEPES with 1 mg/mL fluorescein isothiocyanate–dextran (Sigma). After a 1-hour incubation at 37°C, cells were washed 4 times in ice-cold PBS and analyzed with an EPICS flow cytometer (Coulter Cientı́fica). For a control, cells from each culture condition were also maintained in the same solution for 1 hour at 4°C.
Allogeneic T-lymphocyte proliferation induced by MDDC
Allogeneic T lymphocytes were obtained from peripheral blood samples from healthy adults by using standard procedures. CD4+ T lymphocytes were isolated from umbilical cord blood by immunomagnetic positive selection using CD4 mAb-conjugated beads (Dynal, Oslo, Norway). For T-lymphocyte proliferation experiments, 2 × 105 T cells were stimulated in a 96-well plate with 0.2 × 10,3 0.4 × 10,310,3 2 × 103, or 5 × 103 irradiated (1.5 Gy/min for 10 minutes) allogeneic MDDC matured under the different culture conditions. After a 5-day incubation period, tritium-thymidine was added (0.037 MBq/well) during the last 16 hours of coculture and thymidine incorporation determined to assess the level of T-cell proliferation.
Western blotting
Total cell lysates were obtained in 50 mM HEPES [pH 7.5], 250 mM sodium chloride (NaCl), 1 mM EDTA, 0.5% Triton X-100, 0.5 mM DTT, 10 mM sodium fluoride, 1 mM sodium orthovanadate, 20 mM Pefabloc, and 2 μg/mL aprotinin, antipain, leupeptin, and pepstatin. Then, 10 μg of each lysate were subjected to SDS-polyacrylamide gel electrophoresis under reducing conditions and transferred to an Immobilon polyvinylidene difluoride membrane (Millipore, Bedford, MA). After blocking of the unoccupied sites with 5% bovine serum albumin in 50 mM Tris-HCl (pH 7.6), 150 mM NaCl, and 0.1% Tween-20, protein detection was done with a chemiluminescence system (Supersignal West Pico; Pierce, Rockford, IL). For reprobing, membranes were incubated in stripping buffer (62.5 mM Tris-HCl [pH 6.7], 100 mM β-mercaptoethanol, and 2% SDS) for 30 minutes at 50°C, with occasional agitation. Detection of ERK 1/2, p38, phospho–ERK 1/2, phospho-p38, and IκBα was done by using specific polyclonal antibodies (New England Biolabs, Beverly, MA, and Santa Cruz Biotechnology, Santa Cruz, CA).
Electrophoretic mobility shift assay
Electrophoretic mobility shift assay (EMSA) was done essentially as described previously,25 and nuclear extracts were prepared according to the method described Schreiber et al.26 For competition experiments, unlabeled oligonucleotides (100-fold molar excess) were preincubated with the nuclear extracts at 4°C for 30 minutes before the probe was added. Oligonucleotide probes were nuclear factor κB (NF-κB) consensus 5′-AGTTGAGGGGACTTTCCCAGGC-3′, AP1 consensus 5′-CGCTTGATGAGTCAGCCGGAA-3′, and CAAT enhancer-binding protein (C/EBP) consensus 5′-TGCAGATTGCGCAATCTGCA-3′, which contain consensus-binding sites for NF-κB, AP-1, and C/EBP, respectively.
Results
Serum has a negative regulatory effect on maturation of MDDC
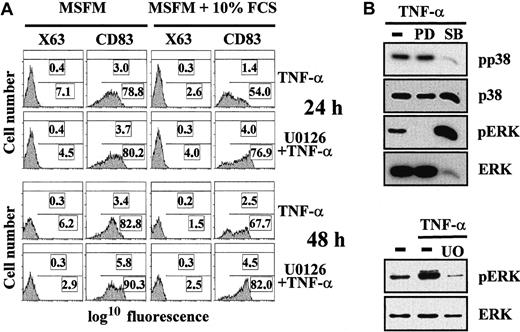
During studies analyzing TNF-α–induced maturation of MDDC, we noticed that acquisition of the maturation marker CD83 was delayed in the presence of serum compared with maturation accomplished in serum-free medium (Figure 1A), a finding in agreement with previously reported results.27 The inhibitory effect of serum, reflected in both the percentage of CD83+ cells and the CD83 MFI of the whole population of MDDC, was obvious at 24 hours and still evident after 48 hours of treatment with TNF-α (Figure 1A). To determine which signal-transduction pathway might be mediating the serum inhibitory effect, maturation of MDDC was done with serum and in the presence of the MEK 1/2 inhibitor U0126, which inhibits activation of the MEK 1/2–ERK MAPK pathway. TNF-α–induced expression of CD83 was augmented in the presence of U0126 at both 24 and 48 hours, reaching levels similar to those obtained in the absence of serum (Figure 1A). Similar enhancing effects were observed when maturation took place in the presence of PD98059,13 an alternative inhibitor of the ERK signaling pathway (data not shown). Conversely, and as previously described,10,18 23 the p38 MAPK inhibitor SB203580 greatly impaired acquisition of the CD83 maturation marker (Figure2). These results suggest that serum delays maturation of MDDC by activating the ERK signal-transduction pathway. To control the specificity of the inhibitors, activation of ERK and p38 MAPK was assessed by Western blot analysis. As expected, ERK pathway inhibitors abolished TNF-α–induced ERK phosphorylation (Figure 1B). Conversely, SB203580 significantly prevented TNF-α–induced phosphorylation of p38 MAPK (Figure 1B). Together, these results suggest that activation of the ERK signaling pathway has an inhibitory role in the maturation of MDDC.
Serum inhibits TNF-α–mediated maturation of MDDC: involvement of the ERK signaling pathway.
(A) Monocytes were cultured in macrophage serum-free medium containing GM-CSF and IL-4. After 5 days, cells were treated with TNF-α in the absence or presence of 10% fetal calf serum and with or without the U0126 MEK 1/2 inhibitor. Expression of CD83 was determined by flow cytometry 24 and 48 hours later. In each panel, the percentage of positive cells (bottom) and the MFI (top) are indicated. A representative experiment is shown. (B) Specificity of the PD98059, U0126, and SB203580 signaling-pathway inhibitors. Immature MDDC were treated with TNF-α in either the absence (minus sign) or presence of the indicated inhibitors (PD indicates PD98059; SB, SB203580; and U0; U0126). After 1 hour of incubation with the inhibitors, cells were stimulated with TNF-α for 10 minutes. Cell lysates (10 μg) were subjected to Western blotting using rabbit polyclonal antiserum specific for ERK 1/2 (ERK) or p38 or against their respective phosphorylated forms (pERK or pp38). The experiment was done on cells from 2 different donors and one experiment is shown.
Serum inhibits TNF-α–mediated maturation of MDDC: involvement of the ERK signaling pathway.
(A) Monocytes were cultured in macrophage serum-free medium containing GM-CSF and IL-4. After 5 days, cells were treated with TNF-α in the absence or presence of 10% fetal calf serum and with or without the U0126 MEK 1/2 inhibitor. Expression of CD83 was determined by flow cytometry 24 and 48 hours later. In each panel, the percentage of positive cells (bottom) and the MFI (top) are indicated. A representative experiment is shown. (B) Specificity of the PD98059, U0126, and SB203580 signaling-pathway inhibitors. Immature MDDC were treated with TNF-α in either the absence (minus sign) or presence of the indicated inhibitors (PD indicates PD98059; SB, SB203580; and U0; U0126). After 1 hour of incubation with the inhibitors, cells were stimulated with TNF-α for 10 minutes. Cell lysates (10 μg) were subjected to Western blotting using rabbit polyclonal antiserum specific for ERK 1/2 (ERK) or p38 or against their respective phosphorylated forms (pERK or pp38). The experiment was done on cells from 2 different donors and one experiment is shown.
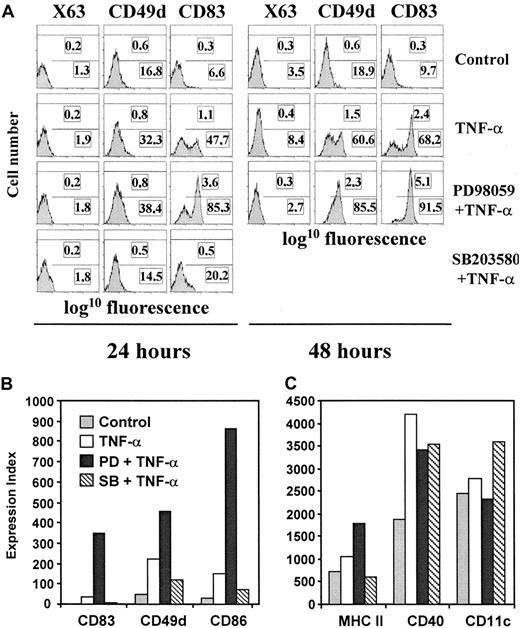
PD98059 and SB203580 differentially regulate the phenotypic changes that take place during TNF-α–induced maturation of MDDC.
Immature MDDC were either not treated (control) or treated with TNF-α in the absence of inhibitors (TNF-α) or in presence of PD98059 (PD and TNF-α) or SB203580 (SB and TNF-α). After 24 hours (A) or 48 hours (B,C), cell-surface expression of CD83, CD49d, CD86, MHC class II, CD11c, and CD40 was determined by indirect immunofluorescence and fluorescence of a negative control antibody (X63). Flow cytometry profiles are shown in panel A, and data are given as an expression index (percentage of marker-positive cells multiplied by their MFI) in panels B and C. Each assessment was done at least 4 times and representative results are shown. The results shown in panels A and B and in panel C were obtained with samples from 2 different donors.
PD98059 and SB203580 differentially regulate the phenotypic changes that take place during TNF-α–induced maturation of MDDC.
Immature MDDC were either not treated (control) or treated with TNF-α in the absence of inhibitors (TNF-α) or in presence of PD98059 (PD and TNF-α) or SB203580 (SB and TNF-α). After 24 hours (A) or 48 hours (B,C), cell-surface expression of CD83, CD49d, CD86, MHC class II, CD11c, and CD40 was determined by indirect immunofluorescence and fluorescence of a negative control antibody (X63). Flow cytometry profiles are shown in panel A, and data are given as an expression index (percentage of marker-positive cells multiplied by their MFI) in panels B and C. Each assessment was done at least 4 times and representative results are shown. The results shown in panels A and B and in panel C were obtained with samples from 2 different donors.
Phenotypic maturation of MDDC is differentially influenced by the ERK and p38 MAPK signaling pathways: effects on maturation markers and costimulatory molecules
To assess the influence of the p38 MAPK and ERK signaling pathways on maturation of MDDC, all subsequent experiments were done in the presence of serum, and TNF-α–induced maturation was carried out in the presence of inhibitors of one of the signaling routes. Maturation of MDDC in the presence of PD98059 resulted in greater expression of the maturation markers CD83 and CD49d (Figure 2A). Moreover, PD98059 markedly increased up-regulation of CD86 and slightly enhanced the maturation-dependent up-regulation of MHC class II molecules but did not affect expression of CD40 or CD11c (Figure 2B-C). All these effects of PD98059 were observed 48 hours after TNF-α was added, although some were seen as early as 24 hours into the maturation process (CD83 in Figure 2A). The inhibitory role of the ERK signal-transduction pathway on phenotypic maturation of MDDC was also indicated by studies using U0126, since this inhibitor also increased expression of CD83, CD49d, and CD86 without affecting expression of CD40 and CD11c (Figure 1A and data not shown). Conversely, the p38 MAPK inhibitor SB203580 blocked TNF-α–induced up-regulation of the maturation markers CD83 and CD49d on MDDC (Figure 2A-B). SB203580 also reduced up-regulation of the costimulatory molecule CD86 and that of MHC class II, but expression of other cell-surface molecules (CD40) was not affected or was even slightly increased (CD11c; Figure 2B-C). No effect on the normal maturation pathway was observed when either PD98059, U0126, or SB203580 was used alone, and the PD98059 enhancing effect was observed at TNF-α concentrations between 2 ng/mL and 600 ng/mL (data not shown). Therefore, PD98059 and U0126 increased TNF-α–induced up-regulation of maturation markers (CD83 and CD49d) and costimulatory molecules (CD86), whereas SB203580 inhibited their expression, suggesting that the ERK and p38 MAPK signaling pathways have opposite effects on the phenotypic maturation of MDDC.
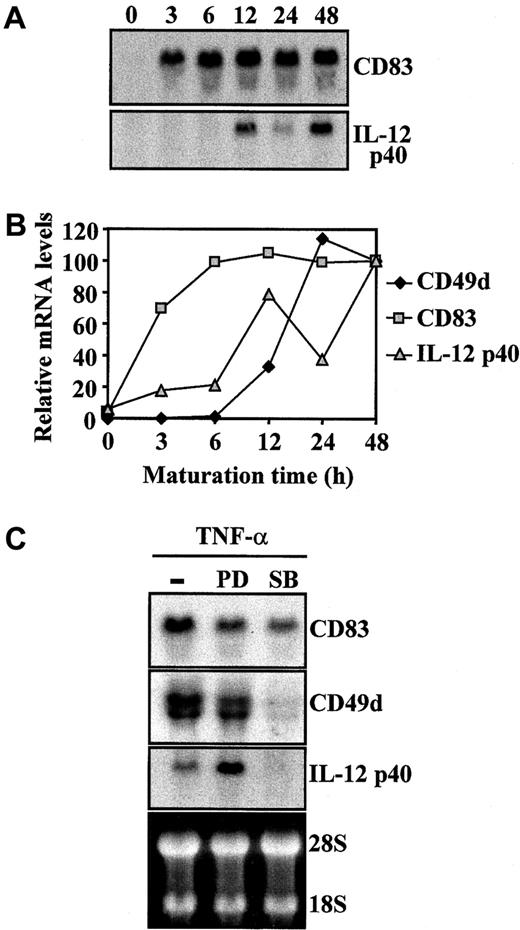
To further analyze the influence of PD98059 and SB203580 on expression of markers of maturation of MDDC, levels of CD83, CD49d, and IL-12 p40 mRNA were determined during TNF-α–induced maturation of MDDC in the presence or absence of the intracellular signaling inhibitors. Northern blot analysis indicated that increased expression of CD83, CD49d, and IL-12 p40 during TNF-α–induced maturation of MDDC correlated with augmented mRNA levels, although their respective mRNA species were up-regulated with different kinetics (Figure3A-B). Whereas CD83 mRNA levels were already detectable 3 hours after TNF-α was added, CD49d mRNA was detected 12 to 24 hours after cytokine treatment,10 and levels of IL-12 p40 mRNA peaked after 12 hours and increased again at 48 hours (Figure 3A-B). The kinetics of mRNA up-regulation of the 3 maturation markers suggested that different transcriptional or posttranscriptional mechanisms regulate their augmented expression on maturation of MDDC. In fact, determination of the respective mRNA levels of the markers 48 hours after TNF-α was added revealed that PD98059 pretreatment increased mRNA levels of IL-12 p40 but reduced those of CD83 and CD49d (Figure 2C). Conversely, SB203580 blocked TNF-α–mediated increase of CD49d mRNA, partly inhibited that of CD83 mRNA (Figure 2C), and prevented TNF-α–triggered IL-12 p40 induction of mRNA, in agreement with previous findings.21,22 28Therefore, ERK signaling-pathway inhibitors increased expression of MDDC maturation markers CD83, CD49d, and IL-12 p40 and differentially affected their mRNA levels at the final stages of the maturation process.
Regulation of CD83, CD49d, and IL-12 p40 mRNA steady-state levels during TNF-α–induced maturation of MDDC.
(A,B) Kinetics of CD83, CD49d, and IL-12 p40 mRNA during maturation of MDDC. (A) Total RNA was isolated from immature or mature MDDC at the indicated times after TNF-α was added and subjected to Northern blotting. (B) Autoradiographs were scanned and measured densitometrically, and the intensity of each band was expressed by using arbitrary units according to the densitometric analysis. (C) Effect of PD98059 and SB203580 on CD83, CD49d, and IL-12 p40 mRNA levels during maturation of MDDC. Determination of CD83, CD49d, and IL-12 p40 mRNA in MDDC treated with TNF-α for 48 hours in the absence (minus sign) or presence of PD98059 (PD) or SB203580 (SB). Ethidium bromide staining of RNA formaldehyde gel is shown as a loading control.
Regulation of CD83, CD49d, and IL-12 p40 mRNA steady-state levels during TNF-α–induced maturation of MDDC.
(A,B) Kinetics of CD83, CD49d, and IL-12 p40 mRNA during maturation of MDDC. (A) Total RNA was isolated from immature or mature MDDC at the indicated times after TNF-α was added and subjected to Northern blotting. (B) Autoradiographs were scanned and measured densitometrically, and the intensity of each band was expressed by using arbitrary units according to the densitometric analysis. (C) Effect of PD98059 and SB203580 on CD83, CD49d, and IL-12 p40 mRNA levels during maturation of MDDC. Determination of CD83, CD49d, and IL-12 p40 mRNA in MDDC treated with TNF-α for 48 hours in the absence (minus sign) or presence of PD98059 (PD) or SB203580 (SB). Ethidium bromide staining of RNA formaldehyde gel is shown as a loading control.
Functional maturation of MDDC is differentially influenced by ERK and p38 MAPK pathway inhibitors
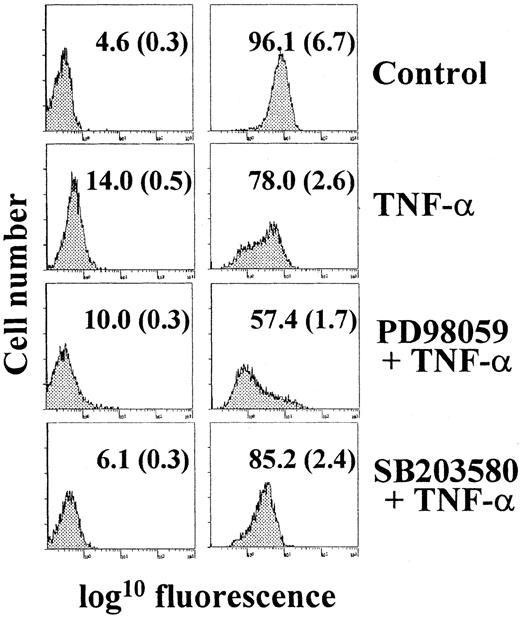
Immature DC capture and process antigens as a consequence of their high endocytic activity, a feature that is lost during maturation.2 29 Compared with immature MDDC, TNF-α–treated cells had a reduced level of mannose-receptor endocytosis (Figure 4). Treatment with PD98059 before the maturation stimulus further increased the loss of endocytic activity during TNF-α–induced maturation (Figure 4). Conversely, no significant change in endocytic activity was observed after 48 hours of maturation in the presence of the p38 MAPK inhibitor SB203580 (Figure 4). These results indicate that blockade of the MAPK-ERK pathway not only potentiates phenotypic maturation but also enhances loss of mannose-receptor–mediated endocytic activity.
Analysis of mannose-receptor–mediated endocytosis during TNF-α–induced maturation of MDDC.
Immature MDDC were cultured for 48 hours in the absence (control) or presence of TNF-α alone (TNF-α) or after pretreatment with either PD98059 (PD and TNF-α) or SB203580 (SB and TNF-α), and endocytic activity was determined by flow cytometry using fluorescein isothiocyanate–dextran. The percentage of positive cells and the MFI (in parentheses) are indicated. In all cases, endocytic activity was also measured at 4°C (left panels) to control for nonspecific fluorescence. Each experiment was done 3 times; a representative experiment is shown.
Analysis of mannose-receptor–mediated endocytosis during TNF-α–induced maturation of MDDC.
Immature MDDC were cultured for 48 hours in the absence (control) or presence of TNF-α alone (TNF-α) or after pretreatment with either PD98059 (PD and TNF-α) or SB203580 (SB and TNF-α), and endocytic activity was determined by flow cytometry using fluorescein isothiocyanate–dextran. The percentage of positive cells and the MFI (in parentheses) are indicated. In all cases, endocytic activity was also measured at 4°C (left panels) to control for nonspecific fluorescence. Each experiment was done 3 times; a representative experiment is shown.
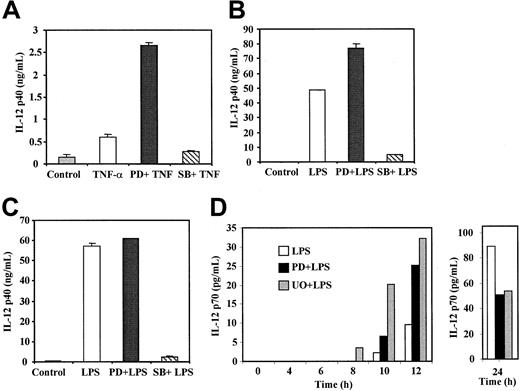
After exposure to maturation stimuli, MDDC produce numerous chemokines and cytokines, including TNF-α, IL-1, and more importantly, IL-12, a critical mediator in the generation of T-helper (Th) 1 responses.29 When IL-12 p40 production and release by TNF-α–treated MDDC was measured, PD98059 produced an average 5-fold increase in the levels of IL-12 p40 (range, 3-7 fold; Figure5A); a similar effect was observed with U0126 (data not shown). Although to a lower extent, PD98059 also increased levels of IL-12 p40 after 24 hours of LPS-induced maturation (Figure 5B). The PD98059 enhancing effect was not observed after 48 hours of LPS treatment (Figure 5C). Conversely, SB203580 caused a considerable reduction in the levels of IL-12 p40 release after maturation induced by either LPS or TNF-α (Figure 5A-C).
IL-12 p40 and p70 production by TNF-α–treated and LPS-treated MDDC in the presence of ERK or p38 MAPK signaling-pathway inhibitors.
Immature MDDC were not treated (control) or were stimulated with either TNF-α (20 ng/mL; A) or LPS (10 ng/mL; B-D) for 24 hours (B), 48 hours (A,C), or the indicated times (D), and IL-12 p40 and IL-12 p70 release was determined by enzyme-linked immunosorbent assay. When PD98059, U0126, or SB203580 were used, cells were preincubated for 1 hour before stimulation. Each assessment was done at least 3 times. In panels A to C, mean and SD results from a representative experiment are shown.
IL-12 p40 and p70 production by TNF-α–treated and LPS-treated MDDC in the presence of ERK or p38 MAPK signaling-pathway inhibitors.
Immature MDDC were not treated (control) or were stimulated with either TNF-α (20 ng/mL; A) or LPS (10 ng/mL; B-D) for 24 hours (B), 48 hours (A,C), or the indicated times (D), and IL-12 p40 and IL-12 p70 release was determined by enzyme-linked immunosorbent assay. When PD98059, U0126, or SB203580 were used, cells were preincubated for 1 hour before stimulation. Each assessment was done at least 3 times. In panels A to C, mean and SD results from a representative experiment are shown.
The differential inhibitory effect of PD98059 at 24 and 48 hours after LPS maturation suggested that inhibition of the ERK signaling pathway may affect the kinetics of IL-12 release. Because of this and because IL-12 p70 is not produced in response to TNF-α30 (A. Puig-Kröger and A.L. Corbı́, unpublished observations, 2001), we analyzed the influence of PD98059 and U0126 on the release of IL-12 p70 at different times during LPS-induced maturation. Kinetic analysis revealed that ERK blockade increased IL-12 p70 production in response to LPS (Figure 5D). In the presence of U0126 or PD98059, LPS induced higher levels (2-6 fold) of IL-12 p70 earlier (8-12 hours; Figure 5D). In contrast, ERK inhibitors reduced IL-12 p70 levels 24 hours after treatment with LPS (Figure 5D). As expected, LPS-induced IL-12 p70 release was completely abolished in the presence of SB203580 (data not shown), in agreement with previously reported results.22 28 Therefore, the ERK and p38 MAPK signaling pathways differentially regulate the release of IL-12, a key factor in determining the outcome of Th differentiation initiated upon antigen presentation.
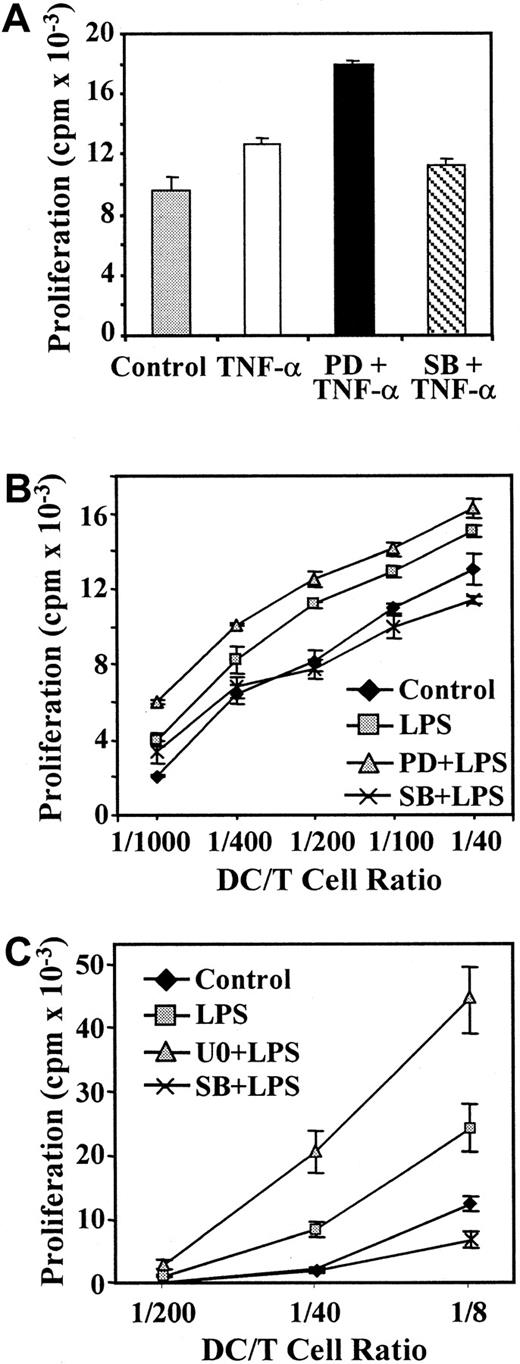
Because p38 MAPK and ERK pathway inhibitors had differential effects on phenotypic maturation of MDDC, and especially on expression of molecules critical for naive T-lymphocyte stimulation, we assessed the effects of the inhibitors on the T-cell response induced by allogeneic MDDC. As expected, MDDC stimulated with TNF-α had higher allostimulatory capacity than immature cells (Figure6A). In experiments using peripheral blood T lymphocytes, proliferation in response to PD98059 and TNF-α–treated MDDC was 30% higher than the allogenic T-cell response elicited by control TNF-α–matured cells (Figure 6A); these findings were in agreement with the increased levels of CD86 costimulatory molecule observed on inhibition of the ERK pathway (Figure 2A). Similarly, PD98059 and LPS-treated MDDC also promoted higher T-cell stimulatory ability than LPS-matured MDDC at all ratios of stimulator to responder tested (Figure 6B).
Influence of signaling-pathway inhibitors on the T-cell stimulatory capacity of mature MDDC.
Maturation of MDDC was induced with TNF-α (20 ng/mL; A) or LPS (10 ng/mL; B,C) and in either the absence or presence of PD98059 (PD; 40 μM), U0126 (U0; 2.5 μM), or SB203580 (SB; 13 μM). After 48 hours, MDDC were irradiated and used to stimulate 2 × 105allogeneic peripheral blood T lymphocytes (A,B) or CD4+cord-blood lymphocytes (C) in 96-well plates. Stimulation was done at a 1:40 ratio (A) or the indicated ratios of MDDC to T cells (B,C). After a 5-day coculture, tritium-thymidine was added to the culture for 16 hours and T-cell proliferation determined by measuring the incorporated thymidine. Each experiment was done twice; representative experiments are shown.
Influence of signaling-pathway inhibitors on the T-cell stimulatory capacity of mature MDDC.
Maturation of MDDC was induced with TNF-α (20 ng/mL; A) or LPS (10 ng/mL; B,C) and in either the absence or presence of PD98059 (PD; 40 μM), U0126 (U0; 2.5 μM), or SB203580 (SB; 13 μM). After 48 hours, MDDC were irradiated and used to stimulate 2 × 105allogeneic peripheral blood T lymphocytes (A,B) or CD4+cord-blood lymphocytes (C) in 96-well plates. Stimulation was done at a 1:40 ratio (A) or the indicated ratios of MDDC to T cells (B,C). After a 5-day coculture, tritium-thymidine was added to the culture for 16 hours and T-cell proliferation determined by measuring the incorporated thymidine. Each experiment was done twice; representative experiments are shown.
On the other hand, SB203580 pretreatment impaired acquisition of T-cell stimulatory activity by mature MDDC, although its effects were more evident when LPS was used as a maturation agent (Figure 6A-B). The effects of the ERK pathway inhibitors on T-cell activation mediated by MDDC were also evaluated by using CD4+ cord-blood T cells, which contain a higher proportion of naive CD45RA+lymphocytes.31 As shown in Figure 6C, U0126 pretreatment increased the T-cell stimulatory ability of LPS-matured MDDC by 90% (24 030 ± 3626 versus 44 207 ± 5122), whereas SB203580 inhibited the T-cell response. The enhancing effect of U0126 was also observed at stimulator:responder ratios of 1:40 (8321 ± 1214 versus 20 310 ± 3116) and 1:200 (1024 ± 118 versus 2709 ± 723; Figure 6C). Together, these results indicate that PD98059 potentiates and SB203580 represses the TNF-α–induced and LPS-induced functional maturation of MDDC, thereby suggesting that the ERK and p38 MAPK signaling pathways exert opposite effects on maturation of MDDC.
The ERK signal-transduction pathway negatively regulates NF-κB DNA- binding and IκBα levels on TNF-α–induced maturation
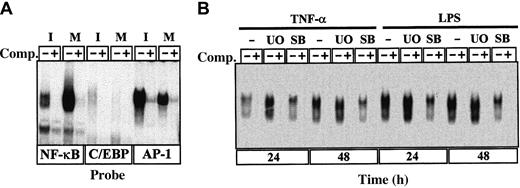
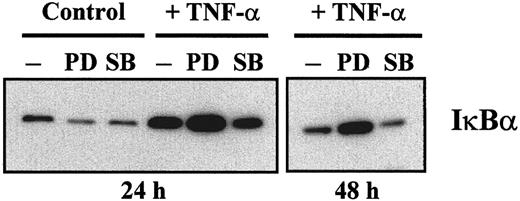
NF-κB transcription-factor family members, especially RelB, are essential for maturation of DC.32 As shown in Figure7A, we found, in agreement with previous studies,33,34 that the DNA-binding activity of NF-κB increased during maturation of MDDC, concomitantly with a decrease in the DNA-binding activity of C/EBP and AP-1. Inhibition of ERK activation before LPS-induced or TNF-α–induced maturation caused a further increase in NF-κB measured either 24 or 48 hours after the maturation agent was added (Figure 7B). Similar effects were obtained by using the PD98059 ERK pathway inhibitor (data not shown), thus providing additional evidence that maturation of MDDC was enhanced by blockade of the ERK signaling pathway before addition of the maturation-inducing agents. Conversely, SB203580 inhibited the increase in NF-κB DNA-binding activity induced by either LPS or TNF-α (Figure 7B). On the other hand, it was previously shown that levels of IκBα and Bcl-3 increase during maturation of MDDC.34Therefore, immunoblotting of cytoplasmic proteins was done to determine the relative levels of IκBα under the different experimental conditions. As expected, IκBα levels increased when measured 24 hours after TNF-α–induced maturation (Figure8). Moreover, IκBα levels increased further in MDDC matured in the presence of PD98059 after 24 and 48 hours, whereas SB203580 caused a reduction in the levels of IκBα with respect to those observed in MDDC matured with TNF-α alone (Figure 8). Therefore, ERK signaling-pathway inhibitors potentiate the increase in NF-κB and IκBα that takes place during maturation of MDDC.
Changes in NFs during TNF-α–induced maturation of MDDC.
(A) Recognition of NF-κB, C/EBP, and AP-1 consensus sequences by nuclear transcription factors from immature (I) and mature (M; treated with TNF-α for 96 hours) MDDC. EMSA was done on the corresponding consensus oligonucleotide probes by using nuclear extracts and in the absence (lanes marked with a minus sign) or presence (lanes marked with a plus sign) of unlabeled competitor (Comp) oligonucleotides at a 100-fold molar excess. (B) Determination of NF-κB DNA-binding activity in total cell extracts from LPS-treated or TNF-α–treated MDDC cultured for 24 or 48 hours in the absence or presence of the indicated inhibitors (U0 indicates U0126; and SB, SB203580). Competition was done by adding cold NF-κB consensus oligonucleotide at a 100-fold molar excess.
Changes in NFs during TNF-α–induced maturation of MDDC.
(A) Recognition of NF-κB, C/EBP, and AP-1 consensus sequences by nuclear transcription factors from immature (I) and mature (M; treated with TNF-α for 96 hours) MDDC. EMSA was done on the corresponding consensus oligonucleotide probes by using nuclear extracts and in the absence (lanes marked with a minus sign) or presence (lanes marked with a plus sign) of unlabeled competitor (Comp) oligonucleotides at a 100-fold molar excess. (B) Determination of NF-κB DNA-binding activity in total cell extracts from LPS-treated or TNF-α–treated MDDC cultured for 24 or 48 hours in the absence or presence of the indicated inhibitors (U0 indicates U0126; and SB, SB203580). Competition was done by adding cold NF-κB consensus oligonucleotide at a 100-fold molar excess.
ERK 1/2 and p38 MAPK differentially affect the levels of IκBα during maturation of MDDC.
Immature MDDC were not treated (control) or were cultured with TNF-α for 24 or 48 hours and in either the absence (minus sign) or presence of inhibitor (PD indicates PD98059; and SB, SB203580). Cytoplasmic extract (10 μg) from each culture condition was subjected to Western blotting using a rabbit polyclonal antiserum specific for IκBα. The experiment was done with samples from 2 different donors and identical results were obtained; a representative experiment is shown.
ERK 1/2 and p38 MAPK differentially affect the levels of IκBα during maturation of MDDC.
Immature MDDC were not treated (control) or were cultured with TNF-α for 24 or 48 hours and in either the absence (minus sign) or presence of inhibitor (PD indicates PD98059; and SB, SB203580). Cytoplasmic extract (10 μg) from each culture condition was subjected to Western blotting using a rabbit polyclonal antiserum specific for IκBα. The experiment was done with samples from 2 different donors and identical results were obtained; a representative experiment is shown.
Discussion
After DC are exposed to inflammatory stimuli or bacterial products, they undergo functional maturation and reenter the circulatory system to home to the T-cell areas of the draining lymphoid organs.2 29 In this study, we assessed the effects of the ERK signaling pathway on the phenotypic and functional maturation of MDDC in response to either TNF-α or LPS. Our results indicate that inhibition of the ERK signal-transduction pathway potentiates acquisition of factors that are usually considered hallmarks for maturation of MDDC, including expression of costimulatory, adhesion, and maturation antigens; loss of mannose-receptor–mediated endocytosis; NF-κB DNA binding; IκBα protein; and, more importantly, IL-12 and T-lymphocyte stimulatory capacity. In contrast, inhibition of the p38 MAPK signaling route prevented acquisition of most of these maturation factors. Therefore, these results show that the ERK and p38 MAPK pathways have opposite effects on DC maturation and suggest a negative regulatory role for ERK during this process.
The opposing roles of the ERK and p38 MAPK pathways in the maturation of DC are evocative of events in other cellular differentiation systems. During cartilage formation, p38 MAPK and ERK 1/2 act as positive and negative regulators of chondrogenesis by conversely regulating expression of different adhesion molecules, including N-cadherin, fibronectin, and the CD49e/CD29 integrin.35Similarly, butyrate-induced erythroid differentiation of K562 cells was found to be distinctly modulated by inhibition of either ERK 1/2 or p38 MAPK activity.36 In both cases, cell differentiation was promoted through the p38 MAPK signal-transduction pathway whereas ERK activation was a negative regulator.35,36 A similar mechanism appears to be present in MDDC. The molecular mechanism by which ERK negatively regulates maturation of MDDC is unknown. However, it has been proposed that a constitutive active MEK-ERK pathway is capable of negatively regulating NF-κB–dependent gene expression in a myeloid cell line through modulation of TATA-binding protein phosphorylation, an obligatory step for NF-κB–driven transcription.37 If this also occurs in MDDC—and given the essential role of NF-κB factors in the generation and maturation of DC32-34—activation of ERK would be preventing maturation of MDDC by decreasing NF-κB–dependent gene transcription. Such a hypothesis would be consistent with the augmented levels of IκBα protein and IL-12 p40 mRNA detected in MDDC treated with PD98059 (Figures 3 and 8), since transcription of both genes depends on NF-κB factors.38 39
Unlike the results with SB203580, maturation of MDDC in the presence of MEK 1/2 inhibitors resulted in augmented expression of MHC class II, costimulatory, and adhesion molecules; faster induction of CD83; increased NF-κB DNA-binding and IκBα protein levels; and increased release of IL-12 p40 and IL-12 p70. In agreement with these results, PD98059 led to an increase in T-cell stimulatory activity of mature MDDC. Therefore, it can be concluded that the ERK 1/2 signaling pathway negatively regulates the maturation of MDDC in response to LPS (10 ng/mL) or TNF-α (2-600 ng/mL). It is worth noting that apart from the reported negative effect of ERK 1/2 on IL-12 p40 mRNA,28 the inhibitory influence of the ERK 1/2 pathway on maturation of DC had not been observed previously, which led to the proposal that ERK 1/2 has no major role in the maturation of MDDC. Previous studies used higher concentrations of LPS (100 ng/mL) as maturation inducer23 and found no effect of ERK pathway inhibitors. A possible reason for this discrepancy might be the maturation stimulus used, since TNF-α appears to be a weaker promotor of maturation of MDDC.30 34 In any event, we found that MEK 1/2 inhibitors enhanced IL-12 p40 and p70 release and T-cell stimulatory activity after maturation with 10 ng/mL LPS (Figures 5 and6). Therefore, it is possible that the higher concentrations of LPS or the use of stronger maturation stimuli obscured the role of the ERK 1/2 signaling pathway in previous studies and that the negative regulatory role of ERK 1/2 activation might be easier to detect on TNF-α treatment. Alternatively, the inhibitory effect of PD98059 may have been missed because the analysis was done at the later stages of maturation of MDDC.
The final outcome of maturation of DC depends on the maturation stimulus and the environmental signals received by DC on activation.40,41 Three functional subsets of DC might be generated: a subset with high costimulatory capacity and IL-12 production (DC1), a subset with high costimulatory capacity but low IL-12 production (DC2), and a subset with low costimulatory capacity and IL-12 production (DC3).40 According to this model, DC1 would promote Th1 polarization, whereas DC2 would drive Th2 differentiation and type 3 would give rise to tolerogenic Th lymphocytes.40 Our results with MDDC indicate that the type of T-cell polarizing activity shown by these cells might depend on the balance between ERK and p38 MAPK activation triggered by maturation stimuli and environmental signals. In this manner, maturation of MDDC in an extracellular context favoring a low ratio of ERK to p38 MAPK activation would give rise to DC with Th1-polarizing capacity, whereas a high ratio would shift the process toward a Th2-tolerogenic outcome. Moreover, it was proposed that the polarizing capacity of DC is determined by the kinetics of activation30: the “DC exhaustion model” proposes that MDDC promote Th1 polarization at the early stages of maturation (active DC) and induce Th2 differentiation once their IL-12 production ability has diminished (exhausted DC). If this model is accurate, it may be that ERK inhibitors accelerate the kinetics of maturation of MDDC and reduce the time frame before exhaustion. We are currently testing this hypothesis by determining the relative proportion of antigen-specific Th1 and Th2 lymphocytes generated by MDDC matured in the presence of ERK or p38 MAPK inhibitors.
We thank Dr Pedro Lastres for flow cytometry and Alba Galán, Patricio Aller, and Jorge Martı́n for helpful discussions and for sharing reagents.
Supported by grants SAF98/0068 from Comisión Interministerial de Ciencia y Tecnologı́a, 08.3/0026/2000.1 from Comunidad Autónoma de Madrid and Fondo de Investigación Sanitaria 01/0063-01 (A.L.C.), and SAF2000/0132 (C.B.). A.P.-K. is the recipient of a fellowship from Instituto Reina Sofı́a (Fundación Renal Iñigo Alvarez de Toledo).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Angel L. Corbı́, Centro de Investigaciones Biológicas, Consejo Superior de Investigaciones Cientı́ficas, Velázquez 144, 28006 Madrid, Spain: e-mail:acorbi@cib.csic.es.