Thrombin is an important agonist for platelet activation and plays a major role in hemostasis and thrombosis. Thrombin activates platelets mainly through protease-activated receptor 1 (PAR1), PAR4, and glycoprotein Ib. Because adenosine diphosphate and thromboxane A2 have been shown to cause platelet aggregation by concomitant signaling through Gq and Gipathways, we investigated whether coactivation of Gq and Gi signaling pathways is the general mechanism by which PAR1 and PAR4 agonists also activate platelet fibrinogen receptor (αIIbβ3). A PAR1-activating peptide, SFLLRN, and PAR4-activating peptides GYPGKF and AYPGKF, caused inhibition of stimulated adenylyl cyclase in human platelets but not in the presence of either Ro 31-8220, a protein kinase C selective inhibitor that abolishes secretion, or AR-C66096, a P2Y12 receptor–selective antagonist; α-thrombin–induced inhibition of adenylyl cyclase was also blocked by Ro 31-8220 or AR-C66096. In platelets from a P2Y12 receptor–defective patient, α-thrombin, SFLLRN, and GYPGKF also failed to inhibit adenylyl cyclase. In platelets from mice lacking the P2Y12 receptor, neither α-thrombin nor AYPGKF caused inhibition of adenylyl cyclase. Furthermore, AR-C66096 caused a rightward shift of human platelet aggregation induced by the lower concentrations of α-thrombin and AYPGKF but had no effect at higher concentrations. Similar results were obtained with platelets from mice deficient in the P2Y12. We conclude that (1)thrombin- and thrombin receptor-activating peptide–induced inhibition of adenylyl cyclase in platelets depends exclusively on secreted adenosine diphosphate that stimulates Gi signaling pathways and (2) thrombin and thrombin receptor-activating peptides cause platelet aggregation independently of Gi signaling.
Introduction
Platelet activation plays a major role in hemostasis and thrombosis. Several agonists, including adenosine diphosphate (ADP), thrombin, and thromboxane A2, can activate platelets.1 These agonists cause platelets to change their shape, to aggregate, and to release the contents of granules. Thrombin, generated at the site of vascular damage by extrinsic and intrinsic coagulation cascades, is an important agonist for platelet activation. Thrombin mediates its cellular effects primarily through a family of G protein–coupled protease-activated receptors (PARs). These receptors are activated by a unique mechanism in which the protease creates a new extracellular amino-terminus that functions as a tethered ligand, resulting in intramolecular activation.2,3 Three of the 4 known PARs, PAR1, PAR3, and PAR4, are activated by thrombin. PAR1 is detected in human platelets and has a major role in activation of human platelets by thrombin, but it plays no role in mouse platelets.4 PAR2 functions as a receptor for trypsin but not for thrombin.5 PAR3 is necessary for mouse platelets to be activated by lower concentrations of thrombin.6 PAR3 functions as a cofactor for the activation of PAR4 by thrombin in mouse platelets.7 There is another receptor, PAR4, which appears to function in both mouse and human platelets.4,8 PAR4 also mediates platelet responses to cathepsin G that might be necessary for neutrophil-dependent platelet activation.9 PAR1 and PAR4 are necessary for normal activation of human platelets by thrombin, and PAR3 and PAR4 mediate normal responsiveness to thrombin in mouse platelets. Thus, a dual thrombin receptor system operates for platelet activation in both human and mouse platelets.10,11 The activation of human platelets by thrombin is mediated predominantly by PAR1, and PAR4-induced platelet responses are less pronounced.12PAR1 is a high-affinity receptor for platelet activation at low concentrations of thrombin, whereas PAR4 is a low-affinity receptor that mediates thrombin signaling at high concentrations. In addition to the PARs, thrombin has been shown to activate platelets through cleavage of glycoprotein V (GPV) and binding to GPIb.13 14
Specific agonist hexapeptides have been designed for the thrombin-independent activation of PAR1 and PAR4. PAR1-activating peptide (PAR1AP), SFLLRN, selectively activates PAR1 in human but not mouse platelets independently of thrombin and receptor cleavage and induces human platelet aggregation and degranulation.15,16The activating peptides GYPGQV and GYPGKF, corresponding to the human and mouse PAR4 tethered ligands, respectively, induce aggregation of human platelets.11 Interestingly, the activating peptide GYPGKF is more effective in inducing human platelet aggregation than is GYPGQV.12 Recently, AYPGKF was shown to be a selective and more potent PAR4AP.17 Although these receptors have been identified, the molecular events leading to PAR agonist–induced platelet aggregation are unknown and the signaling events triggered by the activating peptides remain to be elucidated.
ADP-induced platelet aggregation requires coactivation of both the P2Y1 receptor (platelet ADP receptor coupled to stimulation of phospholipase C) and P2Y12 receptor (platelet ADP receptor coupled to inhibition of adenylyl cyclase) that couple to Gq (heterotrimeric guanosine triphosphate–binding protein that stimulates phospholipase C) and Gi (heterotrimeric guanosine triphosphate–binding protein that inhibits adenylyl cyclase), respectively, and concomitant signaling from Gq and Gi is sufficient and necessary for ADP-induced platelet aggregation.18-20 Thromboxane A2 also causes platelet aggregation by coactivation of Gq and Gi pathways.21 Thrombin and thrombin receptor–activating peptides have been shown to activate both Gq and Gi pathways.22-28 The signal transduction mechanisms of thrombin-induced platelet aggregation are less clear, and it has not yet been defined whether coactivation of Gq and Gi signaling pathways is the general mechanism by which all agonists activate platelet fibrinogen receptor.
Characterization of the signal transduction pathways that mediate thrombin's action on platelets is necessary for understanding hemostasis and thrombosis. To determine the mechanisms of PAR1 and PAR4 in human platelet activation, we performed multiple approaches in human platelets, including analysis of a P2Y12 receptor–defective patient's platelets, utilizing selective P2 receptor antagonists, blocking granule secretion, and the use of P2Y12 receptor–deficient mouse platelets. We report here that thrombin and thrombin receptor–activating peptides cause Gi stimulation through P2Y12 receptor activation by secreted ADP, and that they cause platelet aggregation independently of Gi stimulation.
Materials and methods
Reagents
Apyrase (type V), fibrinogen (type I), and bovine serum albumin (fraction V) were purchased from Sigma (St Louis, MO). Hexapeptides SFLLRN, GYPGKF, and AYPGKF were custom synthesized at Research Genetics (Huntsville, AL). Luciferin-luciferase reagent was purchased from Chrono-Log (Havertown, PA). Imipramine was from ICN (Costa Mesa, CA). Prostaglandin E1 (PGE1) and Ro 31-8220 (bisindolylmaleimide) were from Biomol Research Laboratories (Plymouth Meeting, PA). AR-C66096 and AR-C69931MX were gifts from AstraZeneca (Loughborough, United Kingdom). Yohimbine was from Research Biologicals International (Natick, MA). All other reagents were reagent grade, and deionized water was used throughout.
Preparation of human platelets
Human blood was collected from a pool of healthy volunteers or a patient, after informed consent, in a one-sixth volume of acid citrate dextrose (ACD; 2.5 g sodium citrate, 1.5 g citric acid, and 2.0 g glucose in 100 mL H2O). Platelet-rich plasma (PRP) was prepared by centrifugation of citrated blood at 230g for 20 minutes at room temperature. Acetylsalicylic acid was added to PRP to a final concentration of 1 mM, and the preparation was incubated for 1 hour at 37°C followed by centrifugation at 1000g for 10 minutes at room temperature. The platelet pellet was resuspended in Tyrode buffer (pH 7.4) containing 138 mM NaCl, 2.7 mM KCl, 2 mM MgCl2, 0.42 mM NaH2PO4, 5 mM glucose, 10 mM HEPES, 0.2% bovine serum albumin, and 0.05 U/mL apyrase. This low concentration of apyrase is not enough to block responses to ADP, but will prolong the responsiveness of platelets to ADP by preventing desensitization of the P2Y receptors. These conditions have been standardized in the laboratory. The platelet count was adjusted to 2 × 108cells per milliliter.
Preparation of washed mouse platelets
Studies using P2Y12-null mice were performed under protocols that were approved by the Schering-Plough Animal Use and Care Committee. P2Y12-null mice were generated using standard techniques.29 In-house bred 129Sv × C57BL/6 F2 mice derived from the same 129 Sv parent ES cell line as the P2Y12-null mice that were used as controls. These wild-type mice do not differ genetically from P2Y12-null mice except at the P2Y12-targeted locus. Blood was collected from the vena cava of anesthetized mice into syringes containing ACD as anticoagulant. Red blood cells were removed by centrifugation at 250g for 15 minutes. PRP was recovered, and platelets were pelleted at 1500g for 5 minutes.
Human platelet aggregation
Agonist-induced platelet aggregation was measured using a lumiaggregometer (Chrono-Log) at 37°C with stirring (900 rpm). A 0.5-mL sample of aspirin-treated washed platelets was stimulated with agonist, and change in light transmission was measured. In some experiments, Ro 31-8220 (10 μM), a protein kinase C (PKC) inhibitor, was added and incubated for 5 minutes at 37°C with stirring before agonist stimulation to block secretion. The extent of platelet aggregation was measured 210 seconds after the addition of the agonist, and the maximum extent of aggregation was taken as 100%.
Mouse platelet aggregation
The mouse platelet aggregations were performed using washed platelets from the P2Y12 receptor–deficient mice and the control wild-type mice. The aggregations were performed in 96-well plates at room temperature by the procedure described by Bednar et al.30 Platelets were resuspended in modified Tyrode buffer at 2 × 108 platelets per milliliter. Aggregation was initiated by adding agonist, and the plates were rotated at setting 6 on a microtiter plate shaker (Lab-Line Instruments, Melrose, IL). Optical density readings were obtained at 30-second to 1-minute intervals at 405 nm using a microtiter plate reader (Molecular Devices, Sunnyvale, CA).
Platelet secretion
Platelet secretion was determined by measuring the release of [3H]5-HT. PRP was incubated with [3H]5-HT (1 μCi/mL [37 kBq/mL]) and 1 mM acetylsalicylic acid for 1 hour at 37°C. The PRP was centrifuged, and the platelet pellet was resuspended in HEPES-buffered Tyrode solution containing imipramine at a final concentration of 1 μM to prevent reuptake of secreted [3H]5-HT. Platelet secretion was performed in the lumiaggregometer at 37°C with stirring. The activation of labeled [3H]5-HT platelets was stopped 2 minutes after adding agonist with the addition of stopping solution containing formaldehyde and ethylenediaminetetraacetic acid according to the method previously described.31 Samples were collected and centrifuged at 5000g for 1 minute, and the supernatant was collected to measure the radioactivity using a Wallac 1409 liquid scintillation counter (Gaithersburg, MD).
Platelet secretion was also independently determined by measuring the release of adenosine triphosphate (ATP) by adding luciferin-luciferase reagent to the platelet sample after aggregation is completed. Platelet secretion was performed in the lumiaggregometer at 37°C with stirring, and step change in the luminescence record indicated the amount of ATP released during aggregation.
Measurement of cAMP in human platelets
PRP from normal human blood was incubated with 2 μCi/mL [74 kBq/mL] [3H]adenine and aspirin (1 mM) for 1 hour at 37°C followed by centrifugation at 1000 × g for 10 minutes at room temperature. A 0.5-mL aliquot of washed platelets was stimulated with the following reagents: PGE1, AR-C66096, yohimbine, or Ro 31-8220 and with SFLLRN or GYPGKF. Three and one-half minutes later, the reaction was stopped by addition of 1 M HCl, and 4000 dpm (disintegrations per minute) [14C]cyclic adenosine monophosphate (cAMP) was added as the recovery standard. The level of cAMP was determined as described previously.18 The results were expressed as a percentage of inhibition, taking PGE1-induced cAMP levels as 100%. In the study involving a P2Y12-defective patient, cAMP levels were determined by radioimmunoassay kit from Amersham (Piscataway, NJ).
Measurement of mouse platelet adenylyl cyclase activity
Production of cAMP in platelets was quantitated using the Adenylyl Cyclase Activation FlashPlate Assay Kit (NEN-Life Sciences, Boston, MA) following the manufacturer's instructions. The 100-μL reaction mix contained 50 μL washed platelets (1 × 108/mL) in Stimulation Buffer (NEN-Life Sciences) containing the phosphodiesterase inhibitor isobutylmethylxanthine (Sigma). Receptor agonists and PGE1 were dissolved in Dulbecco phosphate-buffered saline (Gibco, Gaithersburg, MD) supplemented with 0.2% bovine serum albumin, 1 g/L glucose, and 10 mM MgCl2 and were added at the final concentrations indicated in the text and figures. We observed no significant differences in adenylyl cyclase responses between male and female mice.
Results
Evaluation of Gi signaling by PAR1AP and PAR4AP
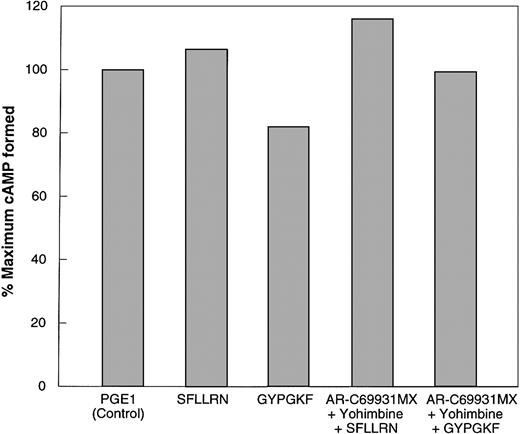
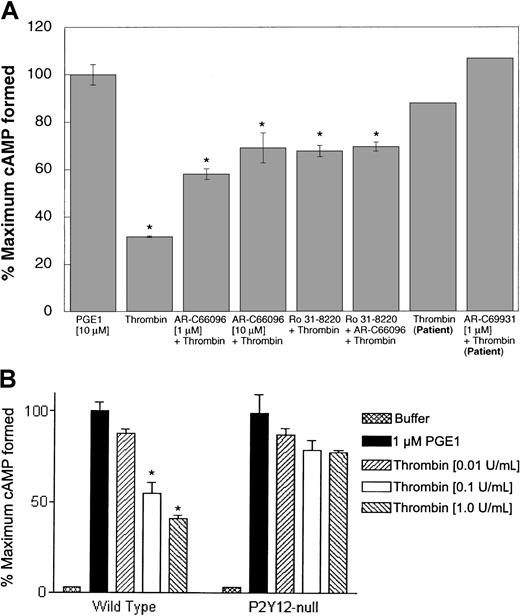
PAR1 and PAR4, similar to thromboxane A2,21 can cause release of dense granule contents, and the ADP thus released could activate the Gipathways through activation of the P2Y12 receptor.11,15,16To determine whether PAR1 and PAR4 receptors can couple to Gi signaling pathways independently of secreted ADP, we utilized multiple complimentary approaches. The first approach was to block secretion, thereby eliminating the contribution of secreted ADP to Gi stimulation. PKC has been shown to play a major role in the induction of platelet secretion.32,33 To assess the role of secreted ADP on cAMP levels upon stimulation of platelets with SFLLRN and GYPGKF, we used Ro 31-8220, a selective inhibitor of PKC isoforms.34-36 The ability of Ro 31-8220 to block agonist-induced dense granule secretion was confirmed by [3H]5-HT release and by the measurement of ATP release using luciferin-luciferase reagent. SFLLRN and GYPGKF caused a decrease in the PGE1-stimulated cAMP levels in the absence of Ro 31-8220 (Figure 1). However, in the presence of Ro 31-8220, neither SFLLRN nor GYPGKF caused inhibition of stimulated adenylyl cyclase (Figure 1). This indicated that the stimulation of PAR1 by SFLLRN and stimulation of PAR4 by GYPGKF does not activate Gi signaling pathways in the absence of secretion.
Effect of Ro 31-8220 or receptor-selective antagonists on PAR1- and PAR4-induced inhibition of adenylyl cyclase in normal human platelets.
Effect of PAR1 and PAR4 agonists on platelet adenylyl cyclase was determined as described in “Materials and methods.” Data are expressed as percentage of total [3H]cAMP. The data are normalized to the level of cAMP in response to 10 μM PGE1 (taken as 100%) or to the level of PGE1-stimulated cAMP in the presence of 10 μM Ro 31-8220. Ro 31-8220 or dimethyl sulfoxide (control) was added to aspirin-treated platelets and incubated for 5 minutes at 37°C with stirring before the addition of either PGE1 alone or PGE1 with agonists; 1 μM AR-C66096 was added 30 seconds prior to agonists, SFLLRN (10 μM) or GYPGKF (700 μM). Each data point is the mean ± SE of at least 3 experiments.
Effect of Ro 31-8220 or receptor-selective antagonists on PAR1- and PAR4-induced inhibition of adenylyl cyclase in normal human platelets.
Effect of PAR1 and PAR4 agonists on platelet adenylyl cyclase was determined as described in “Materials and methods.” Data are expressed as percentage of total [3H]cAMP. The data are normalized to the level of cAMP in response to 10 μM PGE1 (taken as 100%) or to the level of PGE1-stimulated cAMP in the presence of 10 μM Ro 31-8220. Ro 31-8220 or dimethyl sulfoxide (control) was added to aspirin-treated platelets and incubated for 5 minutes at 37°C with stirring before the addition of either PGE1 alone or PGE1 with agonists; 1 μM AR-C66096 was added 30 seconds prior to agonists, SFLLRN (10 μM) or GYPGKF (700 μM). Each data point is the mean ± SE of at least 3 experiments.
Platelet dense granules contain ADP, which inhibits adenylyl cyclase and reduces levels of cAMP following activation of P2Y12.1 Hence, the second approach utilized AR-C66096, a selective antagonist at the Gi-coupled P2Y12 receptor, to eliminate the contribution of signaling from this Gi-coupled receptor to PAR-mediated inhibition of adenylyl cyclase. SFLLRN- and GYPGKF-induced inhibition of PGE1stimulated adenylyl cyclase inhibition, which was blocked by AR-C66096 (Figure 1), suggesting that reversal of cAMP formation is due to the released ADP and that in the absence of secreted ADP, PAR stimulation by SFLLRN or GYPGKF does not couple to Gipathways. It should be noted that α granules can release small quantities of chemokines that can activate Gipathways37 and the P2Y12 receptor antagonist would not affect this phenomenon. The residual inhibition of adenylyl cyclase seen in Figure 1 could be due to Gi stimulation by secreted chemokines.
A patient was described with abnormal responses to ADP due to defective signaling from the P2Y12 receptor.38 In this patient, ADP-induced inhibition of adenylyl cyclase is abolished. To confirm our results obtained with the inhibitors and receptor antagonists, an independent study was performed using the platelets from this P2Y12 receptor–defective patient. The third approach evaluated the effect of SFLLRN or GYPGKF on PGE1-stimulated cAMP levels in platelets from this patient, in the absence and presence of the receptor antagonists AR-C69931MX and yohimbine, an α2A receptor antagonist. In the presence or absence of these antagonists, SFLLRN and GYPGKF failed to cause inhibition of PGE1-stimulated adenylyl cyclase in platelets from the patient with a defective P2Y12 receptor (Figure2), confirming that SFLLRN- and GYPGKF-induced Gi stimulation depends on P2Y12 receptor stimulation by secreted ADP.
Effect of receptor-selective antagonists on SFLLRN- and GYPGKF-induced inhibition of adenylyl cyclase in platelets from a P2Y12-defective patient.
Effects of PAR1 and PAR4 on cAMP accumulation were measured in P2Y12-deficient human platelets. Conditions were identical to those used in Figure 1 except that P2Y12-deficient human platelets were used along with AR-C69931MX and 10 μM yohimbine. The experiment was repeated twice using platelets from the same patient.
Effect of receptor-selective antagonists on SFLLRN- and GYPGKF-induced inhibition of adenylyl cyclase in platelets from a P2Y12-defective patient.
Effects of PAR1 and PAR4 on cAMP accumulation were measured in P2Y12-deficient human platelets. Conditions were identical to those used in Figure 1 except that P2Y12-deficient human platelets were used along with AR-C69931MX and 10 μM yohimbine. The experiment was repeated twice using platelets from the same patient.
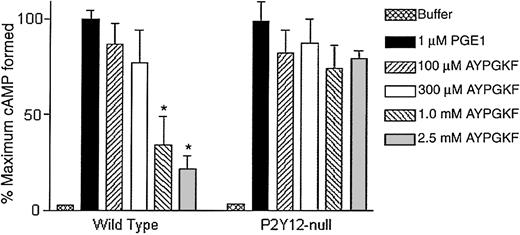
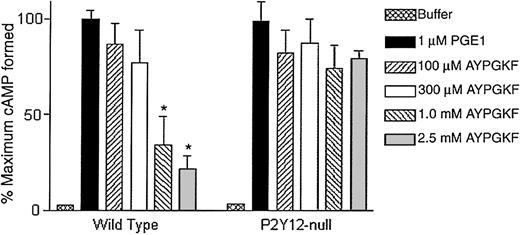
Recently, P2Y12 receptor–deficient mice have been generated and shown to lack Gi stimulation by ADP.29 In this final approach, we used platelets from these mice to evaluate the ability of AYPGKF to stimulate Gi pathways. Mouse platelets do not express PAR1, and hence we did not use SFLLRN. As shown in Figure 3, AYPGKF, in a concentration-dependent manner, caused inhibition of adenylyl cyclase in wild-type mouse platelets but failed to stimulate Gipathways in platelets from the P2Y12 knock-out mice. These data indicate that the PAR1AP SFLLRN and PAR4APs AYPGKF or GYPGKF depend on secreted ADP to stimulate Gi signaling pathways.
AYPGKF-induced inhibition of adenylyl cyclase in P2Y12-deficient mouse platelets.
Effect of varying concentrations of AYPGKF on PGE1-stimulated cAMP levels in platelets from mice deficient in the P2Y12 receptor were determined and compared with wild-type mouse platelets. The data are derived from at least 3 independent experiments. Each data point is the mean ± SE of 3 experiments. *P < .05 by Student t test when compared with the value of PGE1.
AYPGKF-induced inhibition of adenylyl cyclase in P2Y12-deficient mouse platelets.
Effect of varying concentrations of AYPGKF on PGE1-stimulated cAMP levels in platelets from mice deficient in the P2Y12 receptor were determined and compared with wild-type mouse platelets. The data are derived from at least 3 independent experiments. Each data point is the mean ± SE of 3 experiments. *P < .05 by Student t test when compared with the value of PGE1.
Role of secreted ADP in thrombin-induced Gistimulation
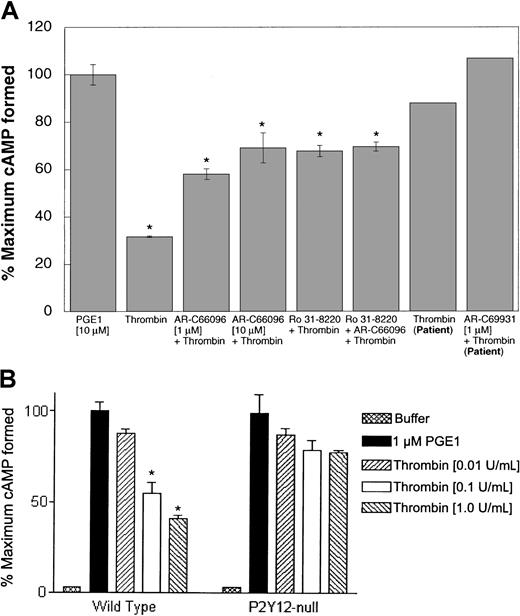
The hexapeptides derived from PAR sequences may not completely mimic the effects of thrombin on PAR1 and PAR4 receptors. For example, YFLLRN stimulates PAR1 receptor but, unlike SFLLRN, does not increase intracellular calcium.39,40 In addition to the PARs, thrombin has recently been shown to signal through GPIb upon cleavage of GPV from the GPIb-V complex.13,14 Furthermore, PAR-activating peptides are excellent research tools, but thrombin is the physiologic agonist. Hence, we evaluated the role of secreted ADP in thrombin-induced Gi stimulation by multiple approaches. Ro 31-8220, which blocks dense granule release, inhibited α-thrombin–induced inhibition of adenylyl cyclase in human platelets (Figure 4A). AR-C66096 (1 μM) also inhibited the Gi stimulation by thrombin in human platelets, and higher concentrations of AR-C66096 inhibited more (Figure 4A). Moreover, thrombin-induced inhibition of adenylyl cyclase was nearly abolished in platelets from the P2Y12 receptor–defective patient, and AR-C69931MX further blocked this effect (Figure 4A). In addition, thrombin-induced inhibition of adenylyl cyclase was almost completely abolished in mouse platelets lacking the P2Y12 receptor (Figure 4B). Thrombin-induced inhibition of adenylyl cyclase in mouse platelets was recently shown to be blocked by a P2Y12 receptor antagonist.41 Because platelets express PAR1, PAR3, PAR4, and GPIb-V-IX complex, these data indicate that thrombin activation of Gi signaling through these thrombin receptors depends primarily on stimulation of the P2Y12 receptor by secreted ADP.
Role of secreted ADP in thrombin-induced Gisignaling.
Effect of α-thrombin (0.5 U/mL) on 10 μM PGE1-stimulated cAMP levels was determined (A) in the presence or absence of Ro 31-8220 (10 μM) and/or AR-C compound in human platelets (as indicated) in normal platelets or in P2Y12-deficient patient platelets (as marked) and (B) in platelets from mice lacking the P2Y12 receptor and wild-type mice. Each data point is the mean ± SE of at least 3 experiments. The experiments with human platelets were repeated at least 3 times using platelets from different donors. *P < .05 by Student t test when compared with the value of PGE1. The experiments with the patient platelets were carried out only once due to problems with the patient's health.
Role of secreted ADP in thrombin-induced Gisignaling.
Effect of α-thrombin (0.5 U/mL) on 10 μM PGE1-stimulated cAMP levels was determined (A) in the presence or absence of Ro 31-8220 (10 μM) and/or AR-C compound in human platelets (as indicated) in normal platelets or in P2Y12-deficient patient platelets (as marked) and (B) in platelets from mice lacking the P2Y12 receptor and wild-type mice. Each data point is the mean ± SE of at least 3 experiments. The experiments with human platelets were repeated at least 3 times using platelets from different donors. *P < .05 by Student t test when compared with the value of PGE1. The experiments with the patient platelets were carried out only once due to problems with the patient's health.
Concentration dependence of thrombin-, SFLLRN-, or PAR4AP-induced platelet aggregation and secretion
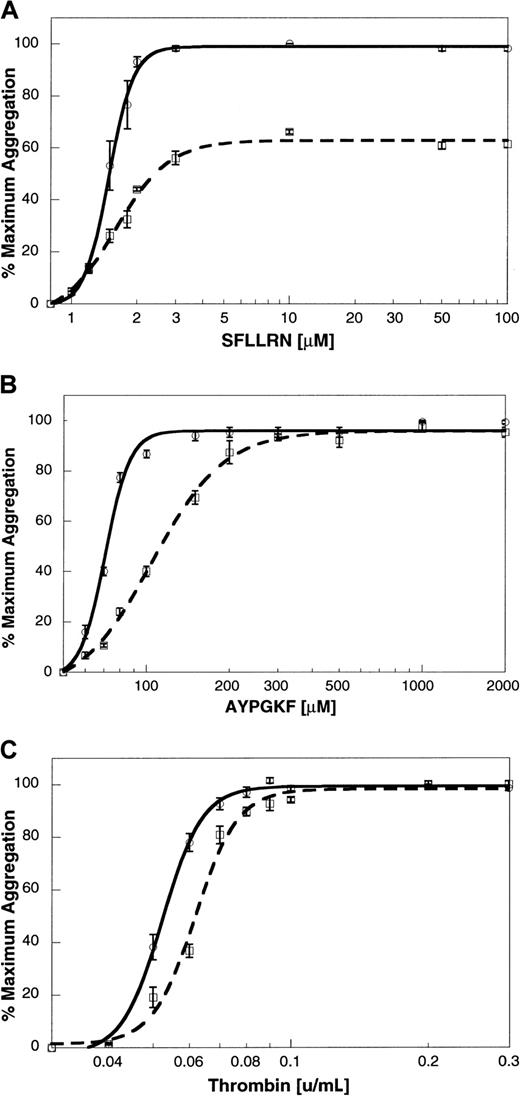
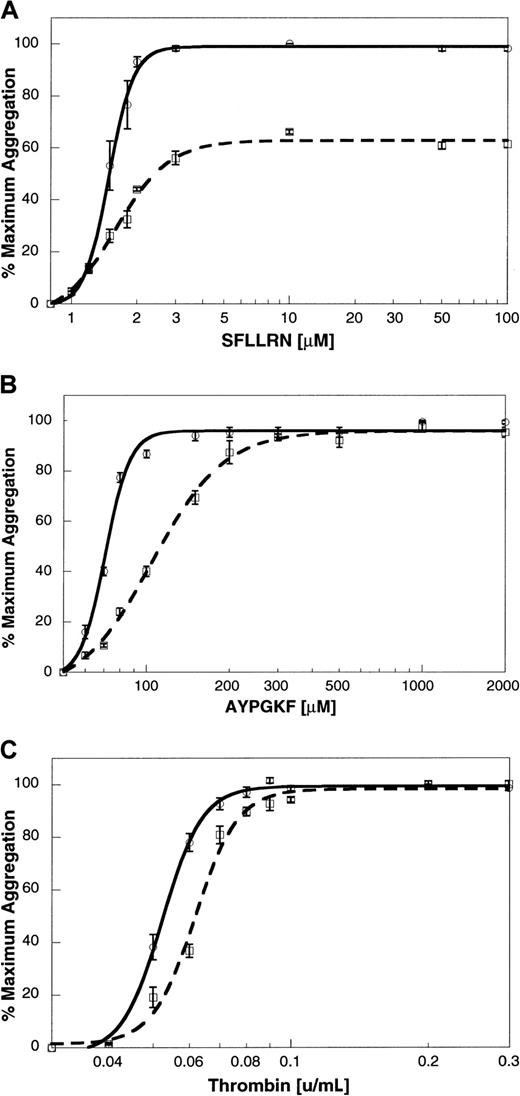
To determine the relationship between platelet aggregation and secretion in response to thrombin, SFLLRN, AYPGKF, and GYPGKF, we exposed platelets to different concentrations of the agonists. The extent of aggregation at 210 seconds after the addition of agonist was measured, and the maximum extent was taken as 100%. Maximal aggregation was observed at concentrations above 2 μM SFLLRN (Figure5A), 200 μM AYPGKF (Figure 5B), 500 μM GYPGKF (not shown), or 0.08 U/mL thrombin (Figure 5C). The dense granule secretion was measured by [3H]5-HT release, and the maximum secretion was taken as 100%. Maximal secretion was observed at concentrations above 3 μM SFLLRN (Figure 5A), 1000 μM AYPGKF (Figure 5B), 700 μM GYPGKF (not shown), or 0.3 U/mL thrombin (Figure 5C).
Concentration response curves for agonist-induced platelet aggregation and secretion.
Platelet aggregation was measured as described previously. Aspirin-treated [3H]serotonin-loaded platelets were stimulated with varying concentrations of (A) SFLLRN, (B) AYPGKF, or (C) thrombin. Platelet aggregation (solid line) and secretion (broken line) were measured as described in “Materials and methods.” The maximum response was taken as 100%, and the remaining data were normalized to this value. The data (mean ± SE) are from at least 3 independent experiments using platelets from different donors.
Concentration response curves for agonist-induced platelet aggregation and secretion.
Platelet aggregation was measured as described previously. Aspirin-treated [3H]serotonin-loaded platelets were stimulated with varying concentrations of (A) SFLLRN, (B) AYPGKF, or (C) thrombin. Platelet aggregation (solid line) and secretion (broken line) were measured as described in “Materials and methods.” The maximum response was taken as 100%, and the remaining data were normalized to this value. The data (mean ± SE) are from at least 3 independent experiments using platelets from different donors.
Effect of receptor-selective antagonists on thrombin-, SFLLRN-, or AYPGKF-induced platelet aggregation
To elucidate the role of Gi-coupled receptors in SFLLRN- and PAR4AP-induced platelet aggregation, the effect of Gi-coupled receptor antagonists AR-C66096 and yohimbine was tested. Yohimbine (10 μM) had no effect on SFLLRN- or GYPGKF-induced aggregation (not shown). AR-C66096 (1 μM) inhibited platelet aggregation induced by SFLLRN (Figure6A). This inhibition reflects the conversion of irreversible platelet aggregation to reversible platelet aggregation by AR-C66096, as reported previously.42However, AR-C66096 inhibited the platelet aggregation induced by lower concentrations of AYPGKF (Figure 6B) or thrombin (Figure 6C), whereas this P2Y12 receptor antagonist had no effect at higher concentrations of AYPGKF or thrombin. These data indicate that thrombin, SFLLRN, or AYPGKF can cause platelet fibrinogen receptor activation without a role for secreted ADP.
Effect of the P2Y12 receptor antagonist, AR-C66096, on agonist-induced platelet aggregation.
The platelet aggregation induced by varying concentrations of (A) SFLLRN, (B) AYPGKF, or (C) thrombin in the absence (solid line) or presence (broken line) of 1 μM AR-C66096 was measured as described in “Materials and methods.” The data (mean ± SE) are obtained from at least 3 independent experiments using platelets from different donors.
Effect of the P2Y12 receptor antagonist, AR-C66096, on agonist-induced platelet aggregation.
The platelet aggregation induced by varying concentrations of (A) SFLLRN, (B) AYPGKF, or (C) thrombin in the absence (solid line) or presence (broken line) of 1 μM AR-C66096 was measured as described in “Materials and methods.” The data (mean ± SE) are obtained from at least 3 independent experiments using platelets from different donors.
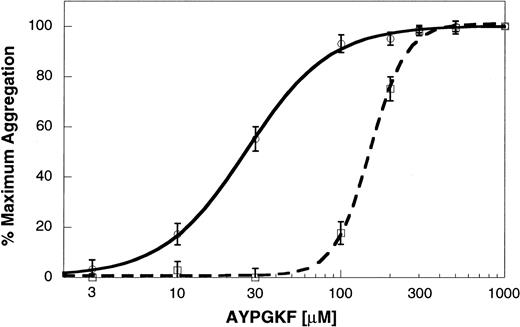
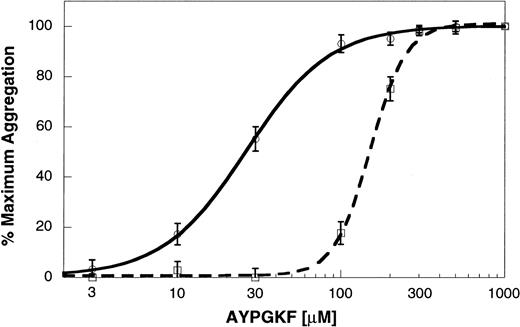
AYPGKF-induced platelet aggregation in P2Y12 receptor–deficient mouse platelets
To confirm the role of secreted ADP and the P2Y12 receptor in PAR4AP-induced platelet activation, we used platelets from mice deficient in the P2Y12 receptor. In wild-type littermates, AYPGKF caused concentration-dependent platelet aggregation. In the P2Y12-deficient mouse platelets, aggregation by lower concentrations of AYPGKF was dramatically inhibited (Figure7). However, aggregation induced by higher concentrations of AYPGKF in the P2Y12-deficient mouse platelets appeared similar to the aggregation in wild-type littermates. Similarly, the concentration-response curve in the P2Y12 receptor–deficient mouse platelets was shifted to the right at the lower concentrations of thrombin but was similar to wild-type littermates with higher thrombin concentrations.29 These results confirm that PAR4-mediated platelet aggregation does not depend on the ability of secreted ADP to activate the P2Y12 receptor.
AYPGKF-induced platelet aggregation in the P2Y12 receptor–deficient mouse platelets.
Platelet aggregation in the washed mouse platelets was determined as described in “Materials and methods.” AYPGKF-induced platelet aggregation was determined in platelets from P2Y12 receptor–deficient mice (dashed line) or wild-type littermates (solid line) using varying concentrations of the agonist. The maximum response (210 seconds after the addition of the agonist) was taken as 100%, and the remaining data were normalized to this value. The data (mean ± SE) are obtained from at least 3 independent experiments.
AYPGKF-induced platelet aggregation in the P2Y12 receptor–deficient mouse platelets.
Platelet aggregation in the washed mouse platelets was determined as described in “Materials and methods.” AYPGKF-induced platelet aggregation was determined in platelets from P2Y12 receptor–deficient mice (dashed line) or wild-type littermates (solid line) using varying concentrations of the agonist. The maximum response (210 seconds after the addition of the agonist) was taken as 100%, and the remaining data were normalized to this value. The data (mean ± SE) are obtained from at least 3 independent experiments.
Discussion
Thrombin is the most powerful activator of platelets, and characterization of the platelet receptors for thrombin is important for understanding thrombosis and hemostasis. Although it is known that cellular response to thrombin in platelets is mediated by PARs, the molecular mechanisms involved in PAR agonist–induced platelet aggregation are yet to be clearly elucidated. It may be possible that platelet activation induced by PAR agonists, similar to ADP and thromboxane A2, requires coactivation of Gq and Gi signaling pathways.2,3,26,28 Previous studies suggested that thrombin and thrombin receptor–activating peptides cause both phosphoinositide hydrolysis and inhibition of adenylyl cyclase via at least 2 G proteins, Gq and Gi.22-28 Benka et al43 demonstrated that thrombin receptor is coupled to a member of the Gqfamily by using anti-Gq antibodies, which inhibited thrombin receptor activation peptide–induced platelet membrane guanosine triphosphatase activation. It also has been shown that platelets from Gq knock-out mice fail to aggregate or to release their granule contents in response to thrombin.44On the other hand, thrombin has been shown to inhibit the adenylyl cyclase activity, which was inhibited by pertussis toxin.22,23,26 Giesberts et al26 also suggested that complete inhibition of cAMP requires activation of both PKC and Gi. It has also been shown that thrombin can couple to Gi in cells stably expressing the PAR1 receptor but not the PAR4 receptor.17 Even though previous studies have provided the evidence of involvement of each pathway, they have not identified the role of each pathway in thrombin-induced platelet activation. We utilized several complementary approaches to characterize the molecular mechanism of PAR1- and PAR4-induced platelet activation. In particular, we focused on the role of secretion and Gi pathways in platelet fibrinogen receptor activation. First, we blocked granule secretion using a specific PKC inhibitor. Second, we used receptor subtype–selective antagonists to eliminate the positive feedback from secreted ADP and epinephrine. Third, we used platelets from a P2Y12 receptor–defective patient. Finally, we utilized the P2Y12 knock-out mouse platelets to evaluate the role of the P2Y12 receptor in thrombin-induced platelet activation. In the last 3 approaches, wherein ADP secretion is preserved but the signaling through the P2Y12 receptor is blocked, activation of the P2Y1 receptor by secreted ADP would still contribute to the Gq-mediated functional responses.
Several groups have demonstrated that PAR1, upon activation by its activating peptide, couples to Gi, leading to inhibition of adenylyl cyclase.26-28,45 To determine whether PAR1– and PAR4–activating peptides can couple to Gi signaling pathways independently of secretion, we have used Ro 31-8220 and receptor-selective antagonists. PKC is essential for platelet secretion, and the PKC inhibitor Ro 31-8220 blocks the secretion of ADP in response to PAR1 and PAR4 agonists.34-36 In our hands, Ro 31-8220 completely blocks secretion (not shown). SFLLRN- or AYPGKF-induced adenylyl cyclase inhibition was dramatically blocked by Ro 31-8220, suggesting that these agonists depend on secreted ADP in Gi stimulation. Previously, Giesberts et al26 showed that a low concentration of thrombin depends on PKC and Gi to cause adenylyl cyclase inhibition. It is conceivable that, in their study, inhibition of PKC blocks secreted ADP, which can stimulate Gi through the P2Y12 receptor.
Blockade of PAR1AP- and PAR4AP-induced adenylyl cyclase inhibition by the P2Y12 receptor–selective antagonist AR-C66096 indicates that PAR receptor–activating peptides cause Gi stimulation through the secreted ADP. Thus, PAR1 and PAR4 fail to directly couple to Gi and inhibit cAMP formation in intact platelets (Figures1 and 2) when activated by receptor-activating peptides. Using a complementary approach involving platelets from a P2Y12 receptor–defective patient,38 we also show that PAR1AP and PAR4AP failed to inhibit adenylyl cyclase. Finally, AYPGKF also failed to cause Gi stimulation in P2Y12 receptor–deficient mouse platelets. Hence, our studies through complementary approaches demonstrate that SFLLRN, through activation of PAR1, and AYPGKF and GYPGKF, through activation of PAR4, cause Gi stimulation in platelets through secreted ADP.
Similar to PAR-activating peptides, thrombin-induced inhibition of adenylyl cyclase in human platelets was blocked when secretion was blocked by Ro 31-8220 or in the presence of AR-C66096 (Figure 4A). In the platelets from the patient lacking the P2Y12 receptor, thrombin failed to cause significant inhibition of adenylyl cyclase. Thus, thrombin cannot cause Gi stimulation in human platelets independently of secreted ADP. Because human platelets express PAR1, PAR3, PAR4, and GPIb-V-IX complex, it can be concluded that thrombin stimulation of these receptors does not activate the Gipathway. These data contradict previous studies demonstrating Gi stimulation by thrombin in human platelet membranes wherein secretion of ADP does not occur.22,23,46 Even though thrombin has been shown to inhibit cAMP formation in HEL cell or platelet membrane preparations where secretion would not be involved, thrombin increases cAMP levels in intact HEL cells.25These studies represent a difference of thrombin responses between intact platelets and membrane preparations and suggest that, in the membrane preparations, thrombin receptors can couple to other G proteins that are not stimulated in the native cell. Our data are also consistent with the studies of Selheim et al,47 who demonstrated that thrombin-induced phosphatidylinositol-3 kinase product formation in platelets depends on secreted ADP. In wild-type mouse platelets, thrombin causes inhibition of adenylyl cyclase but fails to do so in the platelets from P2Y12 receptor–deficient mice (Figure 4B). Recent studies indicated that thrombin-induced inhibition of adenylyl cyclase in mouse platelets could be blocked by a P2Y12 receptor antagonist.41 Mouse platelets do not express PAR1 receptor, and thrombin effects are predominantly mediated by PAR4 receptor in these cells. Consistent with our observations, Faruqi et al17 demonstrated that PAR4 stably expressed in fibroblast cells does not inhibit adenylyl cyclase upon stimulation with thrombin.
Because thrombin, SFLLRN, and PAR4APs depend on secreted ADP for Gi stimulation, and costimulation of Gq and Gi is required for ADP- and U46619-induced platelet aggregation, we investigated the role of secreted ADP in thrombin-, SFLLRN- or PAR4AP-induced platelet aggregation by 2 complementary pharmacologic approaches. The first approach utilized AR-C66096. Platelet aggregation induced by PAR4APs and thrombin was diminished in the presence of AR-C66096 at lower agonist concentrations. However, AR-C66096 has no effect on platelet aggregation induced by higher agonist concentrations of PAR4APs and thrombin, thereby confirming a lack of dependence on secreted ADP. SFLLRN-induced platelet aggregation was rightward-shifted by AR-C66096, indicating a potentiating effect of secreted ADP. In the second approach, the P2Y12 knock-out mouse platelets aggregated normally to high concentrations of AYPGKF, although aggregation was diminished when lower agonist concentrations were used. Similarly, thrombin also has been shown to cause platelet aggregation in P2Y12-deficient mouse platelets.29 These findings correlate with the first approach and provide further evidence that PAR4-mediated platelet aggregation does not depend on Gi stimulation. The primary platelet aggregation mediated by PARs could result from Gq signaling alone or through coactivation of an unidentified G protein–coupled pathway in addition to Gq signaling. Even though it has been shown that both Gq and Gi pathways are necessary for ADP-induced platelet aggregation,18-20 it is conceivable that thrombin-, SFLLRN-, and PAR4AP-induced platelet aggregation do not require these 2 pathways. PAR1 and PAR4 also couple to G12/13 pathways. As result of this coupling, a PAR1AP, YFLLRN, causes human platelet shape change without intracellular calcium mobilization.40In Gq-deficient mouse platelets, where PAR1 is not expressed, thrombin causes platelet shape change through activation of PAR4.44 However, activation of the G12/13 pathway does not result in platelet aggregation.40 44
In conclusion, we demonstrate that, in human platelets, thrombin and SFLLRN depend on secreted ADP to stimulate Gi subsequent to activation of PAR1. In mouse platelets, upon stimulation by thrombin, its receptors GPIb-V-IX, PAR3, and PAR4 fail to couple to Gi in the absence of P2Y12 receptor. Our results also demonstrate that thrombin-, PAR4AP-, or SFLLRN-induced platelet aggregation occurs independently of the Gi-coupled pathway, and concomitant signaling from the Gq and Gisignaling pathways is not required for these agonist-induced primary platelet aggregations.
We thank Ying Zhai, Schering-Plough Research Institute, for her technical assistance.
Supported in part by research grants HL60683 and HL64943 from the National Institutes of Health. T.M.Q. is supported by training grant T32 HL07777 and is the recipient of a postdoctoral fellowship from the Pennsylvania-Delaware affiliate of the American Heart Association.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Satya P. Kunapuli, Dept of Physiology, Temple University School of Medicine, 3420 North Broad St, Philadelphia, PA 19140; e-mail: kunapuli@nimbus.temple.edu.

![Fig. 1. Effect of Ro 31-8220 or receptor-selective antagonists on PAR1- and PAR4-induced inhibition of adenylyl cyclase in normal human platelets. / Effect of PAR1 and PAR4 agonists on platelet adenylyl cyclase was determined as described in “Materials and methods.” Data are expressed as percentage of total [3H]cAMP. The data are normalized to the level of cAMP in response to 10 μM PGE1 (taken as 100%) or to the level of PGE1-stimulated cAMP in the presence of 10 μM Ro 31-8220. Ro 31-8220 or dimethyl sulfoxide (control) was added to aspirin-treated platelets and incubated for 5 minutes at 37°C with stirring before the addition of either PGE1 alone or PGE1 with agonists; 1 μM AR-C66096 was added 30 seconds prior to agonists, SFLLRN (10 μM) or GYPGKF (700 μM). Each data point is the mean ± SE of at least 3 experiments.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/99/10/10.1182_blood.v99.10.3629/6/m_h81022556001.jpeg?Expires=1769892126&Signature=HItVC589en-o4closCv7IQfDCCbPKJL13KVh7cFJwN97aIESaPcA6FwcA26TSEcs-PJZKNrPp~Ci0Le8YTRLZJAyJb1RrpeB3IvkDKrfWcxQVOJ6TPR66t39Uk-8tuK8tGZHghtZcmsruFcxqTAjRT~lhvRRQI92noDTNJlJUHxx5yHpVneD7Ml-MFpZUdHI0vZtAEiboSjQCmkkTYovoZsFE1eapC-IprrHzpGxPYVfwAU65qh5UqDIrvuPuNNh83MezIJYUg4NPfuX3n~CPDZaeiNYeSfcFAIqRIreMF1q-kHCS94KnhjEO3T4iTuw--4fYHied1EhfSetxkdpsA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 5. Concentration response curves for agonist-induced platelet aggregation and secretion. / Platelet aggregation was measured as described previously. Aspirin-treated [3H]serotonin-loaded platelets were stimulated with varying concentrations of (A) SFLLRN, (B) AYPGKF, or (C) thrombin. Platelet aggregation (solid line) and secretion (broken line) were measured as described in “Materials and methods.” The maximum response was taken as 100%, and the remaining data were normalized to this value. The data (mean ± SE) are from at least 3 independent experiments using platelets from different donors.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/99/10/10.1182_blood.v99.10.3629/6/m_h81022556005.jpeg?Expires=1769892126&Signature=ZVc5Up9dxaSbPgUH9EPLrMTA8dTofVHpJ4SWfLfmNx2qjxYWgTW9~IK~4b-d8A-tDgpubDkOXaHqBrdUmZXo-NpEcD4YHOWLdYwO1ugJvujvbqEoWjhI0SgAqfzNqNWGPmKzBsS4aTb1VUN9Io0kRaxGo0jMJocbrr5E9zbg9ShuHo96BCIV~OywnTtbuGuS1YhwsH9aIFZqGW9h1GT98D02yt8JIcgLS4iM5M7eKi4wCMnP4zAX7lmjiOPTOxbWaMSgkY1JhB3KSqkuU4zlW8vtNzExd7RTt-5oBUENEiM8OkyfTLpGRP~0cMeucLShBc8Oo~nQY3rWMMnBaCtrPQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

![Fig. 1. Effect of Ro 31-8220 or receptor-selective antagonists on PAR1- and PAR4-induced inhibition of adenylyl cyclase in normal human platelets. / Effect of PAR1 and PAR4 agonists on platelet adenylyl cyclase was determined as described in “Materials and methods.” Data are expressed as percentage of total [3H]cAMP. The data are normalized to the level of cAMP in response to 10 μM PGE1 (taken as 100%) or to the level of PGE1-stimulated cAMP in the presence of 10 μM Ro 31-8220. Ro 31-8220 or dimethyl sulfoxide (control) was added to aspirin-treated platelets and incubated for 5 minutes at 37°C with stirring before the addition of either PGE1 alone or PGE1 with agonists; 1 μM AR-C66096 was added 30 seconds prior to agonists, SFLLRN (10 μM) or GYPGKF (700 μM). Each data point is the mean ± SE of at least 3 experiments.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/99/10/10.1182_blood.v99.10.3629/6/m_h81022556001.jpeg?Expires=1769892127&Signature=qbCInqDI4d6U8rSc357JvKyGTl0Lfq4wRG3-lCyLIGNhqW7~b7WgTzr0Ky6gSY63~XBOjne6TwbQt2~-erIK7ajWdWurR3PCsSucl8QkPAj~-Kl4viPtnAaz1n27-Gdc8IDq4D4Xp5ZDmTnyygxdNH2J7wjLpUu3SnI2eWlT4JIor8DSUPfz1v36fOSJfGAuZJfNuguSeVsglFxuP0RoXaA2Jo0uzSonlrkx1Xx1P9L9PhhkXf1OmLsn4I4PNA-avovdJpMKE5aeGUWMNFr08L7ORfPvkhEHUuERcvow1iacFOBUwGZUpFA7gP-3M2qI32eijXD2I4KtqxejqQZQ7Q__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 5. Concentration response curves for agonist-induced platelet aggregation and secretion. / Platelet aggregation was measured as described previously. Aspirin-treated [3H]serotonin-loaded platelets were stimulated with varying concentrations of (A) SFLLRN, (B) AYPGKF, or (C) thrombin. Platelet aggregation (solid line) and secretion (broken line) were measured as described in “Materials and methods.” The maximum response was taken as 100%, and the remaining data were normalized to this value. The data (mean ± SE) are from at least 3 independent experiments using platelets from different donors.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/99/10/10.1182_blood.v99.10.3629/6/m_h81022556005.jpeg?Expires=1769892127&Signature=T8FrpH-0wvAykWHc~8jq5m~06hO0LQK2rfQkA0abqZ7yXz2Bci13E0ou-5j98wv0TckorZCcZO6LmoECtWX5ggwtBvd3-TjjCSJvBvgqnwonxGK~n6Iq3k0GcMnnRSW978UPBkr1n~Eu1p3KpEGTLJCX-klamRdqppKX8GBBXQLMs8DVfg2AQrWxIjOwaN7p2tTOdc5rVE95reTewlXjpPWkLuMkf4mBr7f2ASyFyPWZSEuVKVDjn3ht91LoQ3TfKp982Jq93OHB-nz27txofheHc67OzymtHmqMWw96OZ61hGjRIhhI4UirMXsLerILHGWj~JvAsWPg2PErHt7VNg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)