Concomitant use of allogeneic donor granulocyte transfusions and amphotericin B in febrile neutropenic recipients may be limited by the increased incidence of respiratory distress. In vitro effects of amphotericin B and AmBisome were compared on polymorphonuclear leukocyte (PMN) aggregation from PMNs isolated from granulocyte–colony-stimulating factor (G-CSF)/dexamethasone–mobilized allogeneic donors. Six allogeneic donors were mobilized with G-CSF (600 μg subcutaneously) and dexamethasone (8 mg orally) 12 hours before leukopheresis. AmBisome was associated with significantly less PMN aggregation (100 μM [μg/mL]) (0.33% ± 0.33% vs 54.33% ± 5.82%; P < .001) than amphotericin B. Furthermore, with the addition of the PMN agonist, FMLP, AmBisome was also associated with significantly less aggregation (100 μM [μg/mL]) (18.67% ± 1.45% vs 54.67% ± 2.4%;P < .001). In summary, these studies demonstrate that liposomal amphotericin is associated with significantly less in vitro PMN aggregation than amphotericin B and could possibly be administered concomitantly with mobilized allogeneic PMN infusions.
Introduction
Several attempts have been made to collect and transfuse allogeneic donor granulocytes in patients with prolonged and severe neutropenia with systemic infections.1-3 Success has been limited by the small number of granulocytes collected by leukopheresis and the minimal increment in the circulating absolute neutrophil count, especially in large recipients.4-6Although we have previously demonstrated the success of granulocyte transfusions in neonates with sepsis, this has been secondary to the small size of the recipients and the ability to administer 2 granulocyte transfusions per day from a single donor.7
Recently, Price et al8 demonstrated the feasibility of mobilizing and collecting 5- to 10-fold more neutrophils by leukopheresis from allogeneic donors, after screening and after they gave their consent, who had been mobilized with dexamethasone and granulocyte–colony-stimulating factor (G-CSF).8 One of the limitations of this pilot study, however, was the inability to administer granulocyte transfusions concomitantly with systemic amphotericin B (Apothecon, Princeton, NJ) in patients with suspected or confirmed fungal infection. Amphotericin B, when administered concomitantly with granulocyte transfusions, may predispose patients to severe, lethal pulmonary reactions, possibly because of its ability to promote granulocyte aggregation.9 10
AmBisome (Fujisawa, Osaka, Japan) is one of the recent liposomal formulations of amphotericin B; its unilamellar spheric conformation allows better tissue distribution, higher blood levels, and reduced toxicity.11 Walsh et al12recently reported that patients who received liposomal amphotericin B (AmBisome) rather than amphotericin B experienced fewer breakthrough fungal infections (P = .009) and fewer infusion-related fever, chills, cardiorespiratory reactions (P = .001), and nephrotoxicity (P = .001). We hypothesized that liposomal amphotericin B (AmBisome) would also be less toxic to human neutrophils than conventional amphotericin B and would induce significantly less in vitro neutrophil aggregation.
Study design
Neutrophil donors and collection
Allogeneic donors (n = 6) were mobilized with 8 mg dexamethasone orally and 600 μg G-CSF (Neupogen; Amgen, Thousand Oaks, CA) subcutaneously 12 hours before scheduled collections. Neutrophils were collected according to standard apheresis procedures and were depleted of residual red blood cells with 6% hydroxyethyl starch (Hespan; McGaw-Dupont Pharma, Wilmington, DE).8Remaining cells were layered onto Ficoll-Hypaque (Sigma, St Louis, MO) and were centrifuged for 30 minutes to further remove any remaining lymphocytes.
Neutrophil aggregation
Briefly, aggregation of neutrophils was performed using a 4-channel Chronolog 470-VS aggregometer interfaced with Aggro/Link computer software (Chrono-Log, Havertown, PA).13 The aggregometer was calibrated by adjusting to 100% light transmission (T) with 0.45 mL diluted cell suspension (5 × 106cells/mL) and to 0% transmission with an undiluted suspension (1 × 107 cells/mL) in the sample chamber. After 1 minute, 0.05 mL AmBisome or amphotericin B at 0, 25, 50, and 100 μM (μg/mL) or liposome (vehicle component of AmBisome) at 50 μM (μg/mL) was added. The percentage T was recorded continuously. The maximum increase in the percentage T within 5 minutes was used for statistical comparison. To determine the in vitro effects of the neutrophil aggregation after incubation of AmBisome or amphotericin followed byN-formyl-L-methionyl-L-leucyl-L-phenyaline (FMLP) stimulation, neutrophils were incubated with amphotericin B or AmBisome (50 and 100 μM (μg/mL)) before the addition of FMLP (1 × 10−7 M). Light transmission in the test cuvettes was recorded for 3 minutes, and the area of the aggregation curve was calculated. Three replicate experiments were performed using a different allogeneic donor each time.
Myeloperoxidase and lactoferrin assays
Neutrophils (1 × 107 cells/mL) were incubated with 0.05 mL drug or vehicle for 30 minutes at 37°C. Cells were centrifuged, and the supernatant was collected and made into aliquots for enzymatic assay. Myeloperoxidase levels were determined by measuring the colorimetric changes of the oxidation of 4-aminoantipyrine in the presence of hydrogen peroxide.14Activity was expressed as mU/106 neutrophils. Lactoferrin levels were determined using the lactoferrin eicosanoid immunoassay kit (R&D Systems, Minneapolis, MN). Results were expressed as ng/106 neutrophils.
Statistical analysis
Results are expressed as mean ± SEM of 3 to 6 different donors. The probability of significant differences when comparing multiple treatment groups was determined by the analysis of variance followed by the Student Newman-Keuls multiple range test. Statistical analyses were performed using the Instat statistical program (Graph Pad, San Diego, CA). P < .05 was considered significant.
Results and discussion
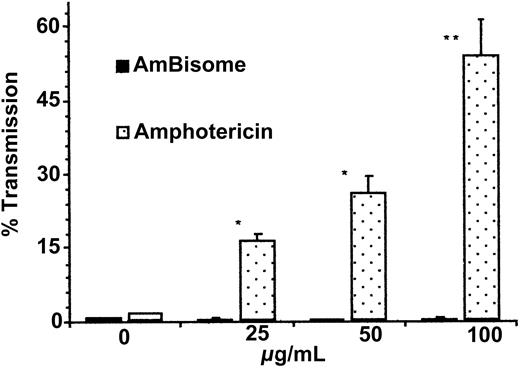
Neutrophil aggregation was significantly greater with amphotericin B than with AmBisome (16.67% ± 7.22% vs 0.33% ± 0.33%,P < .01, n = 3 at 25 μM [μg/mL]; 26.33% ± 8.33% vs 0.5% ± 0.5%, P < .01, n = 3 at 50 μM [μg/mL]; and 54.33% ± 5.82% vs 0.33% ± 0.33%,P < .001, n = 3 at 100 μM [μg/mL]) (Figure1). No significant difference was seen with vehicle alone.
Comparison of neutrophil aggregation after treatment with AmBisome and amphotericin B at escalating doses.
Neutrophils were made into aliquots in siliconized cuvettes and allowed to equilibrate to 37°C with constant stirring. After 1 minute, AmBisome or Amphotericin B at 25, 50, and 100 μM (μg/mL) or vehicle (0) was added, and the maximum increase in percentage light transmission within 5 minutes was measured and compared. Results are expressed as mean percentage light transmission ± SEM (n = 3). *P < .01, amphotericin B versus AmBisome at 25 and 50 μM (μg/mL). **P < .001, amphotericin B versus AmBisome at 100 μM (μg/mL).
Comparison of neutrophil aggregation after treatment with AmBisome and amphotericin B at escalating doses.
Neutrophils were made into aliquots in siliconized cuvettes and allowed to equilibrate to 37°C with constant stirring. After 1 minute, AmBisome or Amphotericin B at 25, 50, and 100 μM (μg/mL) or vehicle (0) was added, and the maximum increase in percentage light transmission within 5 minutes was measured and compared. Results are expressed as mean percentage light transmission ± SEM (n = 3). *P < .01, amphotericin B versus AmBisome at 25 and 50 μM (μg/mL). **P < .001, amphotericin B versus AmBisome at 100 μM (μg/mL).
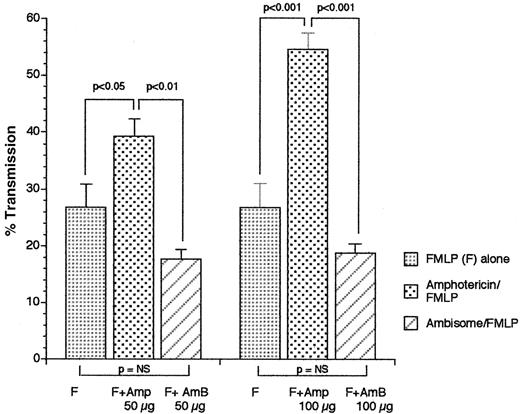
Neutrophils incubated with amphotericin also showed significantly increased FMLP-induced aggregation (39.33% ± 2.9% vs 17.67% ± 1.76%, P < .01, n = 3, 50 μM [μg/mL]; 54.67% ± 2.4% vs 18.67% ± 1.45%,P < .001, 100 μM [μg/mL]) (Figure2).
Comparison of FMLP-induced neutrophil aggregation after preincubation with AmBisome versus amphotericin B at 50 or 100 μM (μg/mL).
Neutrophils were incubated with AmBisome or amphotericin B for 1 minute before the addition of FMLP (1 × 107 M), and the maximum increase in percentage light transmission within 5 minutes was measured and compared. Results are expressed as mean percentage light transmission ± SEM, n = 3. P < .01, amphotericin B versus AmBisome at 50 μM (μg/mL). P < .001, amphotericin B versus Ambisome at 100 μM (μg/mL).
Comparison of FMLP-induced neutrophil aggregation after preincubation with AmBisome versus amphotericin B at 50 or 100 μM (μg/mL).
Neutrophils were incubated with AmBisome or amphotericin B for 1 minute before the addition of FMLP (1 × 107 M), and the maximum increase in percentage light transmission within 5 minutes was measured and compared. Results are expressed as mean percentage light transmission ± SEM, n = 3. P < .01, amphotericin B versus AmBisome at 50 μM (μg/mL). P < .001, amphotericin B versus Ambisome at 100 μM (μg/mL).
Neither myeloperoxidase nor lactoferrin production was significantly affected by AmBisome or amphotericin (myeloperoxidase, 3.3 ± 0.77 vs 5.9 ± 2.3 mU/1 × 106 cells at 100 μM [μg/mL], n = 3, P = NS; lactoferrin, 154.5 ± 7.5 vs 536.5 ± 53.5 mU/1 × 106 cells at 100 μM [μg/mL], n = 2).
In vivo animal studies have demonstrated that amphotericin B can induce in vitro granulocyte aggregation and enhance pulmonary leukostasis.10 Boxer et al15 had previously demonstrated that neutrophils aggregate in the presence of amphotericin B in concentrations achievable in vivo. Myeloperoxidase and lactoferrin were measured to determine the in vitro effects of these drugs on polymorphonuclear leukocyte primary and secondary granules. The results of these studies suggest that although amphotericin B, in comparison with liposomal amphotericin B with and without FMLP, significantly enhances in vitro PMN aggregation with allogeneic donor granulocytes, there is no difference in the 2 drugs on primary or secondary PMN degranulation.
The use of amphotericin B as empirical treatment for documented or suspected fungal infections during febrile neutropenia has become a standard treatment approach.16,17 Its use, however, is limited by infusion-related toxicity and dose-limiting nephrotoxicity.17 This toxicity appears to be secondary to its ability to bind to membrane sterols and to form ion channels with subsequent potassium leakage and cell death.17 Wright et al9 reported the association of respiratory distress in 64% of patients who had received amphotericin B and allogeneic donor granulocyte transfusions, but the direct relationship is unknown. Subsequently, investigators recommend that the interval between the administration of granulocyte transfusions and amphotericin B be at least 12 hours.18
The limited ability to collect large numbers of PMNs from unmobilized donors and the concern of pulmonary systemic reactions associated with allogeneic donor neutrophil transfusions precluded its use in the past.4,5,19 However, using G-CSF/dexamethasone to mobilize donors resulted in a 1-hour posttransfusion neutrophil increment of 2.6 ± 2.6 × 103/μL with normal function and restoration of the neutrophil count to a normal range in 17 of 19 patients.8 20
In summary, the use of amphotericin B with neutrophil transfusions in patients with severe neutropenia with fungal infections has increased.8 21 The ability to separate by 12 hours the concomitant administration of amphotericin B and neutrophil transfusions is difficult. To realize optimal effects of allogeneic donor neutrophil transfusions and amphotericin B, delays in either treatment modality should be avoided. Our in vitro results demonstrate reduced neutrophil aggregation with the liposomal formulation and suggest that the liposomal formulation may be safer to administer concomitantly with neutrophil transfusions. Further in vitro studies with other liposomal amphotericin B derivatives such as amphotericin B cholesteryl sulfate (ABCD) and amphotericin B lipid complex (ABLC) may be warranted. Well-designed and controlled randomized clinical trials investigating pulmonary reactions secondary to the concomitant use of mobilized allogeneic donor neutrophils with liposomal formulations of amphotericin B compared with conventional amphotericin B are needed.
We thank Linda Rahl for her editorial assistance in the preparation of this manuscript.
Supported in part by grants from the Pediatric Cancer Research Foundation and Nexstar Pharmaceuticals.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Mitchell S. Cairo, Pediatric Blood and Marrow Transplantation, Children's Hospital of New York, Columbia University, Irving 7, 161 Fort Washington Ave, New York, NY 10032; e-mail:mc1310@columbia.edu.