The apoptosis and subsequent clearance of eosinophils without histotoxic mediator release is thought to be crucial in the resolution of airway inflammation in asthma. Interleukin-5 (IL-5) is a potent suppressor of eosinophil apoptosis. The mechanism by which IL-5 inhibits spontaneous eosinophil apoptosis was investigated. Freshly isolated eosinophils constitutively expressed the conformationally active form of Bax in the cytosol and nucleus. During spontaneous and staurosporine-induced apoptosis, Bax underwent a caspase-independent translocation to the mitochondria, which was inhibited by IL-5. Eosinophil apoptosis was associated with the release of cytochrome c from the mitochondria, which was also inhibited by IL-5. IL-5 and the cell-permeable caspase inhibitor, benzyloxycarbonyl-Val-Ala-Asp-(OMe) fluoromethyl ketone (z-VAD.fmk), prevented phosphatidylserine (PS) externalization, although only IL-5 inhibited loss of mitochondrial membrane potential (ΔΨm). Peripheral blood eosinophils endogenously expressed “initiator” caspase-8 and -9, and “effector” caspase-3, -6, and -7. Spontaneous eosinophil apoptosis was associated with processing of caspase-3, -6, -7, -8, and -9. IL-5 and z-VAD.fmk prevented caspase activation in spontaneous apoptosis. The results suggest that spontaneous eosinophil apoptosis involves Bax translocation to the mitochondria, cytochrome crelease, caspase-independent perturbation of the mitochondrial membrane, and subsequent activation of caspases. IL-5 inhibits spontaneous eosinophil apoptosis at a site upstream of Bax translocation.
Introduction
Eosinophils play a pivotal role in the pathogenesis of asthma and allergic disease.1,2 Apoptosis and efficient clearance of apoptotic cells without histotoxic mediator release is thought to be crucial in the resolution of airway inflammation,3 and delayed eosinophil apoptosis has an association with asthma, inhalant allergy, and atopic dermatitis.4,5 The accumulation and persistence of eosinophils at sites of inflammation are mediated at least in part by extended survival in response to circulating hematopoietins interleukin-3 (IL-3), IL-5, and granulocyte-macrophage colony-stimulating factor (GM-CSF). Eosinophils rapidly undergo apoptosis unless exposed to these cytokines in vitro6,7and in vivo.8 9 The mechanism by which IL-5 prevents apoptosis in eosinophils is largely unknown.
The Bcl-2 homologues are critical regulators of the apoptotic pathway, with interactions between proapoptotic (Bax, Bik, Bim, Bak) and antiapoptotic (Bcl-2, Bcl-xL) proteins controlling the release of apoptogenic factors from mitochondria10,11 and subsequent activation of caspases, the conserved death proteases of the cell. Previous reports investigating the role of Bcl-2 homologues in eosinophils have concentrated on the level of protein expression. Human peripheral blood eosinophils endogenously express relatively high levels of proapoptotic Bax and antiapoptotic Bcl-xL, with little or no detectable antiapoptotic Bcl-2 expression,12,13 although there is some evidence of higher Bcl-2 expression in eosinophils derived from patients with asthma and hypereosinophilic syndrome,14,15 and stimulation in vitro with IL-5 resulted in detectable up-regulation of Bcl-2 expression in some12,16 but not all studies.17,18 It has been proposed that susceptibility to cell death is determined by the ratio of death agonist to death antagonist and their subsequent interactions by homodimerization and heterodimerization, via conserved BH3 domains.11 The, at best, small increase in Bcl-2 in IL-5–stimulated eosinophils,12 would suggest up-regulation of Bcl-2 alone is unlikely to be solely responsible for the potent survival-enhancing activity of IL-5. This implicates other regulatory mechanisms, possibly involving the well-expressed Bax or Bcl-xL.12,18 Bax is a monomeric, cytosolic protein that has been shown in cell lines to undergo a conformational change and translocate from the cytosol to the outer mitochondrial membrane during apoptosis,19 facilitating the release of cytochrome c20,21 and subsequent activation of caspases. The conformation change allows both oligomerization and membrane insertion of Bax, but also reveals an N-terminal epitope recognized by the monoclonal antibody 6A7.22
Caspases, aspartate-specific cysteine proteases, regulate the execution phase of apoptosis, being responsible for most of the biochemical and morphologic changes associated with the apoptotic phenotype.23 Distinct caspase cascades are initiated dependent on the death stimulus.24,25“Receptor-mediated” apoptosis involves ligation of death receptors, such as CD95 (Fas/Apo-1) and tumor necrosis factor receptor-1 within the plasma membrane of many cell types, and direct processing of caspase-8 on recruitment to the receptor complex.26 A target of caspase-8 is the proapoptotic Bcl-2 homologue, Bid, which on proteolysis translocates from the cytosol to mitochondria, and by a mechanism possibly involving interaction with Bax, potentiates the release of cytochrome c.27 In “chemical/stress-induced” apoptosis, cellular signals induce perturbations of the mitochondria and the release of apoptosis mediators including cytochrome c,28apoptosis-inducing factor (AIF),29 and Smac/Diablo.30,31 Release of cytochrome callows formation of the “apoptosome,” a caspase-9–activating complex, involving cytochrome c, Apaf-1, deoxyadenosine triphosphate (dATP), and procaspase-9.32 33 The activation of “initiator” caspase-8 and -9, results in direct or indirect activation of “effector” caspases, such as caspase-3, -6, and -7.
In this study, we investigated the mechanism by which IL-5 inhibits spontaneous apoptosis in eosinophils. We report that IL-5 prevented Bax translocation and cytochrome c release and mediated eosinophil survival by inhibiting perturbation of the mitochondrial membrane and caspase activation.
Materials and methods
Antibodies and reagents
Cell culture media and recombinant human IL-5 were purchased from Gibco-BRL (Paisley, United Kingdom). Benzyloxycarbonyl-Val-Ala-Asp-(OMe) fluoromethyl ketone (z-VAD.fmk) was from Enzyme Systems (Dublin, CA). Rabbit polyclonal antibodies directed against caspase-3, -7, -8, and -9 were generated as previously described,24 34 and a polyclonal antibody against caspase-6 was purchased from Upstate Biotechnology (Lake Placid, NY). The epitope recognition site of the caspase-6 antibody spanned the prodomain cleavage site; therefore, the subunits were not immunoreactive. Monoclonal antibodies directed against cytochromec (6H2.B4), Bak (G317-2), Bik (C33-1), and amino acids 12 to 24 of the Bax N-terminus (6A7) were from Pharmingen (Oxford, United Kingdom). Anti-Bim polyclonal antibody was from Calbiochem-Novabiochem (San Diego, CA). Antimouse and antirabbit horseradish peroxidase (HRP) conjugates were from Sigma (Poole, United Kingdom) and Dako (Ely, United Kingdom), respectively. Species-specific Alexa 488 secondary antibodies for confocal analysis, tetramethylrhodamine ethyl ester (TMRE), and Mitotracker Red CMXRos were from Molecular Probes (Eugene, OR). Fluorescein isothiocyanate (FITC)–conjugated annexin V was obtained from Bender Medsystems (Vienna, Austria). All other reagents were from Sigma unless otherwise stated.
Cell lines and culture
Jurkat T cells (clone E6-1) were obtained from European Collection of Cell Cultures and maintained in RPMI 1640 supplemented with 10% fetal calf serum (FCS) and 1% Glutamax. Apoptosis was induced by treatment with etoposide (50 μM) for 4 hours.
Isolation of peripheral blood eosinophils and cell culture
Heparinized peripheral venous blood was taken from healthy volunteers with peripheral blood eosinophilia of less than 0.5 × 106/mL. Eosinophils were purified by a 2-step method of density gradient centrifugation, followed by negative immunomagnetic selection as described previously.35Briefly, removal of erythrocytes by dextran sedimentation was followed by centrifugation of the leukocyte-rich supernatant (100g, 15 minutes at room temperature). The cell pellet was resuspended in Hanks balanced salt solution without Ca++ and Mg++, supplemented with 1% bovine serum albumin (BSA) and 20 mM EDTA. The cell suspension was centrifuged on Histopaque 1083 and the mononuclear cell layer carefully removed, prior to lysis of erythrocytes contaminating the granulocyte pellet by hypotonic shock using sterile, ice-cold water. Eosinophils were separated from neutrophils by negative immunomagnetic selection using anti-CD16–coated magnetic beads (Miltenyi Biotec, Auburn, CA). Purity and viability after isolation were routinely more than 99% as assessed by morphology after Kimura stain and trypan blue exclusion, respectively. Peripheral blood eosinophils were cultured in RPMI 1640 supplemented with 1% BSA and 1% Glutamax in the presence of IL-5 (10−10 M), staurosporine (STS; 10−5 M), or z-VAD.fmk (100 μM) where indicated. Preliminary experiments were performed to determine optimum concentrations.
Immunoblotting
Cells (5 × 105) were washed with ice-cold phosphate-buffered saline (PBS) and snap-frozen in dry ice before storage at −70°C. Samples were resuspended in sample buffer with freshly added 5% β-mercaptoethanol and boiled for 5 minutes. Proteins were then separated on 10%/15% sodium dodecyl sulfate–polyacrylamide gels and electrotransferred onto presoaked Hybond-C nitrocellulose filter (Amersham Life Science, Bucks, United Kingdom). Immunoblotting was performed as described previously12 and proteins detected by enhanced chemiluminescence according to the manufacturer's instructions (Amersham Life Science).
Assessment of phosphatidylserine exposure and mitochondrial membrane potential
The exposure of phosphatidylserine (PS) has been shown to be a sensitive marker of apoptosis in eosinophils and other cell types.36,37 Cultured cells were washed once in PBS and incubated with annexin V-FITC (1:1000) according to the manufacturer's instructions. Cells were then incubated with 50 μg/mL propidium iodide for 2 minutes on ice prior to analysis using the FACScan (Becton Dickinson, Oxford, United Kingdom), excitation at 488 nm and detection between 515 and 550 nm. To measure mitochondrial membrane potential (ΔΨm), cells were loaded with 75 nM TMRE for 30 minutes at 37°C. Alterations of ΔΨm can be determined from the fluorescence intensity of TMRE because the transmembrane distribution of this cationic dye is dependent on membrane potential.38 Cells were washed once and resuspended in PBS for FACScan analysis, excitation at 488 nm and detection at 610 nm. Freshly isolated eosinophils were treated with the mitochondrial membrane uncoupler carbonylcyanide m-chlorophenyl hydrazone (mCCP) at 1 μM for 15 minutes at 37°C as a positive control for dissipation of ΔΨm.
Apoptotic morphology
Immunocytochemistry and confocal analysis
Cells were incubated with 75 nM Mitotracker Red CMXRos for 45 minutes at 37°C and washed in RPMI without BSA, and cytospins were performed (650 rpm for 6 minutes) on silane-coated slides at 1 × 106 cells/mL. Slides were fixed in 2% paraformaldehyde for 15 minutes at room temperature and washed 5 times in PBS. Cells were permeabilized for 10 minutes at room temperature in blocking buffer (3% BSA in PBS) plus 0.1% Triton X-100 followed by blocking of nonspecific binding in blocking buffer for 1 hour at room temperature. Cells to be stained with anti–Bax 6A7 were not permeabilized because the recognized Bax conformation has been shown to be sensitive to nonionic detergent.40 Cells were incubated overnight at 4°C with 5 μg/mL primary antibody diluted in blocking buffer. Cells were then incubated for 20 minutes at room temperature with 0.2% chromotrope-2R, which binds to highly basic eosinophil granules thereby reducing nonspecific binding of the secondary antibody. Cells were then incubated with species-specific Alexa 488–conjugated secondary antibody diluted 1:300 in blocking buffer for 50 minutes at room temperature in the dark. The nuclei were stained with Hoechst 33258 (250 ng/mL) for 10 minutes at room temperature in the dark prior to mounting with fluoromount (Dako). Images were collected by confocal laser microscopy (model TCS 4D, Leica, Heidelberg, Germany). The 488- and 568-nm lines of the krypton/argon laser were used for the excitation of Alexa 488 and Mitotracker Red CMXRos, respectively. Excitation of Hoechst 33258 was by UV laser. Cytochrome c release and Bax translocation were quantified on these cytospins by counting at least 300 cells in a blinded fashion in 4 random visual fields and assessing evidence of diffuse cytochrome c or punctate Bax staining.
Statistical analysis
Comparisons between treated and untreated cells were made at individual time points using the Student paired ttest.
Results
Caspase expression and activation in peripheral blood eosinophils
The primary aim of this project was to identify the point in the apoptotic pathway at which IL-5 exerts its antiapoptotic effect. Initial investigation explored the role of caspases in eosinophil apoptosis. Peripheral blood eosinophils endogenously expressed both the “initiator” caspase-8 and -9, and the “effector” caspase-3, -6, and -7 (Figures 1 and2). Treatment of eosinophils for 2 hours with the protein kinase C inhibitor, STS (10−5 M), was sufficient to induce activation of caspase-3, -6, -7, -8, and -9, as evidenced either by the generation of the immunoreactive, catalytically active, large subunits resulting from processing of the caspase proforms, or direct loss of the proform itself (Figures 1and 2).
IL-5 inhibits caspase processing in the spontaneous apoptosis of peripheral blood eosinophils.
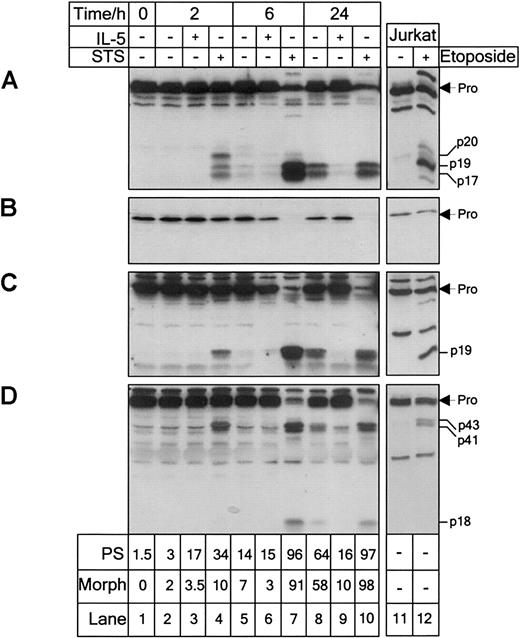
Peripheral blood eosinophils were cultured in the presence or absence of IL-5 (10−10 M) or STS (10−5 M) and analyzed at the times shown for processing of caspase-3 (A), -6 (B), -7 (C), and -8 (D) by immunoblotting. Jurkat T cells treated with or without etoposide (50 μM) for 4 hours were used as controls. The proforms and processed subunits are indicated. Apoptosis was assessed by annexin V binding to determine percentage cells with externalized PS and apoptotic morphology after Kimura stain, as described in “Materials and methods.” Eosinophils constitutively expressed caspase-3, -6, -7, and -8. Culturing in the absence of IL-5 and treatment with STS resulted in activation of all of the caspases. IL-5 prevented caspase activation over the 24-hour culture period. Longer exposure of the caspase-3 (A) blot revealed the presence of p20 and p19 subunits after 6 hours in untreated eosinophils. Shorter exposure of the caspase-8 (D) blot revealed the proform to be a doublet of 53 and 55 kd. Results are representative of 3 separate experiments.
IL-5 inhibits caspase processing in the spontaneous apoptosis of peripheral blood eosinophils.
Peripheral blood eosinophils were cultured in the presence or absence of IL-5 (10−10 M) or STS (10−5 M) and analyzed at the times shown for processing of caspase-3 (A), -6 (B), -7 (C), and -8 (D) by immunoblotting. Jurkat T cells treated with or without etoposide (50 μM) for 4 hours were used as controls. The proforms and processed subunits are indicated. Apoptosis was assessed by annexin V binding to determine percentage cells with externalized PS and apoptotic morphology after Kimura stain, as described in “Materials and methods.” Eosinophils constitutively expressed caspase-3, -6, -7, and -8. Culturing in the absence of IL-5 and treatment with STS resulted in activation of all of the caspases. IL-5 prevented caspase activation over the 24-hour culture period. Longer exposure of the caspase-3 (A) blot revealed the presence of p20 and p19 subunits after 6 hours in untreated eosinophils. Shorter exposure of the caspase-8 (D) blot revealed the proform to be a doublet of 53 and 55 kd. Results are representative of 3 separate experiments.
z-VAD.fmk inhibits caspase processing in IL-5–deprived peripheral blood eosinophils.
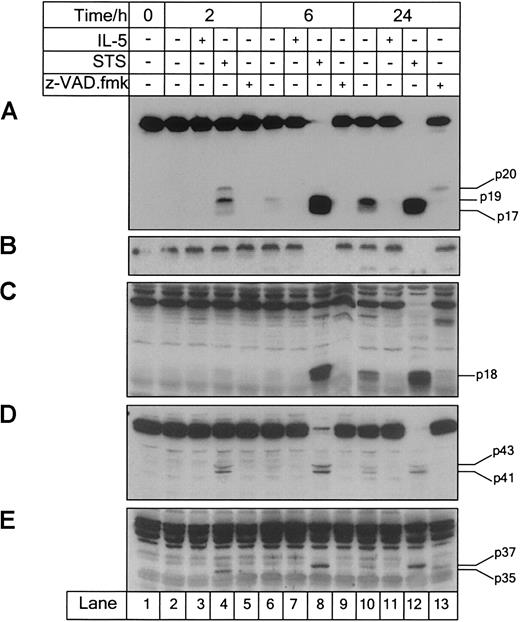
Cells were incubated with IL-5 (10−10 M), STS (10−5 M), or z-VAD.fmk (100 μM), harvested at the times indicated and analyzed for caspase-3 (A), -6 (B), -7 (C), -8 (D), and -9 (E) processing by immunoblotting. Eosinophils constitutively expressed caspase-9, which was activated during STS-induced and spontaneous apoptosis. IL-5 prevented the processing of caspase-9 observed during spontaneous apoptosis over the 24-hour culture period. z-VAD.fmk inhibited caspase activation in IL-5–deprived eosinophils. Results are representative of 3 separate experiments.
z-VAD.fmk inhibits caspase processing in IL-5–deprived peripheral blood eosinophils.
Cells were incubated with IL-5 (10−10 M), STS (10−5 M), or z-VAD.fmk (100 μM), harvested at the times indicated and analyzed for caspase-3 (A), -6 (B), -7 (C), -8 (D), and -9 (E) processing by immunoblotting. Eosinophils constitutively expressed caspase-9, which was activated during STS-induced and spontaneous apoptosis. IL-5 prevented the processing of caspase-9 observed during spontaneous apoptosis over the 24-hour culture period. z-VAD.fmk inhibited caspase activation in IL-5–deprived eosinophils. Results are representative of 3 separate experiments.
Procaspase-3 is expressed as an inactive 32-kd zymogen, which on activation is cleaved initially at D175 (single-letter amino acid codes) generating a p20 subunit, followed by further proteolytic cleavage at D9 and D28 to generate p19 and p17 subunits, respectively.41 In freshly isolated eosinophils, caspase-3 was present as a 32-kd proform (Figure 1A, lane 1). Exposure to STS for 2 hours resulted in the formation of 20-kd (p20), 19-kd (p19), and 17-kd (p17) immunoreactive fragments (Figure 1A, lane 4). After 6 hours, the proform was almost completely processed (Figure 1A, lane 7). Jurkat T cells exposed to etoposide for 4 hours exhibited similar caspase-3 processing (Figure 1A, lane 12).
Procaspase-6 was expressed in eosinophils as its 34-kd zymogen (Figure1B, lane 1). Processing of caspase-6 generates p18 and p11 subunits due to sequential cleavage at D179 and D193.42 Due to lack of immunoreactivity of these subunits, processing of caspase-6 was indicated by the time-dependent loss of the proform after 6 hours of STS treatment (Figure 1B, lane 7). A slight loss of caspase-6 proform was observed in Jurkat cells treated with etoposide for 4 hours (Figure1B, lane 12).
In eosinophils, caspase-7 was expressed as its 35-kd proform (Figure1C, lane 1). Treatment with STS for 2 hours resulted in formation of the immunoreactive 19-kd (p19) fragment (Figure 1C, lane 4), corresponding to the large catalytically active subunit generated due to cleavage at D198 and removal of the prodomain.34 After 6 hours of STS treatment, procaspase-7 was almost completely lost (Figure 1C, lane 7). Similar caspase-7 processing was observed in Jurkat T cells (Figure 1C, lane 12).
Caspase-8 was detectable in freshly isolated eosinophils and Jurkat T cells as the 55- kd proform (Figure 1D, lanes 1 and 12). However, shorter exposure revealed that caspase-8 was expressed as a doublet of 55 and 53 kd, corresponding to the proposed occurrence of 2 isoforms, caspase-8a and caspase-8b.43 After STS treatment for 2 hours, cleavage of the proform resulting in the formation of 43-kd (p43) and 41-kd (p41) subunits was observed corresponding to cleavage between the large and small subunits (Figure 1D, lane 4). After 6 hours of STS treatment, an 18-kd (p18) fragment was observed due to removal of the death effector domains from p43 and p41 (Figure 1D, lane 7). Generation of the p45 and p43 subunits was observed in Jurkat T cells treated with etoposide for 4 hours, although the p18 subunit was not marked (Figure 1D, lane 12).
Caspase-9 is the apical caspase of stress-induced apoptosis. Activated on association with Apaf-1 in the presence of cytochrome c, caspase-9 activates downstream “effector” caspase-3, -6, and -7.44 45 Procaspase-9 was detectable in eosinophils as its 46-kd zymogen (Figure 2E, lane 1), and time-dependent formation of 35-kd and 37-kd (p35 and p37) subunits was observed after 2 and 6 hours of STS treatment, respectively, due to cleavage at D315 and D330 (Figure 2E, lanes 4 and 8).
Detection of caspase processing in eosinophils after treatment with STS for 2 hours was commonly associated with approximately 35% to 50% apoptosis assessed by PS exposure and 10% to 15% cells showing evidence of nuclear condensation by morphology after Kimura stain (Figure 1).
IL-5 inhibits spontaneous eosinophil apoptosis upstream of caspase processing
In the absence of IL-5 for 24 hours, approximately 65% apoptosis assessed by PS externalization was associated with processing of caspase-3, -7, -8, and -9 in eosinophils (Figures 1 and 2). After 2 and 6 hours of IL-5 deprivation, subunit detection was difficult due to low levels of caspase processing. However, longer exposure revealed the presence of caspase-3 subunits p20, p19, and p17 after 6 hours in the absence of IL-5 (approximately 15% apoptosis by PS exposure). Processing of caspase-9 was less marked compared with caspase-3, -7, and -8, but the p37 subunit was detectable after 24 hours of IL-5 deprivation (Figure 2E, lane 10). Processing of caspase-3, -7, -8, and -9 was completely inhibited by IL-5 (10−10 M) over the 24-hour culture period (Figure 1A,C,D, lane 9; Figure 2E, lane 11). Lack of immunoreactivity of caspase-6 subunits made determination of processing in the absence of IL-5 difficult. A slight loss of proform in IL-5–deprived eosinophils compared with IL-5–stimulated eosinophils after 24 hours was observed, but was not marked (Figure 1B, lanes 8 and 9). The broad-spectrum, cell-permeable caspase inhibitor, z-VAD.fmk, inhibited the processing of each caspase in IL-5–deprived eosinophils after 24 hours (Figure 2A-D, lane 13). However, inhibition was not absolute because caspase-9 processing after 24 hours in the presence of z-VAD.fmk was observed, evidenced by the detection of the p35 subunit (Figure 2E, lane 13).
The results demonstrate that caspases were activated during spontaneous eosinophil apoptosis and IL-5 acted at a point prior to caspase activation in inhibiting spontaneous eosinophil apoptosis. The events that precede the activation of caspases in the apoptotic pathway were then investigated. The mitochondria are pivotal in the regulation of apoptotic cell death, with apoptogenic factor release required for the activation of caspase-9. The role of mitochondria in eosinophil apoptosis was investigated.
IL-5 inhibits the loss of mitochondrial membrane potential in eosinophil apoptosis
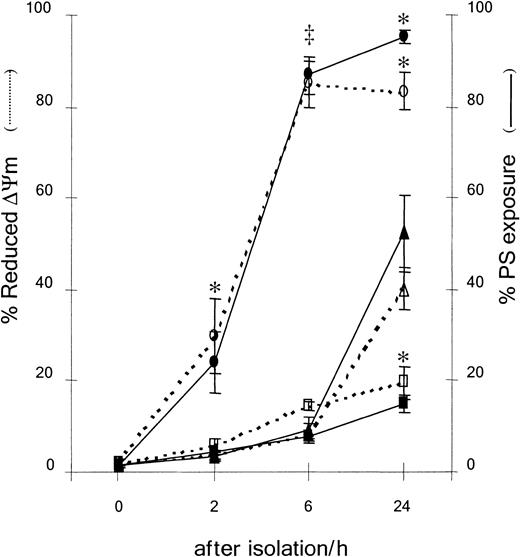
Apoptosis is characterized by the exposure of PS at the cell membrane,36,37 and a reduction in ΔΨm.46Less than 5% of freshly isolated eosinophils exhibited PS externalization or loss of ΔΨm (Figure3A,B). Uncoupling of mitochondrial respiration with mCCP as a positive control caused a marked loss of ΔΨm (Figure 3A, 0 hour, open histogram). Eosinophils undergoing spontaneous apoptosis exhibited an increase in both the percentage of cells with dissipated ΔΨm and the percentage of cells with PS externalization after 24 hours (Figure 3A,B, −IL-5). The increase in spontaneous eosinophil apoptosis assessed by both criteria was observed after 6 hours, but was very marked after 24 hours (Figure4). IL-5 significantly inhibited loss of ΔΨm and externalization of PS compared with medium control (Figure3A, 25% versus 48%, and 13% versus 66% respectively; and Figure3B). Therefore, spontaneous eosinophil apoptosis was associated with a time-dependent externalization of PS and loss of ΔΨm, and the antiapoptotic effect of IL-5 acted at or prior to the loss of ΔΨm. There was no significant difference between the progression of PS externalization and dissipation of ΔΨm during spontaneous eosinophil apoptosis (Figure 4). Apoptosis assessed by both these criteria was significantly increased as early as 2 hours exposure to STS and was almost maximal after 6 hours (Figure 4).
IL-5 and z-VAD.fmk prevent phosphatidylserine externalization, but only IL-5 prevents loss of ΔΨm in eosinophils.
Freshly isolated eosinophils (0 hour) or eosinophils incubated in the absence (−IL-5) or presence of IL-5 (10−10 M) or z-VAD.fmk (100 μM) for 24 hours were assessed for ΔΨm and PS exposure as described in “Materials and methods.” (A) Flow cytometry histograms of 10 000 events showing percentage cells exhibiting reduced ΔΨm or increased PS exposure. At 0 hours, eosinophils were also treated with the mitochondria uncoupling agent, mCCP, as a positive control for loss of ΔΨm (open histogram, figure in parentheses). Results are representative of 6 separate experiments. (B) Percentage eosinophils with reduced ΔΨm (open bars) or PS externalization (filled bars). Results are expressed as mean ± SE of 6 separate experiments. Asterisk indicates that IL-5 and z-VAD.fmk significantly inhibited PS exposure compared with medium control (−IL-5), but only IL-5 inhibited loss of ΔΨm (P < .05).
IL-5 and z-VAD.fmk prevent phosphatidylserine externalization, but only IL-5 prevents loss of ΔΨm in eosinophils.
Freshly isolated eosinophils (0 hour) or eosinophils incubated in the absence (−IL-5) or presence of IL-5 (10−10 M) or z-VAD.fmk (100 μM) for 24 hours were assessed for ΔΨm and PS exposure as described in “Materials and methods.” (A) Flow cytometry histograms of 10 000 events showing percentage cells exhibiting reduced ΔΨm or increased PS exposure. At 0 hours, eosinophils were also treated with the mitochondria uncoupling agent, mCCP, as a positive control for loss of ΔΨm (open histogram, figure in parentheses). Results are representative of 6 separate experiments. (B) Percentage eosinophils with reduced ΔΨm (open bars) or PS externalization (filled bars). Results are expressed as mean ± SE of 6 separate experiments. Asterisk indicates that IL-5 and z-VAD.fmk significantly inhibited PS exposure compared with medium control (−IL-5), but only IL-5 inhibited loss of ΔΨm (P < .05).
IL-5 inhibits and STS accelerates the externalization of PS and loss of ΔΨm during spontaneous apoptosis of peripheral blood eosinophils.
Eosinophils were cultured in the presence of IL-5 (10−10M, ■), STS (10−5 M, ○), or medium alone (▵) for the indicated times. Apoptosis was assessed by PS exposure and ΔΨm as described in “Materials and methods.” IL-5 inhibited PS externalization and loss of ΔΨm observed in spontaneous eosinophil apoptosis after 24 hours (*P < .05). STS accelerated PS exposure and loss of ΔΨm in eosinophils after 2 hours (‡P < .01) and was almost maximal after 6 hours (*P < .05). Results are expressed as mean ± SE of 3 separate experiments.
IL-5 inhibits and STS accelerates the externalization of PS and loss of ΔΨm during spontaneous apoptosis of peripheral blood eosinophils.
Eosinophils were cultured in the presence of IL-5 (10−10M, ■), STS (10−5 M, ○), or medium alone (▵) for the indicated times. Apoptosis was assessed by PS exposure and ΔΨm as described in “Materials and methods.” IL-5 inhibited PS externalization and loss of ΔΨm observed in spontaneous eosinophil apoptosis after 24 hours (*P < .05). STS accelerated PS exposure and loss of ΔΨm in eosinophils after 2 hours (‡P < .01) and was almost maximal after 6 hours (*P < .05). Results are expressed as mean ± SE of 3 separate experiments.
z-VAD.fmk inhibits eosinophil PS exposure after loss of mitochondrial membrane potential
In eosinophils cultured in the absence of IL-5 for 24 hours, incubation with z-VAD.fmk significantly inhibited PS externalization (Figure 3A, 46% versus 66%, and Figure 3B), although less efficiently than IL-5, but exhibited little or no effect on loss of ΔΨm compared with medium control (Figure 3A, 44% versus 48%, and Figure3B). Thus during spontaneous eosinophil apoptosis PS externalization was dependent on caspase activation, whereas the perturbations of the mitochondria leading to loss of ΔΨm were independent of caspases. Prevention of caspase activation inhibited apoptosis assessed by PS externalization, thereby implicating caspases as major effectors of the execution of spontaneous eosinophil apoptosis. To further elucidate the role of the mitochondria in eosinophil apoptosis, cytochromec release was investigated. Cytochrome c release from mitochondria is an early, pivotal event in the apoptosis of many cell types.47
Release of cytochrome c from mitochondria during eosinophil apoptosis
Spontaneous eosinophil apoptosis was associated with caspase-dependent release of mitochondrial cytochrome c, which was inhibited by IL-5. In freshly isolated peripheral blood eosinophils, cytochrome c exhibited a punctate distribution, which colocalized with mitochondria (Figure5A). No staining was observed with the isotype-matched negative control (data not shown). Due to the relatively low level of constitutive cytochrome c expression and number of mitochondria in eosinophils, cells that had undergone cytochrome c release generally appeared diffuse or devoid of green staining entirely (Figure 5B,D, indicated by arrows). Release of cytochrome c was observed in eosinophils cultured in the absence of IL-5 for 6 hours (Figure 5B) and was marked after 24 and 48 hours (Figure 6A). Cytochromec release was commonly associated with eosinophils exhibiting evidence of apoptotic nuclear condensation (Figure 5B). Stimulation with IL-5 for 6 hours maintained the association of cytochromec with the mitochondria (Figure 5C), and inhibited cytochrome c release compared with medium control after 24 and 48 hours (Figure 6A). z-VAD.fmk had no inhibitory effect on cytochrome c release compared with medium control (Figure6A). Cytochrome c release was also observed in eosinophils treated with STS for 2 hours (Figure 5D), and after 6 hours of STS treatment almost 100% of eosinophils exhibited cytochrome crelease, which was not inhibited by z-VAD.fmk (Figure 6B). Due to downstream proteolytic degradation of the cell, accurate determination of cytochrome c release beyond 6 hours of STS treatment was not possible. Therefore, spontaneous and STS-induced eosinophil apoptosis involved caspase-independent cytochrome c release. IL-5 inhibited spontaneous eosinophil apoptosis at or prior to release of cytochrome c. The mechanism by which cytochromec is released from the mitochondria during apoptosis is unclear. We investigated the possible role of Bax in eosinophil apoptosis because its translocation to the mitochondrial membrane facilitates the release of cytochrome c in many models of apoptosis.20 21
Cytochrome c is released from the mitochondria during spontaneous and STS-induced eosinophil apoptosis.
Immunocytochemistry was performed, as described in “Materials and methods,” on fixed cytospins of freshly isolated eosinophils (A) or eosinophils cultured in the absence (B) or presence of IL-5 (10−10 M) for 6 hours (C) or STS (10−5 M) for 2 hours (D). Images were captured by confocal microscopy at × 100 magnification under oil immersion. Cytochrome c was detected using a monoclonal antibody 6H2.B4 and the mitochondria and nuclei stained with Mitotracker CMXRos and Hoechst 33258, respectively. Colocalization (yellow) of cytochrome c (green) and mitochondria (red) was observed in single sections. Eosinophils exhibiting evidence of cytochrome c release are indicated (arrows in panels B and D). Culturing in the absence of IL-5 for 6 hours and treatment with STS for 2 hours induced cytochromec release from the mitochondria. Bar represents 10 μm. Results are representative of experiments performed on 4 separate donors.
Cytochrome c is released from the mitochondria during spontaneous and STS-induced eosinophil apoptosis.
Immunocytochemistry was performed, as described in “Materials and methods,” on fixed cytospins of freshly isolated eosinophils (A) or eosinophils cultured in the absence (B) or presence of IL-5 (10−10 M) for 6 hours (C) or STS (10−5 M) for 2 hours (D). Images were captured by confocal microscopy at × 100 magnification under oil immersion. Cytochrome c was detected using a monoclonal antibody 6H2.B4 and the mitochondria and nuclei stained with Mitotracker CMXRos and Hoechst 33258, respectively. Colocalization (yellow) of cytochrome c (green) and mitochondria (red) was observed in single sections. Eosinophils exhibiting evidence of cytochrome c release are indicated (arrows in panels B and D). Culturing in the absence of IL-5 for 6 hours and treatment with STS for 2 hours induced cytochromec release from the mitochondria. Bar represents 10 μm. Results are representative of experiments performed on 4 separate donors.
IL-5 prevents both cytochrome c release and Bax translocation to the mitochondria during spontaneous eosinophil apoptosis.
Eosinophils were coincubated in the presence or absence of either STS (10−5 M) or IL-5 (10−10 M) and z-VAD.fmk (100 μM) for the indicated times and the cells assessed for evidence of cytochrome c release (A,B) or Bax translocation to the mitochondria (C,D). Cytochrome c and Bax were detected by immunohistochemistry as described in “Materials and methods.” The percentage of eosinophils exhibiting diffuse cytochrome cand aggregated Bax distribution were quantified by fluorescence microscopy. IL-5 inhibited cytochrome c release and Bax translocation observed during spontaneous eosinophil apoptosis after 24 and 48 hours. Quantification of subcellular localization in eosinophils exposed to STS for longer than 6 hours was not possible. STS rapidly induced cytochrome c release and Bax translocation after 2 hours, which was not inhibited by z-VAD.fmk. Results are expressed as the mean ± SE of 3 separate experiments. Asterisk indicates IL-5– or STS-treated eosinophils compared with the relevant untreated or z-VAD.fmk–treated control (P < .05).
IL-5 prevents both cytochrome c release and Bax translocation to the mitochondria during spontaneous eosinophil apoptosis.
Eosinophils were coincubated in the presence or absence of either STS (10−5 M) or IL-5 (10−10 M) and z-VAD.fmk (100 μM) for the indicated times and the cells assessed for evidence of cytochrome c release (A,B) or Bax translocation to the mitochondria (C,D). Cytochrome c and Bax were detected by immunohistochemistry as described in “Materials and methods.” The percentage of eosinophils exhibiting diffuse cytochrome cand aggregated Bax distribution were quantified by fluorescence microscopy. IL-5 inhibited cytochrome c release and Bax translocation observed during spontaneous eosinophil apoptosis after 24 and 48 hours. Quantification of subcellular localization in eosinophils exposed to STS for longer than 6 hours was not possible. STS rapidly induced cytochrome c release and Bax translocation after 2 hours, which was not inhibited by z-VAD.fmk. Results are expressed as the mean ± SE of 3 separate experiments. Asterisk indicates IL-5– or STS-treated eosinophils compared with the relevant untreated or z-VAD.fmk–treated control (P < .05).
Cytosol-to-mitochondria translocation of Bax during eosinophil apoptosis
Spontaneous eosinophil apoptosis was associated with a caspase-independent redistribution of Bax from the cytosol to the mitochondria, which was inhibited by IL-5. Anti–Bax 6A7 detected diffuse cytosolic and nuclear Bax distribution in the majority of freshly isolated eosinophils (Figure 7A), indicating constitutive expression of the conformationally altered form of Bax. Interestingly, Bax expression was observed to localize predominantly to the nucleus compared with the cytosol in freshly isolated eosinophils (Figure 7A). Isotype-matched mouse negative control showed essentially no evidence of nonspecific staining (Figure7B). Punctate Bax distribution that colocalized with the mitochondria was observed in eosinophils deprived of IL-5 for 6 hours (Figure 7C). This punctate distribution was commonly perinuclear and consistently associated with cells exhibiting characteristic apoptotic morphology of condensed nucleus and cytoplasm. Stimulation of eosinophils with IL-5 for 6 hours maintained the cytosolic and nuclear distribution observed in the freshly isolated eosinophils (Figure 7D), and prevented Bax translocation to the mitochondria observed during spontaneous apoptosis after 24 and 48 hours (Figure 6C). In contrast, although z-VAD.fmk inhibited spontaneous apoptosis assessed by PS externalization, it increased the percentage of eosinophils showing Bax translocation to the mitochondria (Figure 6C). Treatment of eosinophils with STS for 2 hours also resulted in the marked appearance of punctate Bax distribution (Figure 7E), and similarly to cytochrome crelease, nearly 100% of eosinophils exhibited translocated Bax after 6 hours (Figure 6D). STS-induced Bax redistribution was not inhibited by z-VAD.fmk (Figure 6D). To determine whether translocation of other proapoptotic Bcl-2 homologues occurred during apoptosis, the expression of Bik, Bak, and Bim was also investigated. Expression of Bik and Bak was undetectable by either immunoblotting or immunocytochemistry in peripheral blood eosinophils (data not shown). Bim expression was detected both by immunoblotting and immunocytochemistry, where it was localized to the cytoplasm. However, Bim did not redistribute during spontaneous or STS-induced apoptosis (data not shown).
Bax translocates to the mitochondria during spontaneous and STS-induced eosinophil apoptosis.
Immunocytochemistry was performed, as described in “Materials and methods,” on fixed cytospins of freshly isolated eosinophils (A,B) or eosinophils cultured in the absence (C) or presence of IL-5 (10−10 M) for 6 hours (D) or STS (10−5M) for 2 hours (E). Images were captured by confocal microscopy at × 100 magnification under oil immersion. Eosinophils were stained with monoclonal anti–Bax 6A7 (A,C-E) or isotype-matched control (B), and the mitochondria and nuclei stained with Mitotracker CMXRos and Hoechst 33258, respectively (A-E). Colocalization (yellow) of Bax (green) and mitochondria (red) was observed in single sections. Culturing in the absence of IL-5 for 6 hours and treatment with STS for 2 hours induced Bax translocation to the mitochondria. Bar represents 10 μm. Results are representative of experiments performed on 4 separate donors.
Bax translocates to the mitochondria during spontaneous and STS-induced eosinophil apoptosis.
Immunocytochemistry was performed, as described in “Materials and methods,” on fixed cytospins of freshly isolated eosinophils (A,B) or eosinophils cultured in the absence (C) or presence of IL-5 (10−10 M) for 6 hours (D) or STS (10−5M) for 2 hours (E). Images were captured by confocal microscopy at × 100 magnification under oil immersion. Eosinophils were stained with monoclonal anti–Bax 6A7 (A,C-E) or isotype-matched control (B), and the mitochondria and nuclei stained with Mitotracker CMXRos and Hoechst 33258, respectively (A-E). Colocalization (yellow) of Bax (green) and mitochondria (red) was observed in single sections. Culturing in the absence of IL-5 for 6 hours and treatment with STS for 2 hours induced Bax translocation to the mitochondria. Bar represents 10 μm. Results are representative of experiments performed on 4 separate donors.
Discussion
We have shown for the first time that IL-5 exhibits its potent antiapoptotic effect in eosinophils by preventing Bax translocation to the mitochondria, cytochrome c release, increased mitochondrial membrane permeability, and subsequent caspase activation. In nonapoptotic cells, Bax is a soluble, cytosolic monomer, with an N-terminus proposed to mask its hydrophobic C-terminus.22During the apoptosis of murine thymocytes, COS-7, and murine fibrosarcoma cell lines, it has been shown that a conformational change exposes the BH3 domain and C-terminus, facilitating oligomerization of Bax and translocation from the cytosol to the mitochondrial membrane, respectively.19,22,48 Bax oligomers have channel-forming activity and trigger the release of cytochrome c from human melanoma cells21 and isolated mitochondria.20,49,50 We, and others, have previously shown that human peripheral blood eosinophils express high levels of Bax.12,13 We now provide evidence that freshly isolated eosinophils constitutively express the conformationally altered form of Bax, detected by the monoclonal antibody 6A7 that specifically recognizes an N-terminal epitope inaccessible in soluble, cytosolic Bax.22 Interestingly, despite diffuse Bax expression being detectable throughout the entire cell, expression was predominantly observed in the nucleus (Figure 7A). Lack of nuclear and cytosolic staining in the isotype-matched negative control would suggest that the staining was not nonspecific (Figure 7B). An association of Bax with the nuclear membrane and matrix in nonapoptotic cells has been previously reported in MCF-7 cells51,52 and a number of human lung cancer cell lines.53 However, the relevance of nuclear Bax expression is currently unclear. During both spontaneous and STS-induced apoptosis, Bax underwent a caspase-independent translocation to the mitochondria in human eosinophils. This colocalization was consistently observed in eosinophils exhibiting the condensed nuclear morphology characteristic of apoptosis and was inhibited by IL-5. Interestingly, Bax redistribution to the mitochondria seemingly increased with z-VAD.fmk (Figure 6C), probably due to the delayed onset of the caspase-mediated execution phase of apoptosis and the consequent proteolytic degradation of cells cultured for up to 48 hours, thereby prolonging the association of Bax with the mitochondria. This is the first report of Bax translocation to the mitochondria during spontaneous apoptosis of human granulocytes due to cytokine deprivation. A recent report has demonstrated that Bax inserts into the mitochondrial membrane and cytochrome c is released during apoptosis of neutrophils, induced by a 15°C to 37°C temperature transition.54 Similarly, in this temperature arrest system, Bax translocation to the mitochondria was shown to be of crucial importance in the execution of granulocyte apoptosis.
Caspase-independent release of cytochrome c from mitochondria was observed in eosinophils undergoing STS-induced and spontaneous apoptosis, with the latter inhibited by IL-5. The mechanism by which cytochrome c is released from the mitochondria remains unclear. It has been proposed that Bax induces the opening of the permeability transition pore complex via interaction with the adenine nucleotide translocator, resulting in mitochondrial membrane depolarization and cytochrome c release.55Spontaneous eosinophil apoptosis clearly involved caspase-independent loss of ΔΨm, which was inhibited by IL-5.
Receptor-mediated and chemical/stress-induced caspase activation are associated with apical processing of caspase-8 and -9, respectively. Although recent studies have reported activation of caspases in eosinophils in response to Fas, growth factor withdrawal, and dexamethasone,17,56,57 caspase processing has not been investigated in detail. We have demonstrated processing of caspase-3, -7, -8, and -9, and possible caspase-6 activation during spontaneous eosinophil apoptosis. Immunoblot analysis seemingly indicated caspase-3 processing proximal to caspase-8 processing. In receptor-mediated apoptosis, perturbations of the mitochondrial membrane are dependent on the activation of caspase-8. The time scale of activation of caspase-3 and -8, coupled with the observation that mitochondrial perturbations were not blocked by z-VAD.fmk, suggested that spontaneous eosinophil apoptosis is mediated by caspase-independent mitochondrial events and the consequent activation of the caspase-9/-3/-7 pathway. The processing and activation of caspase-8 observed after 24 hours may form an amplification loop mediated by caspase-3 and -6 as previously described.45 The processing of caspase-9 was not as marked as the other caspases tested. This may be due to the relative efficacy of the antibodies used, although it must be noted that caspase-9 can be activated without proteolytic cleavage.58 We have demonstrated that caspases are major effectors of the execution phase of spontaneous eosinophil apoptosis, and IL-5 induces eosinophil survival by inhibiting apoptosis before caspase activation.
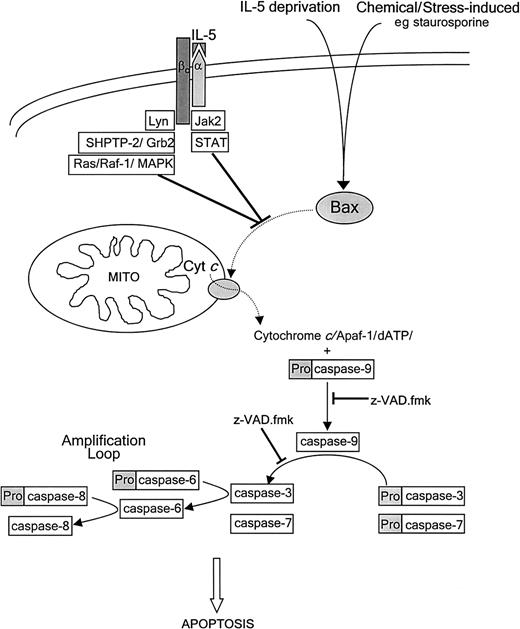
Ligation of the IL-5 receptor induces rapid tyrosine phosphorylation and activation of juxtamembranous tyrosine kinases. The signal is propagated via the Janus kinase2 (Jak2)/signal transducers and activators of transcription (STAT) and Ras-Raf-1–mitogen-activated protein kinase (MAPK) cascades (Figure8).59,60 Activation of Jak2, Lyn, and Syk tyrosine kinases, and Src homology 2 phosphatase 2 tyrosine phosphatase (SHPTP-2) is crucial for IL-5–induced eosinophil survival.60,61 SHPTP-2 activation and association with the adaptor protein Grb2 is proposed to couple the IL-5 receptor to the Ras signaling pathway,61 supported by the requirement for Raf-1 serine/threonine kinase activation in IL-5–mediated suppression of eosinophil apoptosis.60 The mechanism by which these signaling cascades integrate into the apoptotic pathway, thereby regulating Bax translocation, is unknown and the subject of future investigation. Interactions with other proteins, such as the apoptosis suppressing Bcl-xL, the expression of which is down-regulated during spontaneous apoptosis, but maintained or up-regulated on stimulation with GM-CSF and IL-5, may be involved.18
Proposed scheme for spontaneous apoptotic pathway in eosinophils and the inhibitory target of IL-5.
Spontaneous eosinophil apoptosis involves caspase-independent Bax translocation to the mitochondria, cytochrome crelease, and perturbation of the mitochondrial membrane followed by activation of caspase-9, -3, and -7, similar to the pathway of chemical-induced apoptosis. Caspase-3 activation of caspase-6 followed by activation of caspase-8 is proposed to form an amplification loop. IL-5 inhibits eosinophil apoptosis at an as yet undetermined site upstream of Bax translocation, cytochrome c release, and caspase activation. The IL-5 antiapoptotic signal is transduced by recruitment and activation of Jak2 and Lyn tyrosine kinases, and SHPTP-2 to the receptor, resulting in activation of Jak/STAT and Ras-Raf-MAPK pathways (see text for details). Dashed arrows represent protein translocation.
Proposed scheme for spontaneous apoptotic pathway in eosinophils and the inhibitory target of IL-5.
Spontaneous eosinophil apoptosis involves caspase-independent Bax translocation to the mitochondria, cytochrome crelease, and perturbation of the mitochondrial membrane followed by activation of caspase-9, -3, and -7, similar to the pathway of chemical-induced apoptosis. Caspase-3 activation of caspase-6 followed by activation of caspase-8 is proposed to form an amplification loop. IL-5 inhibits eosinophil apoptosis at an as yet undetermined site upstream of Bax translocation, cytochrome c release, and caspase activation. The IL-5 antiapoptotic signal is transduced by recruitment and activation of Jak2 and Lyn tyrosine kinases, and SHPTP-2 to the receptor, resulting in activation of Jak/STAT and Ras-Raf-MAPK pathways (see text for details). Dashed arrows represent protein translocation.
Elucidating the point in the signaling cascade that IL-5 inhibits apoptosis would enable specific inhibitors of growth factor–mediated eosinophil survival to be developed. This could have important therapeutic implications for asthma and related eosinophilic disorders.
We would like to thank Kul Sikand for his expert technical assistance with the confocal microscopy.
Supported by a grant from the National Asthma Campaign.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Andrew J. Wardlaw, Division of Respiratory Medicine, Institute of Lung Health, Clinical Sciences Bldg, Glenfield Hospital, Leicester, LE3 9QP, United Kingdom; e-mail: aw24@le.ac.uk.