In the phase I/II pediatric hydroxyurea safety trial (HUG-KIDS), school-aged children with sickle cell anemia receiving hydroxyurea at the maximally tolerated dose (MTD) had variable increases in the percentage of fetal hemoglobin (%HbF). To identify predictors of the HbF response to hydroxyurea therapy, baseline clinical and laboratory values (age, sex, hemoglobin concentration, %HbF, reticulocytes, white blood cell [WBC], platelets, and serum chemistries), as well as treatment variables (number of toxicities, noncompliance, MTD dose, and MTD blood counts) were analyzed in 53 HUG-KIDS children who achieved MTD. Baseline %HbF values (P = .001), baseline hemoglobin concentration (P = .01), MTD dose (P = .02), and compliance (P = .02) were significantly associated with a higher %HbF at MTD; in contrast, age, sex, number of toxicities, and other baseline hematologic parameters were not. After adjusting for variations in baseline %HbF, the baseline reticulocyte count (P = .05) and baseline WBC count (P = .05) were also significantly associated with a higher %HbF at MTD. Hydroxyurea-induced increases in the hemoglobin concentration and mean corpuscular volume (both higher absolute values at MTD and larger positive changes from baseline values), as well as hydroxyurea-induced decreases in reticulocytes and WBC count, were significantly associated with a higher %HbF at MTD. These data suggest that selected baseline laboratory parameters, a higher MTD dose with attention to compliance, and greater therapy-related changes in blood counts may predict the HbF response to hydroxyurea therapy for children with sickle cell anemia. The HbF response to hydroxyurea is variable and complex, however, and even children with low baseline %HbF values can develop substantial increases in %HbF at MTD.
Introduction
The level of fetal hemoglobin (HbF) expression is one of the most important modifiers of disease expression for patients with sickle cell anemia.1 The percentage of HbF (%HbF) influences both laboratory values and clinical features of children and adults with sickle cell anemia. For example, an elevated %HbF has been significantly associated with fewer painful vasoocclusive events,2 fewer episodes of acute chest syndrome,3 and reduced early mortality.4 5
Pharmacologic enhancement of HbF expression has been accomplished by using myelosuppressive agents, cytokines, and short-chain fatty acids.6-8 To date, however, the prototypic agent for increasing HbF expression is hydroxyurea, based on its ease of oral administration, modest toxicity profile, and rarity of serious side effects. A phase I/II trial of hydroxyurea for adults with sickle cell anemia demonstrated a significant increase in HbF expression, accompanied by a significant increase in hemoglobin concentration and significant decreases in reticulocytes, neutrophils, and platelets.9 The Multicenter Study of Hydroxyurea (MSH), a double-blinded, placebo-controlled phase III trial for adults with sickle cell anemia, demonstrated that hydroxyurea therapy significantly reduced the number of painful events, episodes of acute chest syndrome, transfusions, and hospitalizations.10
Experience to date with hydroxyurea for children with sickle cell anemia suggests that it is also effective in this younger population. Several small trials have reported that hydroxyurea has both laboratory and clinical efficacy in the pediatric age group.11-15 The recently completed multicenter phase I/II pediatric hydroxyurea trial (HUG-KIDS) confirmed that hydroxyurea at a maximally tolerated dose (MTD) has a similar efficacy and toxicity profile for children with sickle cell anemia as observed for adults.16 For all participants in HUG-KIDS, the mean %HbF increased significantly from 7.3% at entry to 17.8% after 12 months of hydroxyurea therapy. There was, however, a wide variability in the HbF response; a few children who reached MTD had %HbF levels that were persistently below 10%, whereas several others had levels that exceeded 25%. To investigate this variable HbF response, we examined a variety of clinical, laboratory, and treatment characteristics of children enrolled in HUG-KIDS to identify factors that significantly influence the HbF response.
Patients, materials, and methods
Study overview
In the phase I/II Pediatric Hydroxyurea Trial (HUG-KIDS), a cohort of school-aged African American children with homozygous sickle cell anemia was administered hydroxyurea therapy, with the stated goal of treating 50 children at the MTD for 1 year. Entry criteria, clinical monitoring, and laboratory measurements have been described in detail previously.16 Briefly, all patients had severe disease, defined as 3 or more painful vasoocclusive events in the year before entry, 3 or more episodes of acute chest syndrome requiring hospitalization in the previous 2 years before entry, or any combination of 3 episodes of painful events or acute chest syndrome within 1 year of enrollment. Patients were monitored every 2 weeks with blood counts and an interval history, and every 4 weeks with a physical examination. The %HbF was determined by high-pressure liquid chromatography as previously described.17 Noncompliance with hydroxyurea therapy was assessed by counting any returned pills at each visit.
The hydroxyurea dose was given orally once a day, initially at 15 mg/kg/d, then escalated every 8 weeks as tolerated to the MTD or maximum dose of 30 mg/kg/d. Dose escalation was limited by predefined hematologic toxicities, which included a hemoglobin concentration less than 5 g/dL, absolute neutrophil count less than 2.0 × 109/L, absolute reticulocyte count less than 80 × 109/L (unless the hemoglobin concentration was > 9 g/dL), or platelet count less than 80 × 109/L. Nonhematologic toxicity included an elevated serum alanine aminotransferase (twice the upper limit of normal) or a doubling of the serum creatinine.16 MTD was defined as the dose 2.5 mg/kg below which 2 successive hematologic toxicities occurred, or when the daily dose reached 30 mg/kg sustained without toxicity for 8 weeks.16
Patient data for analysis
Children were enrolled in HUG-KIDS from December 1994 through March 1996, and data accrual was closed in March 1998. Of the 84 pediatric patients originally enrolled in HUG-KIDS, 68 children achieved MTD during the study period, and 52 were treated at MTD for at least 12 months.
The current analyses on predictors of HbF response were performed only for children who achieved MTD during the study. Of the 68 children who reached MTD, baseline and follow-up data sufficient for analysis were available for 53 patients; 15 children were excluded because of missing baseline %HbF values (n = 13) or missing %HbF values at MTD (n = 2). This patient population of 53 children included 32 boys (60%) and 21 girls (40%), with a mean age at entry of 9.5 years. The average length of time at MTD for these 53 children was 11.7 months. Each available baseline variable from these 53 children was compared with baseline data from the other 15 children who reached MTD but had missing data, using t tests for continuous variables and chi-square tests for discrete variables. No statistically significant (P < .05) differences were found, and most differences had highly nonsignificant P values, suggesting that the missing data from these 15 children were missing at random.
The response variable of interest (%HbF at MTD) was calculated as the mean of the %HbF measurements at MTD performed on each study participant. The median number of %HbF measurements during MTD was 5, with a range of 1 to 7 measurements, although only 4 children had a single measurement. Values of %HbF within 90 days of a blood transfusion were not included in the analyses. The %HbF at MTD was analyzed as a continuous response variable and also as a categorical outcome by dividing the mean MTD %HbF into quartiles. For some analyses, the absolute change in %HbF (Δ %HbF = %HbF at MTD − %HbF at baseline) was used as a continuous response variable.
Statistical methods
Clinical patient characteristics at baseline, including age and sex, were analyzed initially for statistical associations with the mean %HbF at MTD. Baseline laboratory values for hemoglobin concentration, %HbF, mean corpuscular volume (MCV), white blood cell (WBC) count, red blood cell count, absolute neutrophil count, absolute reticulocyte count, platelet count, aspartate aminotransferase, alanine aminotransferase, total bilirubin, and lactate dehydrogenase were then analyzed for an association with mean MTD %HbF. Finally, treatment characteristics (MTD dose, MTD blood counts, toxicity, and noncompliance) were studied in relationship to %HbF at MTD. Only MTD blood counts measured at least 90 days after a blood transfusion were included. Each clinical, laboratory, and treatment characteristic was analyzed as a continuous variable and also as a categorical variable by separating the values at the median into high versus low categories.
Statistical associations were first assessed by computing simple Pearson correlation coefficients (simple correlations) between mean MTD %HbF and each continuous study variable. For some variables, simple correlations were also computed by using the Δ %HbF. Partial correlations between mean MTD %HbF and all study variables were then calculated after adjusting for baseline %HbF, WBC, and reticulocyte count. For categorical variables, the Fisher exact test, Mantel-Haenszel row mean score test, and logistic regression were used to analyze the association between the baseline indicator variables (dichotomized at their median) and quartiles of %HbF at MTD. Thet tests were used to compare the mean %HbF at MTD between the high and low baseline predictor groups. Analyses were performed by using SAS software (SAS, Cary NC) on a data file closed March 1998. No adjustments were made for multiple comparisons. P ≤ .05 is described as significant.
Results
HbF response in HUG-KIDS
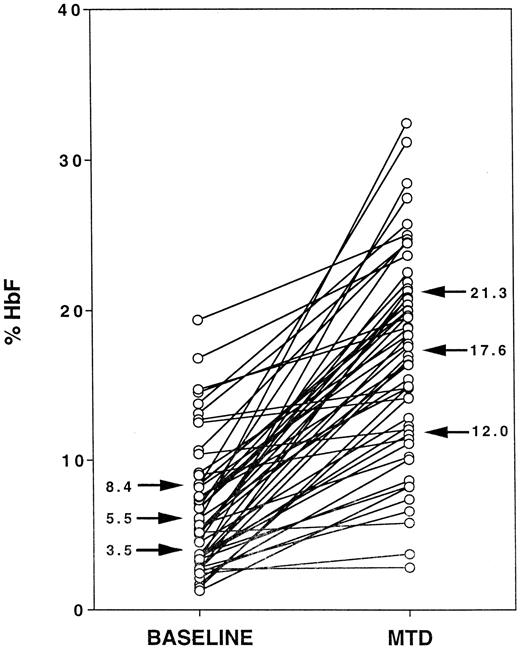
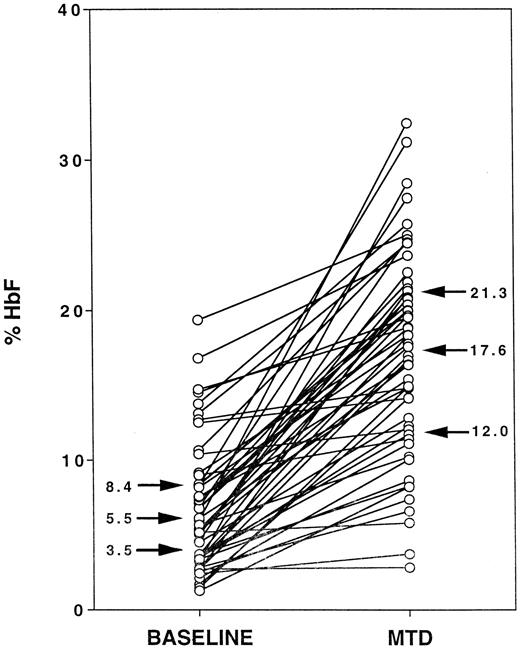
Figure 1 illustrates %HbF levels for the cohort of 53 HUG-KIDS children, with each child's baseline %HbF value connected by a line segment to the MTD %HbF value. At baseline, the 25%, 50%, and 75% quartile values for %HbF (indicated by arrows) were 3.5%, 5.5%, and 8.4%, respectively, with a minimum %HbF value of 1.3% and a maximum value of 19.4% HbF. At MTD, the 25%, 50%, and 75% values for %HbF (arrows) were 12.0%, 17.6%, and 21.3%, respectively, with a minimum %HbF value of 2.9% and a maximum value of 32.4%. In response to hydroxyurea therapy, most of the lines show a substantial upward trend, ie, an increase in %HbF. However, the response was highly variable, ranging from an increase in %HbF of 0.1% to 26.4% (median increase, 9.6%). No patient experienced a decline in %HbF over the treatment period, but 5 children had an increase in %HbF of less than 2.0%.
Fetal hemoglobin (HbF) responses to hydroxyurea therapy in the phase I/II hydroxyurea trial for children (HUG-KIDS).
The baseline %HbF level for each child is connected to the corresponding average %HbF level at MTD. Arrows indicate the 25%, 50%, and 75% values for each group.
Fetal hemoglobin (HbF) responses to hydroxyurea therapy in the phase I/II hydroxyurea trial for children (HUG-KIDS).
The baseline %HbF level for each child is connected to the corresponding average %HbF level at MTD. Arrows indicate the 25%, 50%, and 75% values for each group.
Statistical analysis using continuous variables
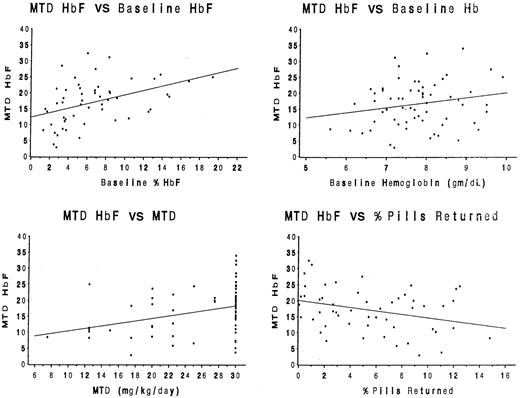
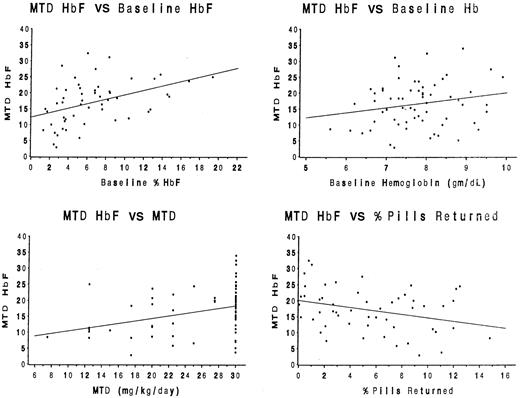
For the entire cohort of 53 children, baseline clinical and laboratory data, as well as selected hydroxyurea treatment characteristics (MTD dose, number of toxicities, percentage of pills returned), were analyzed for associations with the %HbF at MTD (Table1). Simple Pearson correlation coefficients revealed statistically significant positive associations between %HbF at MTD and the baseline %HbF value (P = .001), the baseline hemoglobin concentration (P = .01), and the MTD dose (P = .02), but a negative association between the %HbF at MTD and the percentage of pills returned (P = .02). These associations are illustrated by scatter diagrams in Figure2. No other baseline value or treatment characteristic was significantly associated with MTD %HbF.
Statistical associations between baseline laboratory parameters and treatment characteristics with the MTD %HbF.
Significant positive associations (r) were identified for the baseline %HbF value (panel A, r = 0.43,P = .001), the baseline hemoglobin concentration (panel B,r = 0.34, P = .01), and the MTD dose (panel C, r = 0.33, P = .02). A significant negative association was identified between the percentage of pills returned and the MTD %HbF value (panel D, r = −0.32,P = .02).
Statistical associations between baseline laboratory parameters and treatment characteristics with the MTD %HbF.
Significant positive associations (r) were identified for the baseline %HbF value (panel A, r = 0.43,P = .001), the baseline hemoglobin concentration (panel B,r = 0.34, P = .01), and the MTD dose (panel C, r = 0.33, P = .02). A significant negative association was identified between the percentage of pills returned and the MTD %HbF value (panel D, r = −0.32,P = .02).
Because the baseline %HbF varied widely among patients and might influence other variables, the analysis was repeated after adjustment for the baseline %HbF (Table 1, column PR). These partial correlations revealed that the MTD dose and noncompliance remained significant, but the baseline hemoglobin concentration did not. After adjustment for baseline %HbF, both the baseline reticulocyte count and WBC count were significantly associated with a higher %HbF at MTD (P = .05 for each; Table 1). Finally, the analysis was repeated after adjustment for baseline WBC and reticulocyte counts, and the %HbF at MTD remained significant in each case with the baseline %HbF, baseline hemoglobin concentration, MTD dose, and compliance.
When the data were analyzed by using the Δ %HbF as the response variable instead of %HbF at MTD, significant correlations were again noted with MTD dose (P = .0006), baseline WBC count (P = .02), and baseline reticulocyte count (P = .02). The Δ %HbF was not significantly associated, however, with the baseline %HbF value or the baseline hemoglobin concentration.
Statistical analysis using %HbF quartiles
For analyses using methods for categorical variables, the MTD %HbF was divided into quartiles, and baseline clinical and laboratory parameters were dichotomized at their median. Similar to the results described above using continuous variables, children with a higher baseline %HbF (ie, above the median baseline HbF value of 5.5%) reached a higher %HbF at MTD (20.0% versus 14.2%,P = .001 by t test). Children with an MTD of 30 mg/kg/d also had a higher overall mean %HbF at MTD than those children whose MTD was below 30 mg/kg/d (18.5% versus 14.8%,P = .05 by t test). Similar results were obtained when participants in the 2nd and 3rd quartiles of %HbF at MTD were excluded from these analyses, leaving only those children in the extreme quartiles (1st versus 4th).
Statistical associations of HbF response with treatment parameters
The next analyses were performed to determine if the %HbF response was associated with treatment variables, especially changes observed in other laboratory parameters. A higher MTD %HbF was associated with a higher MTD dose (P = .001) but not with age, sex, or number of hematologic toxicities (Table2). Treatment blood counts were analyzed according to their absolute value at MTD as well as their change from baseline. A higher hemoglobin concentration and a higher MCV (both the absolute MTD values and the positive changes from baseline) were significantly associated with a higher MTD %HbF response. A lower reticulocyte count and WBC count (both absolute MTD values and negative changes from baseline) were significantly associated with a higher %HbF at MTD. No significant associations were noted between the %HbF response and MTD treatment values for neutrophils or platelets.
Discussion
Over the past decade, hydroxyurea has emerged as a therapeutic option for both children and adults with sickle cell anemia. Early studies documented that hydroxyurea has a pronounced effect on HbF parameters, and it typically increases both the %HbF level and the percentage of F cells.9,16,18 Subsequent studies have reported additional potentially beneficial effects of hydroxyurea therapy, including diminished adhesiveness of sickle erythrocytes, improved rheology of circulating erythrocytes, and altered expression of surface adhesion markers.19-21 All studies to date have documented a variable response to hydroxyurea, however, especially with regard to elevation of %HbF. Identification of predictors of the HbF response to hydroxyurea might help clinicians maximize the efficacy of hydroxyurea therapy while possibly reducing its side effects and toxicities.
In the phase III, randomized, double-blinded, placebo-controlled MSH study, adults with sickle cell anemia who received hydroxyurea therapy at MTD had a modest but variable %HbF response. When the MSH data were analyzed according to the intention-to-treat principle, the mean 2-year change in %HbF was only 3.6 ± 5.4%, ranging from −1.5 ± 1.1% in the first quartile to +11.7 ± 3.8% in the fourth quartile.22 Somewhat surprisingly, half of the MSH patients treated with hydroxyurea actually had no increase in HbF after 2 years of therapy. The investigators speculated that this was due to genetic variation, bone marrow exhaustion, variability in drug bioavailability or metabolism, and (perhaps most likely) poor compliance with the treatment regimen.22 Baseline laboratory predictors of the HbF response in MSH included a higher initial reticulocyte count (≥ 300 × 109/L) and neutrophil count (≥ 7.5 × 109/L), but the baseline %HbF did not reach statistical significance (P = .07). Treatment-related predictors of the HbF response included noncompliance with hydroxyurea therapy and a higher number of toxic events, as well as greater hydroxyurea-associated changes in the MCV, reticulocyte count, and granulocyte count.22
The analyses of HbF parameters for children enrolled in the HUG-KIDS study differ from the MSH results in several areas. Importantly, we chose to use the %HbF at MTD as the primary response variable rather than Δ %HbF. Children with sickle cell anemia typically have higher %HbF baseline values than adults, hence the Δ %HbF could fail to identify a substantial response for those with high baseline values. Unlike MSH, our analysis of HUG-KIDS data identified the baseline %HbF level as the most powerful predictor of the MTD %HbF response (P < .001; Figure 2A). When analyzed by several different statistical methods, including continuous variables and quartiles, children with higher baseline %HbF levels developed higher MTD %HbF levels. This association was previously noted in phase I/II pilot trials of hydroxyurea therapy for adults with sickle cell anemia9,18 and suggests that the level of HbF production is strongly influenced by genetic modifiers. It should be noted, however, that the HbF response to hydroxyurea therapy in HUG-KIDS was highly variable (Figure 1), and even patients with a low baseline %HbF level occasionally had a substantial response to hydroxyurea therapy (eg, one patient whose baseline %HbF was 3.3% and mean MTD %HbF was 28.5%). In addition, a higher baseline reticulocyte count and higher WBC count also were associated with a higher MTD %HbF level, supporting an earlier claim that better “marrow reserve” may be associated with, or possibly necessary for, a robust response to hydroxyurea.22
Analysis of treatment characteristics revealed that children who achieved a higher hydroxyurea MTD dose had higher MTD %HbF levels (Figure 2C). This observation should not be overinterpreted because all patients were escalated to MTD, but it suggests that the highest tolerated daily hydroxyurea dose may yield the greatest HbF response. Treatment-associated changes in laboratory variables also were highly predictive of the HbF response: greater positive changes in hemoglobin concentration and MCV, as well as greater negative changes in reticulocyte and WBC counts, were associated with a higher MTD %HbF (Table 2). The predictive power of most of these therapy-associated changes has been recognized previously9,16 18 and suggests that hydroxyurea dose escalation to mild myelosuppression is desirable for maximal HbF response.
Our data indicate that selected baseline laboratory parameters (%HbF, reticulocyte count, and WBC count) identify the children most likely to have a robust HbF response to hydroxyurea therapy. A higher MTD dose of hydroxyurea, coupled with good compliance with the treatment regimen, will further identify children who will have the best HbF response. Finally, greater treatment-associated changes in selected laboratory variables (hemoglobin concentration, MCV, reticulocyte count, and WBC count) correlate with better HbF responses to hydroxyurea therapy. Taken together, these data demonstrate that almost all children with sickle cell anemia will respond to hydroxyurea therapy with increases in %HbF, but the response is complex and cannot be predicted accurately. Because even children with low baseline %HbF values can develop substantial increases in %HbF at MTD, however, clinicians should consider every severely affected child with sickle cell anemia to be a potential candidate for hydroxyurea therapy.
We thank Dr Kazumi Horiuchi for measurement of %HbF levels for the HUG-KIDS trial, Marsha McMurray and Dr Sharyne Donfield for data management services, and the many patients and families who participated in this study. Investigators for the Pediatric Hydroxyurea Trial (HUG-KIDS) are listed in the .
Clinical Centers (Numbers of patients enrolled in the study)—Children's Hospital, Boston, MA: O. Platt, B. Gee, S. Kurth (4); Duke University Medical Center, Durham, NC: T. Kinney, R. Ware, E. O'Branski, W. Schultz, A. Walker (17); Oakland Children's Hospital, Oakland, CA: E. Vichinsky, L. Styles, E. Hackney Stephens (18); Children's Hospital of Philadelphia, Philadelphia, PA: K. Ohene-Frempong, K. Smith-Whitley, L. Moore, L. Parkin, S. Whitehead (24); East Carolina University, Greenville, NC: C. Daeschner, D. Gordon, C. Brown (11); University of North Carolina at Chapel Hill, Chapel Hill, NC: R. Redding-Lallinger, S. Jones (7); St Jude Children's Research Hospital, Memphis, TN: W. Wang, K. Cupples, L. Wynn (7).
Statistical Center—University of North Carolina, Department of Biostatistics and the Frank Porter Graham Child Development Center: R. Helms, L. Brooks, K. Gover, E. Gunn, M. Helms, M. McMurray.
Data and Safety Monitoring Board—George Buchanan, MD (Chair); Robert Baumiller, SJ, PhD; George Dover, MD; Bertil Glader, MD, PhD; Genell Knatterud, PhD; Doris Wethers, MD.
Project Office (National Heart, Lung, and Blood Institute, Bethesda, MD)—Duane Bonds, MD (Project Officer); Clarice Reid, MD; Myron Waclawiw, PhD.
Supported in part by Comprehensive Sickle Cell Center Awards from the National Heart, Lung, and Blood Institute, National Institutes of Health, Bethesda, MD (Boston P60 HL15157, Duke-UNC P60 HL28391, Philadelphia P60 HL38632, and Northern California P60 HL20985).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Russell E. Ware, PO Box 2916, DUMC, Durham, NC 27710; e-mail: ware0005@mc.duke.edu.