Rituximab is a chimeric murine/human monoclonal antibody that binds to CD20 on B lymphocytes. Although binding of the Fab domain may induce apoptosis, the Fc domain recruits immune effector functions to mediate cell lysis. Interleukin-12 (IL-12) facilitates cytolytic T-cell responses, enhances the lytic activity of natural killer (NK) cells, and induces the secretion of interferon γ (IFN-γ) by both T and NK cells. Therefore, the hypothesis was considered that combining IL-12 with rituximab would augment the immune-mediated cell lysis induced by rituximab. A phase 1 study of IL-12 in combination with rituximab was conducted in 43 adults with B-cell lymphoma to determine the optimal immunologic dose of this combination. Rituximab was administered at a dose of 375 mg/m2 by intravenous infusion weekly for 4 weeks, and IL-12 was given subcutaneously twice weekly. The starting dose of IL-12 was 30 ng/kg and this was escalated to 500 ng/kg. Constitutional symptoms and liver enzyme elevations at 500 ng/kg of IL-12 were dose limiting. A greater than 20-fold increase in the serum levels of IFN-γ and a 2.5- to 5-fold increase in inducible protein 10 (IP-10) levels was seen at IL-12 doses of 100 ng/kg or greater. Objective responses occurred in 29 of the 43 patients (69%), with 8 of 11 complete responses seen at IL-12 doses of 300 ng/kg or greater. The optimal immunologic dose of IL-12 in combination with rituximab was determined to be 300 ng/kg subcutaneously twice weekly starting on day 2. These data suggest that IL-12 and rituximab is an active combination and further studies of this combination in B-cell non-Hodgkin lymphoma are warranted.
Introduction
B-cell non-Hodgkin lymphoma (NHL) is the sixth most common cause of cancer-related deaths in the United States and the incidence of this disease is increasing. Although approximately half the patients with aggressive lymphomas may be cured with cytotoxic therapy, most indolent lymphomas are incurable with current therapy. Novel effective therapies are therefore needed to treat patients with NHL.
Interleukin-12 (IL-12) is a cytokine that exerts a number of regulatory effects on T lymphocytes and natural killer (NK) cells.1,2These include facilitating specific cytolytic T-cell responses; promoting the development of Th1-type helper T cells, thereby contributing to the development of cell-mediated immune responses; enhancing the lytic activity of NK cells; and inducing the secretion of interferon γ (IFN-γ) by both T and NK cells.3-6 A further activity of IL-12 that may contribute to an antitumor effect is the ability to inhibit angiogenesis, an effect mediated through the induction of IFN-γ and inducible protein 10 (IP-10).7
Interleukin-12 has also shown antitumor activity in a number of in vivo murine tumor models,6,8 and the antitumor effects were demonstrated in microscopic disease models as well as animals bearing large established tumors. Antitumor activity was reduced in cytotoxic granule-deficient mice, but was nearly eliminated in T- cell–deficient mice.6 Depletion of either CD8+ or CD4+ T cells reduced antitumor activity in animal models.8
The recruitment of immune cells to the areas of tumor appears to be mediated by various chemokines. The role of the chemokines IP-10 and Mig (monokine induced by IFN-γ) in antitumor activity induced by systemic treatment with IL-12 was examined in mice bearing the murine renal adenocarcinoma RENCA, the CSA1M fibrosarcoma, and the OVHM ovarian carcinoma.9 10 IL-12 treatment produced a potent antitumor effect that was associated with tumor infiltration by CD4+ and CD8+ T lymphocytes. The regression of tumor was associated with the elevated expression of the IFN-γ–inducible chemokines IP-10 and Mig within the tumor tissue. In animals treated with rabbit polyclonal antibodies specific for IP-10 and Mig, the IL-12–induced regression of RENCA tumors was partially abrogated. Thus, it appears that IL-12–dependent, T-cell–mediated antitumor activity requires the intermediate expression of IP-10 and Mig to recruit antitumor effector T cells to the tumor site.
Rituximab is a genetically engineered chimeric murine/human monoclonal antibody that binds specifically to the antigen CD20 (human B lymphocyte–restricted differentiation antigen, Bp35) located on pre-B and mature B lymphocytes.11,12 The antigen is expressed on more than 90% of B-cell NHLs13 but is not found on hematopoietic stem cells, pro-B cells, normal plasma cells, or other normal tissues.14 The Fab domain of rituximab binds to the CD20 antigen on B lymphocytes and the Fc domain recruits immune effector functions to mediate lysis of the B cell.15 The antibody also induces apoptosis,16,17 and in clinical studies it has induced objective responses in approximately 30% to 50% of patients treated.18-28
We have shown that the patient's immunologic response to the B-cell lymphoma (specifically the number of infiltrating CD4+ T cells) predicts duration of response to treatment and survival.29 Because IL-12 plays a pivotal role in enhancing the cytotoxicity of T and NK cells and in promoting antibody-dependent cellular cytotoxicity (ADCC), we postulated that the addition of IL-12 to the rituximab treatment regimen would augment the antitumor effect seen in patients with B-cell lymphoma treated with this anti-CD20 monoclonal antibody.
Patients, materials, and methods
Patient eligibility
To be eligible for participation in this study, patients were 18 years of age or older with histologic proof of CD20+ B-cell NHL. If patients had a recurrent lymphoma, a biopsy to confirm the recurrence was required except in patients with only inaccessible sites of disease. Patients with intermediate- or high-grade lymphoma were required to have received at least one prior anthracycline-containing chemotherapy regimen and first be considered for stem cell transplantation. Patients with low-grade lymphoma (with or without prior therapy) felt to be incurable with standard therapy were also eligible. Previously untreated patients with low-grade lymphoma that was incurable with current therapy were eligible for this phase 1 study because rituximab was a reasonable consideration as front-line therapy for these patients and IL-12 was administered at immunologic doses below the maximum tolerated doses (MTDs) shown in other studies.
The following laboratory values, completed 14 days or less before registration, were required: an absolute neutrophil count (ANC) of at least 1500/μL, a platelet count of at least 75 000/μL, hemoglobin of at least 10 g/dL, total bilirubin no more than 3 times the upper limit of normal (ULN), serum creatinine no more than 2 times ULN, aspartate aminotransferase (AST) less than 3 times ULN, and alanine aminotransferase (ALT) less than 3 times ULN. All patients were required to return to the Mayo Clinic (Rochester, MN) for treatment and follow-up, to have a life expectancy of 12 weeks or more, and a European Cooperative Oncology Group (ECOG) performance status of 0 to 1. Patients treated with rituximab within the previous 12 months, previously treated with IL-12, or not yet fully recovered from effects of prior chemotherapy were not eligible. Patients known to be positive for human immunodeficiency virus and patients requiring concurrent steroid therapy were not eligible. Patients previously treated with fludarabine or 2-chlorodeoxyadenosine (2-CDA) were required to have a CD4 count within the normal range.
Other exclusion criteria included uncontrolled infection, central nervous system involvement by lymphoma, autoimmune-related phenomena, and peptic ulcer disease. Pregnant and nursing women were not eligible for the study. All patients were required to give informed consent and the Institutional Review Board of the Mayo Clinic/Mayo Foundation approved the study.
Study design
This modified standard phase 1 design used initial cohorts of 6 patients at each dose level.30 This was done using the same determination of MTD as is used typically in the standard “cohorts of 3” design. Specifically, if 2 or more patients of 6 (or 9) patients at any given dose level experience dose-limiting toxicity (DLT), the MTD was defined as the preceding lower dose level. This provided a slightly more conservative estimation process for the MTD, but also allowed for further characterization of the laboratory-related variables.
Treatment regimen and dose escalation
Rituximab was administered at a dose of 375 mg/m2 by intravenous infusion weekly for 4 weeks; IL-12 was given subcutaneously twice weekly for a maximum of 24 weeks. IL-12 was obtained from the Clinical Trial Evaluation Program (CTEP) of the National Cancer Institute (NCI) and rituximab was obtained commercially. The starting dose of IL-12 was 30 ng/kg and this was escalated with each cohort of 6 patients to a maximum of 500 ng/kg (Table1). Because of a concern for excessive toxicity if IL-12 and rituximab were given concurrently at the initiation of treatment, rituximab was given alone for the first 2 weeks (ie, days 1 and 8) for the first 5 patient cohorts. IL-12 was added on day 16 after the third dose of rituximab (day 15). However, the first cohort of patients was given all 4 doses of rituximab (days 1, 8, 15, and 22) prior to receiving IL-12 and these patients were used to assess the immunologic response to rituximab alone. Beginning on day 29, the patients in the first cohort were given IL-12 twice weekly subcutaneously. The patients were treated with rituximab at a fixed dose with escalating doses of IL-12 used in each subsequent cohort. No unexpected toxicities were seen when IL-12 was given on day 16 in combination with rituximab and the final 2 cohorts of patients (cohorts 6 + 7) received IL-12 the day after initiation of rituximab (day 2). There was no dose escalation within a given cohort. To avoid the excessive toxicity experienced in previous studies and to define the optimal immunologic dose, the doses of IL-12 were initially less than one tenth of the MTD found in other studies. The dose of IL-12 was increased with each subsequent cohort; however, the goal of the study was to determine the optimal immunologic dose of IL-12 in combination with rituximab rather than the MTD of IL-12 in this combination.
Toxicity and response evaluation
Six patients were treated at each dose level and evaluated at weeks 2, 4, 8, and 12 to assess toxicity. Doses were not escalated in any individual patient. Known rituximab toxicities (of any grade) occurring prior to IL-12 administration were not included in toxicity calculations. With this exception, the following toxicities were regarded as DLTs: hematologic, any grade 4 toxicity; renal, serum creatinine 3 times or more the ULN; cardiac, any grade 2 toxicity; neurologic, any grade 2 toxicity; or any other nonhematologic toxicity grade 3 or higher according to the NCI Common Toxicity Criteria (CTC) version 2.0. Grade 3 nausea and grade 4 vomiting with maximal antiemetic treatment and grade 3 diarrhea with maximal antidiarrheal therapy were also considered dose-limiting. As per the NCI CTC version 2.0, an elevation of at least 5 times the ULN in the serum alkaline phosphatase (ALP), AST, and ALT was regarded as a DLT. An elevation in the serum bilirubin of at least 3 times the ULN was also regarded as a DLT.
If the liver function tests (LFTs) increased to at least 2 times the baseline value, the IL-12 would be held until the values returned to baseline. If the values returned to baseline in 7 days or less, no dose reduction in the IL-12 would be required. If the values returned to baseline in 8 to 21 days, the dose of IL-12 would be decreased one dose level and restarted. If the values took 21 days or more to return to baseline, the IL-12 would be discontinued. For patients experiencing grade 2 or above cardiac toxicity or neurotoxicity, the IL-12 would be discontinued. For severe constitutional symptoms, gastrointestinal bleeding, or a serum creatinine at least to 2 times the baseline value, the IL-12 would be held for up to 21 days until resolved and then decreased one dose level. There were no dose reductions for lymphopenia. For grade 4 neutropenia, granulocyte colony-stimulating factor could be added.
Should a patient have stable disease or be responding to treatment at the time of the evaluation at 12 weeks, IL-12 could be continued twice weekly at the physician's discretion until progression or up to 24 weeks. Patients having objective progression of disease or clinical deterioration were taken off the study. Patient responses to therapy were assessed using the International Working Group recommendations for response criteria for NHL.31
Serum levels of IFN-γ and IP-10 after treatment with IL-12 in combination with rituximab
To evaluate the immunologic effects of the combination of IL-12 and rituximab, the serum levels of 2 cytokines induced by IL-12 were measured. IFN-γ and IP-10 were evaluated in the first patient cohort at baseline and after the administration of rituximab alone to evaluate whether rituximab would increase their expression. For subsequent patients, blood was drawn for the determination of serum IFN-γ and IP-10 levels prior to IL-12 administration to serve as a baseline. Serum levels of IFN-γ and IP-10 were determined again at 0, 8, and 24 hours after the administration of IL-12. A single serum level of IFN-γ and IP-10 was determined on days 28 and 56 (14 and 40 days after the IL-12 was started) to assess whether a tachyphylaxis effect was present. These serum samples were tested for the presence of IFN-γ and IP-10 by a sandwich enzyme-linked immunosorbent assays (ELISA). The ELISA used a goat antihuman IP-10 or IFN-γ antiserum (R & D Systems, Minneapolis, MN) diluted at 1:250 and a purified rabbit antihuman IP-10 or IFN-γ polyclonal antiserum to capture bound IP-10 or IFN-γ followed by an ALP-conjugated goat antirabbit antibody (Sigma, St Louis, MO).
Immunohistochemistry to evaluate T-cell infiltration
The T-cell recruitment to areas of B-cell lymphoma was also evaluated. The number of T cells in an area of B-cell lymphoma was determined either by flow cytometry or by immunohistochemistry. Specifically, we determined the levels of CD3+, CD4+, CD8+, and CD20+ cells in tumor biopsies. This was done on the original biopsy at the time of registration into the study. Using the same methods, a repeat core needle or excision biopsy of accessible areas of lymphoma was done on day 28 to evaluate the extent of T-cell infiltration in response to therapy.
T-cell and B-cell subsets in peripheral blood
Quantitative determinations of the T-cell and B-cell subsets in the peripheral blood by flow cytometry were performed on all patients. These determinations included quantitation of CD4+ and CD8+ T-cell subsets, NK cells, and circulating B cells before treatment and repeated on days 15 and 28 to assess the effect of the treatment combination not only on B cells but also on the T-cell subsets and NK cells.
Statistical methods
The primary goal of this single-arm phase 1 study was to determine the optimal immunologic dose for the combination of IL-12 and rituximab. The primary outcome measures of this study were to determine the dose at which there was a maximal immunologic response to the combination and the number of toxicity incidents associated with the combination. The maximal immunologic response was defined as the dose level of IL-12 that induced at least a 10-fold increase in serum cytokines and the maximum amount of T-cell recruitment to the B-cell lymphoma (defined as at least a 50% increase in the number of T cells in areas of B-cell lymphoma). If the T-cell infiltration and increase in the levels of the above cytokines were similar between 3 dose levels, the middle dose level would be defined as the optimal dose. Should the MTD be reached prior to the maximum immunologic response, the optimal dose would be defined as the highest safely tolerated dose where 1 patient of 6 experienced DLT with the next higher dose having at least 2 patients who experience DLT.
Hematologic toxicity measures of thrombocytopenia, neutropenia, and leukopenia were assessed using the continuous variables as the outcome measures (primarily nadir and percent change from baseline values) as well as categorization via CTC standard toxicity grading. Nonhematologic toxicities such as diarrhea and stomatitis were evaluated via the ordinal CTC standard toxicity grading only. Frequency distributions and other descriptive measures formed the basis of the analysis of these variables.
A secondary outcome measure for the study was the number of clinical responses. Although an estimated response rate will further be determined by evaluation of the combination of IL-12 and rituximab in a phase 2 setting, the responses were summarized by simple descriptive summary statistics delineating complete and partial responses as well as stable and progressive disease.
Standard paired comparisons methodologies (paired t tests, Wilcoxon signed rank tests, and Fisher exact tests for interval, ordinal, and nominal level data, respectively) were used to compare the average intrapatient levels of the laboratory correlates (IFN-γ, IP-10, T-cell recruitment, etc) between baseline values and after treatment.
These data were correlated with toxicity data using bivariate scatterplots and Spearman correlation coefficients. An analysis was carried out on time-related variables including time until immunologic response, time until any treatment-related toxicity, time until treatment-related grade 3+ toxicity and time until hematologic nadirs (white blood cells, ANC, platelets). The summary statistics were supplemented with Kaplan-Meier survival estimates and related confidence intervals.32 The effect of dose and ancillary dichotomized covariates such as sex or age (< 50 years versus ≥ 50 years) were explored using log-rank testing involving one covariate at a time.
The study data set was monitored and updated, and summary toxicity and demographic reports provided to the clinical investigators on a weekly basis. Standardized routines for analysis of phase 1 trials at the Mayo Clinic were used.33
Results
Patient characteristics
Forty-three adult patients (22 women, 21 men) with CD20+ NHL were treated. The patient characteristics are shown in Table 2. Twenty-six patients had indolent lymphoma and 17 had aggressive histologies. The median age of the patients was 54 years (range, 34-84 years). Thirty-four patients were previously treated including 6 patients who had previously undergone autologous stem cell transplantation. Nine patients with low-grade lymphoma were previously untreated or had been previously managed with only local radiation therapy or splenectomy. Two patients had previously received 2-CDA and one patient had previously been treated with fludarabine. All patients had an ECOG performance score of 0 to 1 and all patients had advanced-stage disease.
Dose escalation and toxicity
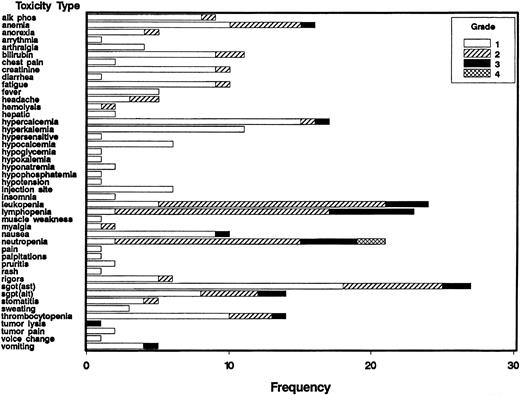
Six patients were treated at each dose level and that number was increased if toxicity related to the combination was seen. The numbers of patients requiring dose modifications in each cohort are shown in Table 3; the frequency of the various toxicities by grade for all patients is shown in Figure1. All patients have completed therapy. The first cohort of patients received all 4 doses of rituximab prior to the initiation of IL-12 on day 29. One patient developed thrombocytopenia just before the start of IL-12 that subsequently proved to be immune thrombocytopenic purpura. The patient required steroids and the IL-12 was discontinued as per protocol. Because this is a previously described side effect of rituximab and started prior to IL-12, it was not regarded as a DLT and the dose of IL-12 was escalated to the next dose level.
Frequency of toxicities classified as related to treatment by grade in patients receiving IL-12 and rituximab.
Frequency indicates number of patients; alk phos, alkaline phosphatase; SGOT (AST), aspartate aminotransferase; SGPT, (ALT), alanine aminotransferase.
Frequency of toxicities classified as related to treatment by grade in patients receiving IL-12 and rituximab.
Frequency indicates number of patients; alk phos, alkaline phosphatase; SGOT (AST), aspartate aminotransferase; SGPT, (ALT), alanine aminotransferase.
For patients treated in cohorts 2 to 5, IL-12 was started on day 16, the day after the third dose of rituximab, due to concerns about potential excessive toxicity when the 2 agents were started simultaneously. The dose of IL-12 was initially 30 ng/kg and was escalated to 500 ng/kg. At 100 ng/kg, one patient developed a pleural effusion on day 64 of treatment. Due to safety concerns that this may constitute a DLT, 3 more patients were treated at this dose level. The effusion subsequently was demonstrated to contain malignant cells and was felt to be evidence of progressive disease rather than DLT. At 300 ng/kg, one patient developed Coomb-positive hemolytic anemia on day 44 of treatment that was felt to be a DLT and 3 more patients were therefore treated at this dose level. The IL-12 was discontinued in the patient who developed the autoimmune hemolytic anemia and this patient responded to steroids. Two other patients treated at this dose level required a dose reduction for elevated LFT results and constitutional symptoms. At the dose of 500 ng/kg IL-12, 2 of the first 3 patients treated had a more than 10 times increase in their LFTs that constituted a DLT. Both of these patients required a dose reduction, although the first patient treated at this dose level also required a dose reduction but did not experience a DLT.
Because no unexpected increase in toxicity was seen when IL-12 was added to rituximab on day 16, IL-12 was subsequently started on day 2, after the first dose of rituximab, for patients treated in cohorts 6 and 7. At 300 ng/kg using this schedule, 2 of the 6 patients required a dose reduction due to elevated LFTs and constitutional symptoms, but none of the patients had a DLT. At an IL-12 dose of 500 ng/kg given on day 2, 2 of the 4 patients treated had a DLT and all 4 required a dose reduction.
The total number of IL-12 doses given per dose level for dose levels 1 to 7 were 193 doses given to 6 patients, 208 doses to 6 patients, 352 to 9 patients, 278 to 9 patients, 71 to 3 patients, 229 to 6 patients, and 49 to 4 patients, respectively. The median number of IL-12 doses per patient per dose level is shown in Table 3. Patients who tolerated IL-12 received this agent for 24 weeks and the IL-12 was then discontinued. The administration of IL-12 was not continued beyond 24 weeks in any patient. Twenty patients completed 24 weeks of IL-12; the others discontinued IL-12 due to toxicity or progression of disease. The IL-12 dose was held in 12 patients due to toxicity, 8 of whom required that the IL-12 be restarted at the next lower dose level. Four of these 8 patients received 300 ng/kg IL-12 and 4 received 500 ng/kg. A further 3 patients treated at the 500-ng/kg dose level experienced toxicity that did not resolve within 21 days and the IL-12 was not restarted.
For patients experiencing LFT elevations, the median time from the initiation of IL-12 to an increase in LFTs at least 2 times the baseline value was 13 days. The commonly elevated LFTs were the ALP, AST, and ALT. The median number of days of elevated values for all patients was 6 days for the ALP, 4 days for the AST, and 3 days for the ALT. For patients experiencing grade 3 hepatic toxicity, the median duration of elevated LFTs above baseline was 21 days.
Due to the dose-limiting constitutional symptoms and liver enzyme elevations seen at the 500-ng/kg dose level, it was concluded that 300 ng/kg was the MTD of IL-12 when given in combination with standard doses of rituximab. No difference in the toxicity profile was seen depending on whether the IL-12 was started on day 2, 16, or 29. Because of the convenience of starting the 2 agents together, starting IL-12 on day 2 was determined to be the preferred schedule.
T-cell infiltration in areas of B-cell lymphoma
Patients with accessible lymph nodes, and who gave informed consent, had a repeat biopsy of an involved disease site after 1 month on treatment and the T-cell infiltration in the area of lymphoma after therapy was compared to a biopsy specimen before treatment. The patients treated in the first cohort had the second biopsy performed before the IL-12 was started, and served as a control to assess the T-cell infiltration caused by rituximab alone. Seventeen of the 43 patients had another biopsy specimen taken and the extent of the immune infiltrate in the area of lymphoma was evaluated. Although there was a suggestion that the therapy increased T-cell infiltration, a greater than 50% increase in the extent of the immune cell infiltration could not be documented (results not shown).
Serum levels of IFN-γ and IP-10
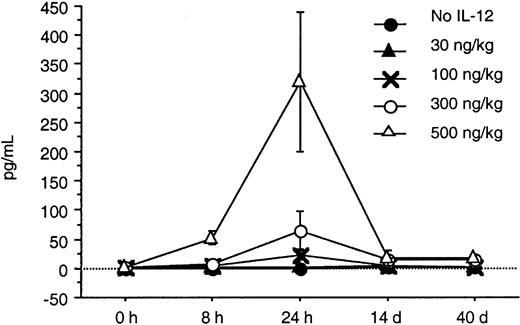
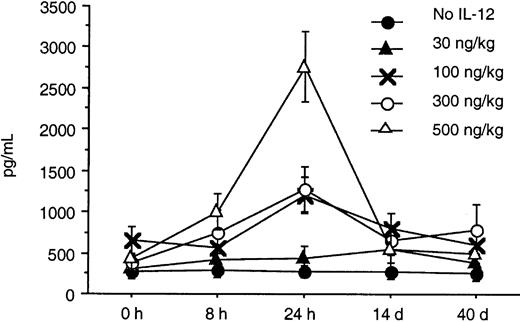
Serum levels of IFN-γ and IP-10 were determined by an ELISA at baseline, 8 and 24 hours after IL-12 was initiated, and again on days 14 and 40. A substantial increase from baseline in the serum levels of IFN-γ and IP-10 was seen in response to doses of IL-12 of 100 ng/kg or more within the first 24 hours (Figures2 and 3). A dose-dependent increase in both IFN-γ and IP-10 was seen at IL-12 doses of 100, 300, and 500 ng/kg. The increase in IFN-γ was seen as early as 8 hours after initiation of IL-12, whereas the increase in IP-10 was seen at 24 hours. This was expected because IP-10 is known to be induced by IFN-γ. The serum levels of IP-10 and IFN-γ when tested on days 28 and 56 remained significantly elevated with sustained IL-12 administration and did not return to baseline. Although the increase in IFN-γ correlated with increased toxicity, the peak increase in IFN-γ 24 hours after IL-12 and the IFN-γ levels on days 28 and 56 did not correlate with the clinical response in this study.
T- and B-lymphocyte subsets in peripheral blood
The numbers of B, T, and NK cells in the peripheral blood were measured at baseline and then again on days 14 and 28. No clinically or statistically significant change in the numbers of CD4+, CD8+, or NK cells in the peripheral blood was seen over this period. The numbers of circulating B cells dropped to almost undetectable levels by day 14 and the B-cell numbers remained extremely low when retested on day 28 as expected with rituximab.
The median pretreatment CD4+ cell count for patients enrolled in the study was 396 cells/μL (range, 80-2072; normal, 401-1532). The CD4+ cell count was lower than the normal range in 21 patients but only returned to normal in 3 patients. There was no significant difference in the response to therapy or toxicity when patients with a low CD4 count were compared to those with a normal count. The median peripheral blood NK cell count was 204 cells/μL (range, 27-1537; normal, 80-597). Five patients had NK cell counts below the normal range before treatment and in 2 patients the NK cell count returned to normal by day 28. The median peripheral pretreatment CD8+ T-cell count was 304 cells/μL (range, 65-2677; normal, 152-838). Six patients had CD8 counts below the normal range before treatment and these returned to normal by day 28 in 3 patients. The pretreatment NK cell and CD8+ T-cell counts also did not correlate with response or toxicity.
Clinical responses
Objective responses were seen in 29 of 43 patients (69%, 95% confidence interval of 55%-83%). Eleven patients had a complete response (25%), and 18 patients had a partial response (42%) to this therapeutic combination. Responses were seen in all histologic types. Three of the 4 patients (75%) with small lymphocytic lymphoma, 13 of 20 patients (65%) with follicular lymphoma, 5 of 6 patients (83%) with mantle cell lymphoma, 7 of 11 patients (64%) with large cell lymphoma, and 1 of 2 (50%) patients with lymphoplasmacytic lymphoma had an objective response to therapy. Three of 6 patients (50%) who had previously received an autologous transplant responded. The complete responses were seen in 3 patients with follicular lymphoma patients, 3 with mantle cell lymphoma, and 5 with large cell lymphoma, including a patient with T-cell–rich large B-cell lymphoma. Four of the complete responses were seen at the 300-ng/kg dose, 4 at the 500-ng/kg dose, 2 at the 30-ng/kg dose, and 1 at the 100-ng/kg dose. Of note, there was 1 complete response and 3 partial responses in the 6 patients in the first cohort who first received rituximab and then IL-12. Although clinical efficacy was not a primary end point of the study, these data suggested a trend toward a higher complete response rate at IL-12 doses of 300 and 500 ng/kg. Only 1 of the 11 complete responders has had disease progression to date, whereas 10 of the 18 patients who had a partial response have shown disease progression. With a median follow-up of 11 months (range, 6-16 months), 11 of 29 responding patients (38%) have had a relapse and the median response duration is at least 8 months (range, 5-12+ months).
Determination of the optimal immunologic dose of IL-12 in combination with rituximab
The combination of IL-12 and rituximab was generally well tolerated, but DLT was seen in patients receiving IL-12 at a dose level of 500 ng/kg. Although dose reductions were required in 33% of patients given IL-12 at the 300-ng/kg dose level, this dose was well tolerated with dose reductions required predominantly for asymptomatic LFT elevations and constitutional symptoms. The optimal immunologic dose of IL-12 to administer in combination with rituximab was originally defined as the dose that caused a more than 10-fold increase in the IP-10 and IFN-γ levels with a maximal increase in immune cell infiltration. A more than 10-fold increase in, particularly, IFN-γ was seen in patients receiving IL-12 at doses of 100, 300, and 500 ng/kg. A significant increase in immune cell recruitment in response to the increase in IFN-γ and IP-10 could not, however, be documented at any dose level. Of note, 8 of 11 complete responses were seen at IL-12 dose levels of 300 ng/kg or more (4 complete responses at 300 ng/kg and 4 at 500 ng/kg), although substantial DLTs were seen at 500 ng/kg. In view of the acceptable toxicity, the number of complete responses seen, and the substantial immunologic effect as measured by the increase in the levels of IP-10 and IFN-γ, the recommended immunologic dose of IL-12 in combination with standard doses of rituximab was determined to be 300 ng/kg subcutaneously twice weekly.
Discussion
The chimeric anti-CD20 monoclonal antibody, rituximab, is a commonly used and effective treatment for follicular and other low-grade NHLs.18-20,24,27,28 Despite its clinical efficacy, the mechanisms of action of this agent are only now being established. Although the antibody has been shown to directly induce apoptosis,16,17 the Fc domain has also been shown to recruit immune effector functions to mediate lysis of the B cell.15 Recent studies have concluded that complement-mediated cytotoxicity and ADCC are major mechanisms of action of rituximab in B-cell lymphomas.34 35
Interleukin-12 plays a pivotal role in controlling cell-mediated immunity through a number of important biologic activities such as the induction of IFN-γ. IL-12 as a single agent has potent antitumoral activity in animal models and objective tumor responses have been seen in patients with renal cell carcinoma, melanoma, and lymphoma.36-39 These clinical responses appeared to correlate with the induction of IFN-γ.38 This biologic effect of IL-12 in up-regulating cell-mediated immunity was the reason for combining IL-12 with rituximab in this phase 1 study.
Significant toxicities have been reported in the previous phase 1 and phase 2 studies of IL-12 as a single agent.40,41Substantial toxicities have also been reported when IL-12 and other monoclonal antibodies such as Herceptin are given concurrently at the initiation of treatment.42 For this reason, in this phase 1 trial, we originally administered rituximab alone for the first 2 weeks and then initiated IL-12 on a twice-weekly subcutaneous schedule while continuing with rituximab weekly for 2 more weeks. Subsequent cohorts of patients received rituximab and IL-12 administered on days 1 and 2, respectively, without any increase in toxicity. In view of the significant toxicities seen with IL-12 in previous studies, patients in our phase 1 trial received IL-12 at a starting dose of 30 ng/kg, a dose more than 10-fold lower than the MTD reported in previous studies.
The most common toxicities seen in this phase 1 trial of IL-12 plus rituximab were liver enzyme elevations and cytopenias secondary to therapy. Constitutional symptoms and elevated LFTs were found to be the DLTs. Due to the dose-limiting constitutional symptoms and liver enzyme elevations seen at the dose level of 500 ng/kg, 300 ng/kg was felt to be the MTD of IL-12 when given in combination with standard doses of rituximab. No difference in the toxicity profile was seen depending on whether the IL-12 was started on day 2, 16, or 29 (data not shown). Due to the convenience of starting the 2 agents together, starting IL-12 on day 2 is the preferred schedule.
In this study, we found that twice-weekly subcutaneous administration of IL-12 maximally increased the serum levels of IFN-γ and IP-10 24 hours after the IL-12 was given. The increase in IFN-γ was seen as early as 8 hours after initiation of IL-12, whereas the increase in IP-10 was seen at 24 hours. This was expected because IP-10 is known to be induced by IFN-γ. These increases in serum IFN-γ and IP-10 levels were seen at doses of IL-12 that were 100 ng/kg or higher. The toxicities seen in this study appeared to be predominantly associated with the serum levels of IFN-γ. One third of the patients at the dose level of 300 ng/kg required dose reductions because of constitutional symptoms and liver enzyme abnormalities, whereas all but one patient receiving 500 ng/kg required dose reductions and 4 had DLT. The IFN-γ levels in patients treated at the 300- and 500-ng/kg dose levels increased to levels 25 and 100 times their baseline value after IL-12 administration.
Sixty-nine percent of the patients treated with IL-12 and rituximab had an objective response to this therapy. In view of the fact that the standardized response criteria described by Cheson et al31used in this study are less stringent than criteria used in other studies evaluating rituximab, and the study included a heterogeneous population of patients, the high response rate seen in this study should be interpreted with caution. Responses seen in this study, however, were seen in patients with both low-grade and aggressive lymphomas. Heavily pretreated patients as well as those who had relapsed after transplantation responded to this therapy. Responses were also seen in patients with mantle cell lymphoma and diffuse small lymphocytic lymphoma. Although this study included a heterogeneous population of patients with lymphoma, many of the patients had poor prognostic features of their disease. Of the 29 responding patients, 11 had a complete response and the median duration of response is more than 8 months. The majority of the complete responses were seen at dose levels of IL-12 of 300 ng/kg or higher. Due to the increased complete response rate, the significant induction of IFN-γ and IP-10 and the acceptable toxicity, 300 ng/kg IL-12 in combination with rituximab was felt to be the optimal dose.
Although response to therapy was not a primary end point of this study, the response to the combination of IL-12 plus rituximab in this group of patients is encouraging. IL-12 has shown activity as a single agent in other malignancies37-41 and appears to have activity in lymphoma as a single agent in an ongoing study at M. D. Anderson Cancer Center (Houston, TX). Preliminary data on the first 11 evaluable patients with relapsed indolent NHL treated with single-agent IL-12 show an overall response rate of 27% (Anas Younes, personal written communication, July 2001). Response rates with rituximab alone in NHL have ranged between 30% and 73%, depending on the histology and extent of the disease.18,20,21,23-27,43 44Although the response rate to the combination of IL-12 and rituximab appears promising, confirmation of a possible synergistic effect between these 2 agents will require further study.
This study has demonstrated that IL-12 in combination with rituximab is a well- tolerated and biologically active combination, and the recommended dose of IL-12 for use in combination with standard doses of rituximab is 300 ng/kg starting on day 2. However, this combination requires further study to demonstrate its superiority when compared to rituximab alone.
The authors wish to thank Janell Hovey and Susan Steinmetz for data management, Michelle Daiss for protocol management, and Britta Jasperson for assistance with data analysis.
Supported in part by National Institutes of Health grants UO1 CA69912 and CA15083. Presented in part at the American Society of Hematology 42nd annual meeting, San Francisco, CA, December 2000.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Stephen M. Ansell, Division of Hematology, Mayo Clinic, 200 First St SW, Rochester, MN 55905; e-mail:ansell.stephen@mayo.edu.