Abstract
Congenital or immunomediated deficiencies of the metalloprotease that cleaves physiologically von Willebrand factor (vWF) reduce or abolish the degradation of ultralarge vWF multimers that cause the formation of intravascular platelet thrombi in patients with thrombotic thrombocytopenic purpura (TTP). There is little knowledge on the behavior of the protease in other physiological and pathologic conditions. Such knowledge is important to evaluate the specificity of low protease plasma levels in the diagnosis of TTP. Using an enzyme immunoassay, the protease was measured in 177 control subjects of different ages, in 26 full-term newborns, and in 69 women during normal pregnancy. Because TTP is often associated with multiorgan involvement and acute phase reactions, clinical models of these pathologic conditions were also investigated, including decompensated liver cirrhosis (n = 42), chronic uremia (n = 63), acute inflammatory states (n = 15), and the preoperative and postoperative states (n = 24). Protease levels were lower in healthy persons older than 65 than in younger persons. They were low in newborns but became normal within 6 months, and they were lower in the last 2 trimesters of pregnancy than in the first. Protease levels were also low in patients with cirrhosis, uremia, and acute inflammation, and they fell in the postoperative period. There was an inverse relation between low protease and high plasma levels of vWF antigen and collagen-binding activity. In conclusion, low plasma levels of the vWF cleaving protease are not a specific beacon of TTP because the protease is also low in several physiological and pathologic conditions.
Introduction
Thrombotic thrombocytopenic purpura (TTP) and the hemolytic uremic syndrome (HUS) are diseases with features in common, such as thrombocytopenia, hemolytic anemia, and thrombotic occlusions in arterioles and capillaries, that result in ischemic organ damage.1 In TTP, thrombi are composed mainly of platelets with little fibrin, and they react strongly to the adhesive glycoprotein von Willebrand factor (vWF) on immunohistochemistry.2 Peculiar clinical features in TTP are the presence of focal neurologic symptoms; in HUS, they are of renal impairment, often associated with diarrhea and fever.1 In practice, differences are seldom clear-cut, and in individual patients differential diagnosis is often so uncertain that it has been proposed that the syndromes be grouped under the comprehensive term of thrombotic microangiopathies.3 There are primary thrombotic microangiopathies, such as the acute, the chronic relapsing, and the familial forms, and there are secondary forms associated with disseminated malignancies, bone marrow transplantation, human immunodeficiency infection, drug intake, andEscherichia coli 0157-associated diarrheal disease.1
Recently, the possibility of making an accurate differential diagnosis between TTP and HUS has been revived by the independent demonstration by Furlan et al4,5 and Tsai and Lian6 that a metalloprotease of large molecular weight that cleaves vWF physiologically7-9 is deficient in the plasma of patients with acute sporadic, chronic relapsing, and familial TTP but is measurable in normal amounts in acute sporadic HUS.4-6 In the absence of the plasma protease, ultralarge forms of vWF released from damaged vascular endothelium10,11 circulate undegraded in TTP plasma12 and may cause thrombus formation because they aggregate platelets under conditions of high fluid shear stress in the terminal arterial circulation.13
Whether low plasma levels of the protease are a specific beacon of TTP or also occur in physiological and pathologic conditions other than TTP is unknown, perhaps because the original protease assays4-6 were too complex and unsuitable for the study of a large number of samples. Recently, Furlan and colleagues14 developed a more facile assay based on the lower binding affinity to human collagen type III of protease-cleaved vWF compared with uncleaved vWF. The new method precludes the need for purified vWF as protease substrate and for the lengthy electrophoretic techniques used in the original method to assess the degree of degradation of larger vWF forms by the protease.14 Using this method, we evaluated the plasma changes of the protease in physiological states and in pathologic conditions associated with organ failure or acute phase reactions because these are often present in patients with thrombotic microangiopathies and may confound the diagnosis. Our results indicate that low protease levels are not a specific beacon for TTP given that protease levels are low or very low in plasma in other physiological and pathologic conditions.
Patients, materials, and methods
Physiological conditions
The protease was measured in 177 healthy control subjects, almost equally split between the 2 sexes, in the age groups 20 to 35 years (n = 52), 36 to 50 years (n = 48), 51 to 65 years (n = 38), and older than 65 years (n = 39). They were ostensibly healthy and were chosen among those used as controls for other laboratory measurements at the Hemophilia and Thrombosis Center, usually spouses or friends of patients referred for investigation of thrombotic and bleeding disorders. We also investigated 69 women during normal pregnancy (18 in the first, 19 in the second, and 32 in the third trimester), who attended routine clinical visits at the hospital, and 26 full-term healthy newborns of both sexes within 4 days of birth. Consent for blood sampling was obtained from the control subjects or their custodians, who were informed of the research use of their plasma.
Pathologic conditions
We chose to investigate the effect on protease levels of clinical conditions typically associated with multiorgan involvement and acute phase reactions—decompensated (Child C) liver cirrhosis (n = 42), chronic renal insufficiency treated with regular hemodialysis (n = 63; samples taken immediately before dialysis), acute inflammatory states (n = 15; usually respiratory tract bacterial infections, with serum levels of C-reactive protein higher than 5 mg/dL), and the postoperative state (samples obtained before and 4 days after surgery in 24 patients undergoing abdominal surgery for benign or malignant conditions, mainly cholecystectomy, gastrectomy, and colectomy).
To evaluate the relation between changes in plasma vWF and protease levels, we also studied 7 healthy controls before and after the infusion of desmopressin (0.3 μg/kg), a pharmacologic compound that increases vWF for 8 to 10 hours and elicits the transient appearance in plasma of ultralarge vWF forms15; 5 patients with type 3 von Willebrand disease (vWD) with unmeasurable levels of plasma and platelet vWF; 4 patients with type 2A vWD carrying vWF gene defects associated with enhanced susceptibility to proteolysis of the dysfunctional vWF16; and 6 patients with vWD type 2M Vicenza, characterized by the inherited persistence in plasma of ultralarge vWF forms.17 Plasma samples were also obtained from 2 unrelated patients followed at the Hemophilia and Thrombosis Center for chronic relapsing TTP, diagnosed during recurrences on consistent findings such as thrombocytopenia, hemolytic anemia, high serum lactate dehydrogenase levels, and transient focal neurologic symptoms. Informed consent was obtained from patients and control subjects.
Plasma preparation
Venous blood was collected into 0.1 vol 0.129 M sodium citrate, and platelet-poor plasma was prepared by double centrifugation at 1500g. A pool of plasma obtained from 50 healthy women not pregnant and not taking oral contraceptives and from 50 healthy men was used as reference for the assays in this study and were arbitrarily defined to contain 100% of the protease and vWF measurements. Control subjects contributing to the pool were different from those who comprised the healthy population of 177 adults.
Assay of the protease
The enzyme immunoassay described by Gerritsen et al14 was carried out with no substantial modification. The source of vWF used as substrate for the protease was pooled normal plasma in which protease activity was neutralized by the addition of both EDTA and an inhibitor of serine proteases (4-[2-aminoethyl]-benzenesulfonyl fluoride, hydrochloride; Pefabloc SC, Roche, Mannheim, Germany). The substrate was then dialyzed to eliminate EDTA. Serial dilutions in a buffer containing urea of the plasma samples to be tested were incubated with BaCl2 to achieve partial degradation of endogenous vWF, particularly of larger vWF multimers that might interfere with the assay by preferentially binding to type III collagen. Digested samples were then incubated for 2 hours with the vWF substrate and centrifuged at 2500g for 3 minutes. Supernatants were added to microtiter plates coated with human collagen type III, and vWF bound to collagen was quantitated using a peroxidase-labeled rabbit anti–human vWF polyclonal antibody (Dako, Glostrup, Denmark). Protease values were read from a dose-response curve obtained for each assay run by testing, as described above, serial dilutions of the reference plasma from 1:5 to 1:320. Within-assay (n = 18) coefficient of variation was 8%, and between-assay (n = 74) coefficient of variation was 14%; the lower limit of sensitivity of the method was 6% of the normal protease levels. The mixing procedure used to evaluate the presence of an inhibitor of the protease in plasma samples was that described by Gerritsen et al.14 The presence of an inhibitor was also indirectly evaluated by checking whether the dose-response curve of test plasma samples were parallel to those of reference plasma.
vWF antigen
vWF antigen (vWF:Ag) was measured by enzyme immunoassay using rabbit anti–human vWF polyclonal antibodies (Dako) as first and second antibody.
vWF collagen-binding activity
vWF collagen-binding activity (vWF:CB) was measured by enzyme immunoassay according to the method described by Favaloro et al18 with small modifications. Collagen type 1 (95%) and type 3 (5%) (Collagen reagent Horm; Nycomed, Munich, Germany) from equine tendon was diluted to a final concentration of 25 μg/mL, and the coated plates were then left overnight for a maximum of 18 hours at 22°C before the assay.
vWF cryoprecipitation
Selected citrated plasma samples stored at −70°C were thawed in an ice-melting bath at 0 to 1°C for 20 to 30 minutes and centrifuged immediately at 10 000g for 10 minutes at 5°C, and the supernatants were tested for protease and vWF:Ag and CB values.
Data analysis
For descriptive purposes, the values of each measurement are given as means ± standard deviations and ranges for each subgroup of patients or control subjects. Student t test for paired or unpaired samples was used to compare each group of patients with the corresponding controls, who were age- and sex-matched from the 177 healthy controls. Correlation coefficient (r) was used to determine the relation between protease and vWF measurements.
Results
Table 1 summarizes the behavior of the vWF cleaving protease in physiological and pathologic conditions. Corresponding values of vWF:Ag and vWF:CB and the ratios of these 2 measurements are also given.
Physiological conditions
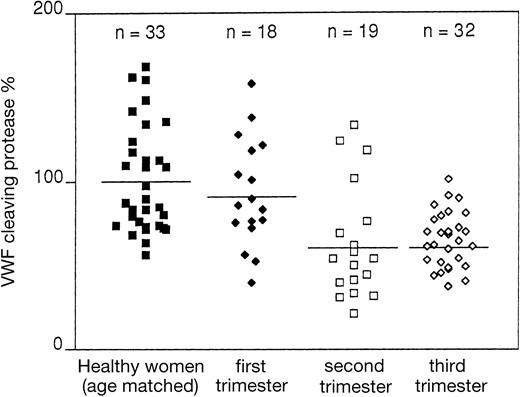
Figure 1 shows that in the whole group of 177 healthy controls, the protease had a wide distribution, ranging from 40% to 170%. No healthy adult with protease values lower than 40% was found in this study. Protease levels were slightly lower in those older than 65 years than in those younger than 65 (P = .03). In newborns, compared with the younger age group (between 20 and 35 years), there was a decrease of protease level (P = .0001) that in some cases reached as low as 25% to 30% (Figure 1). In a small group of 6 newborns, protease levels were measured again 6 months after birth. In all cases values increased and reached the normal laboratory limits (data not shown). In pregnant women, protease levels—similar in the first trimester to those of a sex- and age-matched group of healthy nonpregnant women—were on average lower in the second and third trimesters (P = .0001), reaching values as low as 25% to 30% in some instances (Figure 2). There was no statistically significant correlation between protease levels and platelet count (r = .016).
Values of the vWF cleaving protease, expressed in percentages, in healthy control subjects of different age groups and in full-term newborns.
Horizontal solid lines indicate the mean values for each group.
Values of the vWF cleaving protease, expressed in percentages, in healthy control subjects of different age groups and in full-term newborns.
Horizontal solid lines indicate the mean values for each group.
Values of the vWF cleaving protease, expressed in percentages, in women during pregnancy.
These values were compared with those of healthy nonpregnant women matched for age with pregnant women.
Values of the vWF cleaving protease, expressed in percentages, in women during pregnancy.
These values were compared with those of healthy nonpregnant women matched for age with pregnant women.
vWF:Ag and vWF:CB were significantly higher in older healthy adults, newborns, and pregnant women than in the corresponding comparison groups (Table 1).
Pathologic conditions
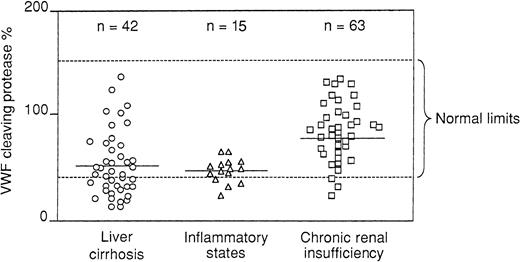
All patients with liver cirrhosis had decompensated disease with ascites and severe impairment of protein synthesis reflected by low levels of serum albumin and prolonged prothrombin times. Protease levels were lower than in an age- and sex-matched comparison group (P = .0001), but there was a wide range of values that spanned from 6% to 15% to the upper normal range (Figure3). Patients with chronic renal insufficiency on regular hemodialysis had reduced protease levels (P = .0004), though in general they were much higher than in patients with liver cirrhosis and with a similarly wide range of values (15%-140%) (Figure 3). There was no significant correlation between protease and serum creatinine levels. A decrease of the protease was also seen in patients with acute inflammatory states (P = .0001) (Figure 3). At variance with patients with cirrhosis and patients with uremia, these patients had a narrow range of protease levels, usually less than 50% (Figure 3). In patients scheduled to undergo major surgery, baseline protease levels had a wide range of values but were not significantly different from those in the matched control groups. However, in most patients protease levels fell on the fourth postoperative day (P = .0001) to values as low as 20% to 25% (Figure 4). Both vWF measurements were high in patients with liver cirrhosis (vWF:Ag more than vWF:CB) and, to a lesser extent, in patients with acute inflammatory states and renal insufficiency and during the postsurgical period (Table 1).
Values of the vWF cleaving protease, expressed in percentages, in patients with decompensated liver cirrhosis, acute inflammatory states, and chronic renal insufficiency; patients were on regular hemodialysis.
Horizontal broken lines indicate the upper and lower normal limits of protease values.
Values of the vWF cleaving protease, expressed in percentages, in patients with decompensated liver cirrhosis, acute inflammatory states, and chronic renal insufficiency; patients were on regular hemodialysis.
Horizontal broken lines indicate the upper and lower normal limits of protease values.
Changes of the vWF cleaving protease in 24 patients.
Before and 4 days after major abdominal surgery.
Changes of the vWF cleaving protease in 24 patients.
Before and 4 days after major abdominal surgery.
Relation between the protease levels and vWF measurements
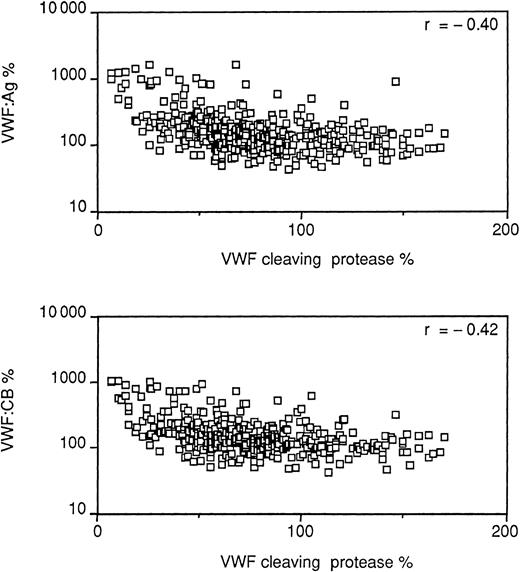
The opposite pattern of changes between protease (low) and vWF:Ag and CB (high) led to a calculation of the correlation coefficients between these measurements. There was a statistically significant (P < .001) inverse correlation between the protease and vWF:Ag (r = −0.40) and CB (r = −0.42) when all the values obtained in the physiological and pathologic conditions were included in the analysis (Figure 5). These findings prompted evaluation of the possibility of artefacts2 in the assay and particularly the possibility that the endogenous vWF forms of larger molecular weight, highly reactive with collagen type 3, might not be sufficiently degraded by the assay step meant to avoid this interference (preincubation of the diluted test samples with barium chloride).8 Samples from patients selected because they had particularly low protease and high vWF levels were tested before and after cryoprecipitation, which removed from the plasma a large proportion of vWF, particularly the largest multimeric forms. Table 2 shows that, as expected, vWF:Ag values and, to a higher degree, vWF:CB were much lower in cryoprecipitate supernatants than in intact plasma. Protease values were slightly higher in supernatants than in intact plasma, but mean values were not statistically significant (Student t test for paired data); in no instance did postcryoprecipitation values reached normal values (greater than 40%).
Correlation between vWF measurements and protease levels.
vWF:Ag and vWF:CB, vertical axis; protease levels, horizontal axis.
Correlation between vWF measurements and protease levels.
vWF:Ag and vWF:CB, vertical axis; protease levels, horizontal axis.
Protease in conditions associated with changes in vWF levels
To further evaluate the effect of changes in vWF levels on the protease measurement, a few conditions known to be associated with particularly low or high vWF levels were chosen as models. In the plasma samples of 7 healthy controls taken before and 60 minutes after the beginning of desmopressin infusion (0.3 μg/kg), at the time of 3-fold to 4-fold rises in plasma vWF levels and of the transient appearance in plasma of ultralarge vWF forms, protease values remained substantially unchanged (from values of 91% to 139%, 80% to 70%, 78% to 53%, 98% to 125%, 97% to 117%, 56% to 54%, and 78% to 71%). We also measured protease levels in 6 patients with type 2M Vicenza vWD, with ultralarge vWF forms in plasma since birth, and found plasma levels within the normal laboratory limits (data not shown). Normal protease values were also found in 5 patients with type 3 vWD, with no measurable plasma or platelet levels of vWF, and in 4 patients with type 2A vWD previously characterized genotypically and biochemically16 to have an abnormal vWF protein highly susceptible to proteolysis (data not shown).
Protease in chronic relapsing TTP
In 2 patients, the protease level was always below the lower limit of sensitivity of the assay (6%), whether they were examined during clinical recurrences or in the intervals between recurrences when they were asymptomatic and had normal platelet counts. Assays of mixtures of patient and reference plasma gave unmeasurable protease values, and the corresponding dose-response curves were not parallel, indicating that in these cases protease deficiency was caused by the presence of an inhibitory antibody. Both patients responded clinically to plasma exchange, and, during the periods of this treatment, the protease became transiently measurable in plasma.
Discussion
The recent finding that the metalloprotease that cleaves vWF physiologically is at low or unmeasurable levels in patients with TTP but at normal levels in patients with HUS4-6 has for the first time identified a laboratory index for the differential diagnosis between the 2 syndromes. The findings of Furlan et al4 and Tsai and Lian,6 originally obtained in patients with primary forms of TTP and HUS, have been subsequently confirmed in secondary forms of TTP (HIV, ticlopidine, and clopidogrel associated)19-21 and HUS (E coliassociated).22 However, the paradigm that in thrombotic microangiopathies low protease level means TTP and normal protease level means HUS has been challenged. van der Plas et al,23 for instance, have shown that TTP occurring after bone marrow transplantation is associated with normal protease levels in plasma. The assumption that VWF-cleaving protease deficiency is specific for TTP has also been challenged by Oleksowicz et al,24 who found a severe deficiency of the protease in patients with metastatic malignancies in the absence of TTP, even though others have found normal or slightly reduced protease levels in similar patients.25 Our study, carried out in a broad inclusive population of healthy controls from newborns to elderly and in several physiologic inflammatory and disease states, demonstrates that the protease is low or very low in an array of clinical conditions unrelated to TTP, even though it was completely unmeasurable only in the 2 patients with chronic relapsing TTP.
Current scant knowledge of the metabolic features of the protease makes uncertain the mechanisms underlying the low plasma levels found in so many physiological and pathologic conditions. It is known that metal ions are needed for its full activity,7,8 and that transfusion experiments in patients with TTP without autoantibodies have shown an unusually long plasma half-life of the protease.26 Therefore, it cannot be established in this study whether low plasma levels are caused by decreased synthesis or increased turnover or to other mechanisms. Mixing experiments and parallelism of the dose-response curves of the assays make unlikely inactivation by autoantibodies a major mechanism, even though the presence of low-activity antibodies cannot be ruled out completely. In severe liver failure, decreased synthesis is a plausible mechanism, but a few patients had normal protease levels in the presence of clear signs of defective protein synthesis by the liver. In patients with uremia, the moderately reduced protease levels were not related to serum creatinine taken as an index of renal function. In patients undergoing major abdominal surgery, protease levels fell consistently in the postoperative period. The protein seems to be influenced by high levels of estrogens because plasma levels decreased progressively from the first trimester of pregnancy, albeit moderately. The low values found in full-term newborns can perhaps be explained as another phenotypic expression of the immaturity of the newborn liver, as indicated by the return of the protease to normal levels within 6 months of birth.
The most important correlate of protease level was the plasma level of its natural substrate vWF. Measured both as immunoreactive protein and for its capacity to bind to collagen, vWF was almost always high when the protease was low. This inverse relation was unlikely to have resulted from the influence on the protease assay of high levels of endogenous vWF, undegraded or partially degraded by barium chloride, because the protease remained low after selected plasmas with particularly high vWF levels were exposed to cryoprecipitation to remove vWF measured as vWF:Ag, particularly its larger forms highly reactive with collagen (vWF:CB). We can only speculate on the inverse relation between protease and its substrate, surmising that the protease is “consumed” as a consequence of a compensating phenomenon meant to dispose of the more platelet-adhesive and agglutinating forms of vWF. This hypothesis—that the protease level decreases as a consequence of the demand to degrade vWF—is apparently contradicted by the observation that plasma levels did not change after desmopressin infusion, at the time of the peak rise of vWF levels and the transient appearance in plasma of ultralarge vWF forms. Perhaps the protease did not change in this short-term situation of vWF rise because the stimulus for vWF rise and hence for protease utilization/consumption was short-lasting (8-10 hours), at variance with the long-lasting conditions of vWF rise examined by us.
In conclusion, this study on the behavior of the protease in physiological and pathologic conditions other than thrombotic microangiopathies clearly shows that low protease values are far from a specific beacon for these syndromes. Liver and renal diseases and inflammatory states are not unusual in thrombotic microangiopathies; therefore, these situations may confound the paradigm that low protease means TTP. On the other hand, the protease might be low in patients with HUS and renal or liver failure, challenging the paradigm that HUS is accompanied by normal protease levels. Another corollary of this study is that the protease level is usually low when vWF is high. Because there is some evidence that high vWF plasma levels are indicators of increased risk for atherothrombosis, it will be of interest to establish the contribution of the protease to this association.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Note added in proof
In the September 15, 2001, issue of Blood 2 articles report on the purification of the von Willebrand factor–cleaving protease, and its identification as a new member of the ADAMTS family of metalloproteinases. The gene encoding this enzyme is located in chromosome 9q34 (Gerritsen et al 2001;98:1654-1661; Fujikawa et al 2001;98:1662-1666). In the same issue 2 clinical papers provide evidence that low protease activity levels are not fully specific for TTP (Veyradier et al, 2001;98:1765-1772; Moore et al 2001;98:1842-1846).
Author notes
Pier Mannuccio Mannucci, Angelo Bianchi Bonomi Hemophilia and Thrombosis Center, IRCCS Maggiore Hospital, University of Milan, Via Pace 9, 20122 Milano, Italy; e-mail:piermannuccio.mannucci@unimi.it.