Immunizations using the endoplasmic reticulum–resident heat shock protein Gp96 induce specific immune responses. Specificity is based on the major histocompatibility complex class I–restricted cross-presentation of Gp96-associated peptides derived from endogenous proteins. Initiation of the immune response depends on the ability of Gp96 to induce the production of proinflammatory cytokines by macrophages and dendritic cells (DCs) and of their maturation in a fashion presumably independent of associated peptide. Both events are mediated by Gp96 receptors on antigen-presenting cells. It is known that Gp96 is released from cells at necrosis induced, for example, by virus infection. Although this event supports the efficient induction of immune responses, it might also interfere with processes that are susceptible to chronic inflammation, such as wound healing after tissue damage. Therefore, Gp96-mediated stimulation of the immune system requires tight regulation. Here we show that human thrombocytes specifically interact with Gp96 and that binding of Gp96 to platelets is enhanced more than 10-fold on activation by thrombin. Gp96 interferes with neither thrombin-induced platelet activation nor platelet aggregation. However, the presence of platelets during Gp96-mediated DC activation reduces the secretion of proinflammatory cytokines and the activation of DCs. This effect is independent of soluble platelet factors and cell-to-cell contact between DCs and thrombocytes. Thus, we provide evidence for a regulatory mechanism that neutralizes Gp96 molecules systemically, especially in the blood. This effect might be of significance in wounds in which chronic inflammation and immune responses against autoantigens have to be prevented.
Introduction
The endoplasmic reticulum (ER)–resident heat shock protein (HSP) Gp96 plays multiple roles in mammalian organisms. As a chaperone, it assists protein folding and prevents aggregation of partially unfolded proteins in the ER.1 In this key compartment of the major histocompatibility (MHC) class I presentation pathway, Gp96 is also one of the major peptide-binding proteins, and it associates with a peptide pool representative for the protein content of the cell.2,3 Gp96 is released from cells after tissue damage caused by severe injury and resulting from necrotic cell death induced by freeze–thaw cycles4 or virus infection.5 Its presence in the extracellular space reveals surprising immunostimulatory properties: immunization with Gp96 preparations from tumor cells has been shown to elicit protective and therapeutic immune responses against the tumor from which the HSP had been purified.6,7 The specificity of this immune response is attributed to tumor-derived peptides associated with Gp96.6 After receptor-mediated endocytosis of Gp96 by professional antigen-presenting cells (APCs),8 these peptides are presented on MHC class I molecules.9 This process is usually referred to as cross-presentation,10,11and it is one of the key events during the priming of naive T cells.12 In addition, Gp96-mediated APC activation results in the up-regulation of costimulatory molecules and in the release of the pro-inflammatory and TH1-promoting cytokines tumor necrosis factor (TNF)-α and interleukin (IL)-12.4 13 The release of the anti-inflammatory and TH2-promoting cytokine IL-10 is only slightly stimulated. The resultant cytokine milieu is an important prerequisite for the triggering of a cytotoxic T-cell response.
So far, receptor-mediated interactions for Gp96 have only been described for professional APCs comprising dendritic cells (DCs), macrophages, and B cells. The α2-macroglobulin receptor (CD91) has been identified as one receptor on a macrophage cell line.14 Binding of Gp96 to the scavenger receptor CD36 has been shown by Panjwani et al.15 More recently, physical interaction between Gp96 and Toll-like receptors 1, 2, and 4 in the ER has been described,16 and we were able to show that Gp96 signals through the Toll-like receptor 4 pathway, at least in bone marrow–derived mouse DCs.17 However, the potent ability of Gp96 to induce a proinflammatory milieu might be a mixed blessing for the organism in some situations. After injuries, transient inflammation during the first phase of wound healing is helpful, but chronic inflammation prevents proper tissue formation.18In this scenario, the proinflammatory potential of Gp96 has to be regulated, or wound healing might be impaired and the uncontrolled maturation of DCs would favor the development of autoimmunity.
A major difference between, for example, virus-induced cell lysis and cell death caused by injury is the presence of platelets. These small, non-nucleated cells play a key role in hemostasis by forming a plug that physically stops blood loss. In addition, activated platelets release several mediators from their granules that contribute to proper wound healing. Platelet aggregation and activation are triggered by many different stimuli, of which the most prominent are thrombin, collagen, and adenosine diphosphate (ADP). The latter 2 substances are normally not visible to platelets unless blood vessels are disrupted. From this perspective, collagen and ADP might be considered messengers of cell death and injury, with platelets as appropriate sensors. Although platelets carry MHC class I molecules on their surfaces, they do not themselves stimulate primary T-cell responses.19 However, thrombocytes have several immunomodulatory properties. Activated platelets have been shown to induce an inflammatory reaction on vascular endothelial cells through CD40 ligand (CD40L), which was originally identified on activated CD4+ T cells.20 In addition, the maturation of DCs by fixed, activated platelet preparations was demonstrated.21 On activation thrombocytes secrete proinflammatory cytokines and chemokines (eg, platelet factor 4, RANTES) and anti-inflammatory mediators (eg, transforming growth factor-β).22-26
In this work, we show for the first time that human platelets express receptors for the ER-resident heat shock protein Gp96. We investigate in detail the binding of Gp96 to the surfaces of human platelets and the consequences thereof concerning platelet function. Furthermore, we analyze the influence of human platelets on the Gp96-mediated activation of DCs.
Materials and methods
Materials
Purified mouse Gp96 and fluorescein isothiocyanate (FITC)–labeled Gp96 were provided by Immatics Biotechnologies (Tübingen, Germany). Gp96 lots were tested endotoxin-free with a limulus amebocyte lysate assay (QCL-1000; BioWhittaker, Verviers, Belgium). Recombinant ovalbumin and FITC-labeled bovine serum albumin (BSA) were purchased from Sigma-Aldrich (Taufkirchen, Germany). Ovalbumin was labeled with FITC according to standard protocols and was purified by gel filtration on a Sephadex G-25 column (Amersham Pharmacia Biotech, Freiburg, Germany).
Platelet isolation
Nine volumes of freshly drawn blood from healthy donors were mixed with one volume of 110 mM sodium citrate as anticoagulant. The citrated blood was centrifuged at 100g for 15 minutes at room temperature to obtain platelet-rich plasma (PRP) in the upper phase. For activation, flow cytometry, and coculture experiments with DCs, platelets were separated from plasma according to the following procedure. The PRP was overlaid on top of a 2-mL 34% (wt/vol) BSA cushion and was centrifuged at 550g for 10 minutes. Platelets were collected from the interphase and were washed twice with serum-free cell culture medium. For activation, platelet suspensions were incubated for 3 minutes with 0.2 U/mL thrombin at 37°C if not stated otherwise. Thereafter, cells were fixed for 2 minutes on ice by the addition of paraformaldehyde (PFA) to a final concentration of 1% (wt/vol). Residual PFA was removed by 2 additional washing steps. Nonfixed platelets were used within 3 hours of preparation. Contamination by other blood cells (mainly erythrocytes) in thrombocyte preparations used for DC cocultures was always lower than 0.5%.
Dendritic cell preparation
Human DCs were prepared from freshly drawn blood from healthy donors. Peripheral blood mononuclear cells (PBMCs) were isolated using a Ficoll density gradient (Lymphoprep; Nycomed, Oslo, Norway). The obtained cells were washed twice with phosphate-buffered saline (PBS) and resuspended in X-Vivo 15 medium (BioWhittaker) supplemented with 2 mM l-glutamine, 50 U/mL penicillin, and 50 μg/mL streptomycin. PBMCs were plated at a density of 6 × 106cells/mL. After 2 hours at 37°C, nonadherent cells were removed by washing with PBS. Adherent monocytes were cultured for 6 days in medium supplemented with 1% (vol/vol) human serum (Peel-Freez, Brown Deer, WI), 1000 U/mL IL-4 (R&D Systems, Minneapolis, MN), and 20 ng/mL granulocyte macrophage–colony-stimulating factor (Leukomax; Novartis Pharma GmbH, Nuremberg, Germany). The differentiation state of DCs was examined by flow cytometry. Only immature DCs CD14−, CD83−, and CD86low were used for activation experiments. The amount of CD1a on DCs varied in different DC preparations between low expression and complete absence, in accordance with earlier findings.27 The fraction of activated DCs, as analyzed by CD83 expression, was always less than 5%.
Antibodies and staining for flow cytometry
The following antibodies were used for fluorescence-activated cell sorter (FACS) analysis: FITC-labeled monoclonal antibody (mAb) against CD41 and phycoerythrin (PE)-labeled CD40L-mAb (both from Coulter Immunotech, Marseilles, France); PE-labeled mAb specific for the α subunit of CD91 (Research Diagnostics, Flanders, NJ); and PE-CD1a mAb, PE-CD14 mAb, PE-CD36 mAb, PE-CD83 mAb, PE-CD86 mAb (all from BD Biosciences, Heidelberg, Germany). For FACS analysis, aliquots of 1 × 107 platelets were incubated with labeled antibodies or proteins in FACS buffer (PBS, 1% [wt/vol] BSA, 0.02% [wt/vol] sodium azide) for 30 minutes on ice. Staining with Gp96-FITC was performed in cell culture medium supplemented with 10% (vol/vol) FCS. Platelets were washed 3 times with FACS buffer and were fixed in 1% (wt/vol) PFA before analysis on a FACScalibur cytometer (BD Biosciences). Appropriate mouse isotype controls were included to evaluate background staining. If indicated, platelets were preincubated with competitors for 30 minutes on ice. For competition experiments, the anti-CD36 antibody clones CB38 (BD Biosciences) and SM0 (Sigma-Aldrich) and an mAb against the 85-kd subunit of CD91 (clone 5A6; Research Diagnostics) were used.
Analysis of platelet function
Freshly isolated platelets from different donors were preincubated for 15 minutes at 37°C with different effectors. ADP (2.5 μM), a weak inducer of platelet activation at this concentration, was used as positive control. Thereafter, thrombin was added in different concentrations (0, 1, 5 or 100 mU/mL). After 5 minutes of incubation at 37°C, platelets were fixed by the addition of PFA to a final concentration of 1% (wt/vol). Residual PFA was removed by 2 washing steps with FACS buffer. Platelets were stained with a PE-labeled antibody specific for the platelet activation marker CD40L. CD40L expression levels after activation with 500 mU/mL thrombin were set as 100%. For aggregation assays, freshly prepared PRP from healthy donors who had not taken acetylsalicylic acid for 10 days was used. Platelet concentration was adjusted to 2.5 × 105/μL with platelet-poor plasma that had been obtained by centrifugation (2500g, 15 minutes, room temperature) of the remaining blood after PRP preparation. Aggregation was analyzed using an APACT 4 aggregometer (Labor GmbH, Ahrensburg, Germany). Stirred PRP (300 μL) was incubated at 37°C with 50 μg/mL Gp96, 50 μg/mL ovalbumin, or buffer alone for 3 minutes. Thereafter, ADP, collagen, or adrenaline was added, and aggregation was measured for 6 additional minutes.
Platelet–dendritic cell coculture
Platelets (2 × 104/μL) in 200 μL serum-free medium were preincubated for 45 minutes with 20 μg/mL Gp96 or 20 ng/mL lipopolysaccharide (LPS) in a 96-well plate. Thereafter, 2 × 105 immature DCs were added. After 24 and 48 hours, 100 μL cell culture supernatant was assayed for IL-10, TNF-α, and IL-12 by sandwich enzyme-linked immunosorbent assay with antibodies obtained from BD Biosciences. In addition, the maturation state of DCs was measured by determining the amount of CD83+ and CD86high cells by flow cytometry after 48 hours. To exclude endotoxin contamination as the reason for DC activation in the Gp96 lots used, boiled Gp96 was included because Gp96 is heat sensitive whereas LPS is not.13 In some experiments direct cell-to-cell contact was prevented with transwell inserts for 96-well plates (Nunc, Roskilde, Denmark). In these experiments, platelets filled into the chamber of the insert were separated from DCs by a membrane (0.2-μm pore diameter) allowing only the exchange of soluble factors. Total culture volume in transwell experiments was 230 μL.
Results
Gp96-FITC binds specifically to human platelets
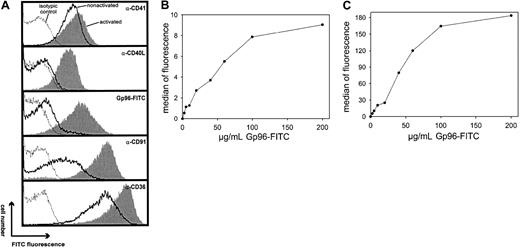
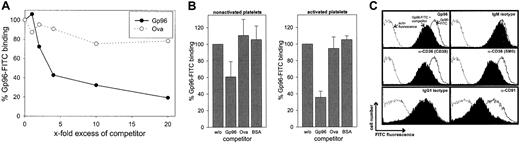
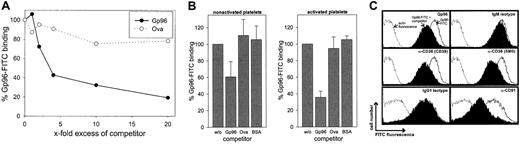
We first investigated whether Gp96 molecules interact with human platelets. Freshly purified platelets were fixed directly or were stimulated by 0.2 U/mL thrombin for 3 minutes at 37°C before fixation. To control the homogeneity of the cells used, thrombocytes were stained with an antibody against the platelet-specific marker CD41 (Figure 1A). This marker identified a homogenous platelet preparation with few variations in fluorescence intensity between activated (filled gray) and nonactivated (black line) cells. In contrast, only activated thrombocytes showed staining with an antibody against CD40L. This is in line with previous findings reporting CD40L expression exclusively on activated platelets.20 Gp96-FITC binds to nonactivated and to thrombin-activated platelets (Figure 1A, third panel). Comparing fluorescence intensities, binding of Gp96 was enhanced approximately 10-fold on activated platelets. As a control, FITC-labeled ovalbumin showed no binding on thrombocytes (data not shown). Figure 1A (fourth panel) shows that CD91, which has been already identified as a Gp96 receptor, is also expressed on human platelets and is strongly up-regulated after thrombin-induced activation, correlating with the binding characteristics of Gp96-FITC. The scavenger receptor CD36, which has been suggested recently as an additional receptor for Gp96,15 is already present at high levels on nonstimulated platelets and is only slightly up-regulated after activation (Figure1A, last panel). To analyze the specificity of the observed binding of Gp96 to platelets, we tested whether saturation could be achieved. Nonstimulated and thrombin-activated thrombocytes showed typical saturation curves when incubated with different concentrations of Gp96-FITC (Figure 1B-C). Maximal binding is again more than 10-fold higher on activated than on nonactivated platelets. In both cases, half-maximal binding is achieved approximately at 40 μg/mL Gp96-FITC, which is comparable to the binding characteristics on monocytes and DCs.9 Another feature of specific binding of a labeled ligand to its receptor at saturating concentrations is that it can be specifically competed by the same unlabeled ligand. Figure2A shows the binding of 50 μg/mL Gp96-FITC to activated platelets in the presence of different amounts of unlabeled Gp96. Increasing amounts of competitor reduced the Gp96-FITC binding as expected for specific ligand-receptor interactions. Ovalbumin and BSA as control proteins were unable to compete with Gp96-FITC binding (Figure 2A-B). The same competition pattern was observed for nonstimulated platelets (Figure 2B). We tried to compete Gp96-FITC binding with monoclonal antibodies against CD36 and CD91, to investigate whether these 2 receptors are also involved in Gp96 binding to platelets. Although competition with appropriate isotypic controls did not result in reduced Gp96-FITC staining, 2 anti-CD36 clones and an antibody against the 85-kd subunit of CD91 competed with Gp96 binding (Figure 2C). An antibody against the platelet marker CD41 did not alter Gp96-FITC staining (data not shown), supporting the specificity of the observed competition. This suggests that CD36 and CD91 are involved in Gp96 binding to platelets.
Gp96 binds to nonactivated and thrombin-activated human platelets.
Freshly prepared platelets from human blood were incubated for 3 minutes at 37°C with 0.2 U/mL thrombin or without effector and were fixed with paraformaldehyde. (A) After extensive washing, platelets were stained with antibodies specific for CD41, CD40L, CD91, and CD36 or with 50 μg/mL FITC-labeled Gp96 and were analyzed by flow cytometry. Isotypic controls are shown as dotted lines, stainings of nonactivated platelets are shown as solid lines, and thrombin-activated platelets are shown as filled gray lines. Nonactivated (B) or thrombin-activated (C) platelets were stained with different concentrations of Gp96-FITC for 30 minutes at 4°C and were analyzed by flow cytometry. Data shown are representative for at least 4 independent experiments.
Gp96 binds to nonactivated and thrombin-activated human platelets.
Freshly prepared platelets from human blood were incubated for 3 minutes at 37°C with 0.2 U/mL thrombin or without effector and were fixed with paraformaldehyde. (A) After extensive washing, platelets were stained with antibodies specific for CD41, CD40L, CD91, and CD36 or with 50 μg/mL FITC-labeled Gp96 and were analyzed by flow cytometry. Isotypic controls are shown as dotted lines, stainings of nonactivated platelets are shown as solid lines, and thrombin-activated platelets are shown as filled gray lines. Nonactivated (B) or thrombin-activated (C) platelets were stained with different concentrations of Gp96-FITC for 30 minutes at 4°C and were analyzed by flow cytometry. Data shown are representative for at least 4 independent experiments.
Gp96-FITC binding to human platelets can be specifically competed by unlabeled Gp96 and by antibodies against CD36 and CD91.
Platelets were fixed and incubated with 50 μg/mL Gp96-FITC after preincubation with competitor for 30 minutes on ice. Fluorescence of stained cells was analyzed by flow cytometry. (A) Different amounts of unlabeled Gp96 or ovalbumin were used for competition on thrombin-activated cells. Data shown are representative for 3 independent experiments. (B) Nonstimulated and thrombin-activated platelets were stained using a 10-fold excess of the indicated competitors. Fluorescence intensity without competitor was set as 100%. Means of triplicates are shown, and error bars represent SEM. (C) Unlabeled Gp96 (500 μg/mL), isotypic controls, and monoclonal antibodies against CD36 (CB38, SM0; both IgM isotype) or against the 85-kd subunit of CD91 (IgG1 isotype) were used for competition before staining of thrombin-activated platelets with 50 μg/mL Gp96-FITC (concentration of competing antibodies: 50 μg/mL). Autofluorescence is indicated by the dotted line, and uncompeted and competed staining are indicated by the solid line and the filled histogram. Experiments were performed in triplicate.
Gp96-FITC binding to human platelets can be specifically competed by unlabeled Gp96 and by antibodies against CD36 and CD91.
Platelets were fixed and incubated with 50 μg/mL Gp96-FITC after preincubation with competitor for 30 minutes on ice. Fluorescence of stained cells was analyzed by flow cytometry. (A) Different amounts of unlabeled Gp96 or ovalbumin were used for competition on thrombin-activated cells. Data shown are representative for 3 independent experiments. (B) Nonstimulated and thrombin-activated platelets were stained using a 10-fold excess of the indicated competitors. Fluorescence intensity without competitor was set as 100%. Means of triplicates are shown, and error bars represent SEM. (C) Unlabeled Gp96 (500 μg/mL), isotypic controls, and monoclonal antibodies against CD36 (CB38, SM0; both IgM isotype) or against the 85-kd subunit of CD91 (IgG1 isotype) were used for competition before staining of thrombin-activated platelets with 50 μg/mL Gp96-FITC (concentration of competing antibodies: 50 μg/mL). Autofluorescence is indicated by the dotted line, and uncompeted and competed staining are indicated by the solid line and the filled histogram. Experiments were performed in triplicate.
Gp96 does not influence platelet activation and aggregation
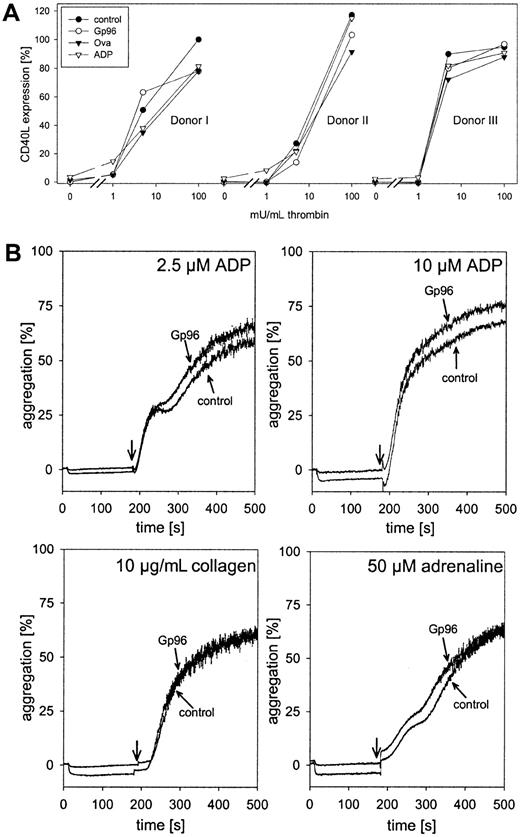
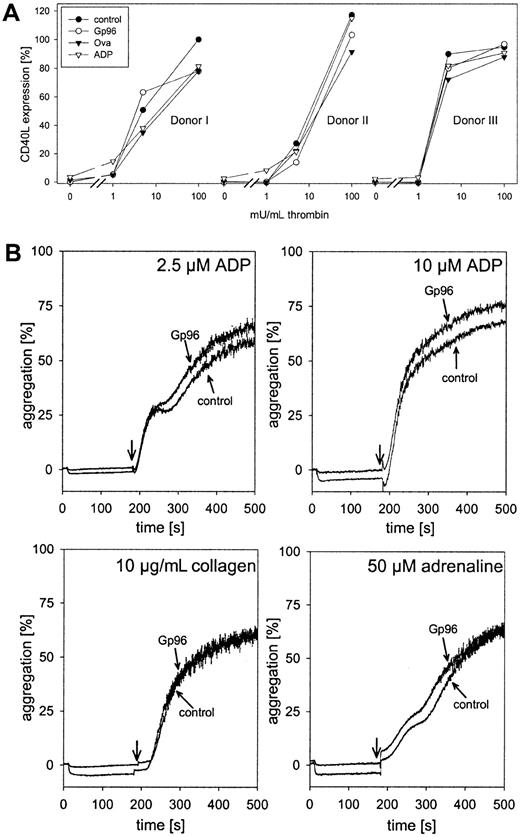
We tested whether Gp96 was able to interfere with thrombin-induced platelet activation. Freshly isolated platelets were pre-incubated with 100 μg/mL Gp96 or ovalbumin as control for 15 minutes at 37°C or were left untreated. ADP (2.5 μM), a weak inducer of platelet activation at this concentration, was included as a positive control. Thereafter, thrombin was added in different concentrations. After 5 minutes of incubation with thrombin, cells were fixed and expression of the platelet activation marker CD40L was measured by flow cytometry. Because of variations between different donors in their responses to thrombin, data for individual donors are shown (Figure3). Without thrombin, only ADP-treated platelets showed a slight increase in CD40L expression. Therefore, Gp96 did not trigger thrombocyte activation. Moreover, compared to control samples, Gp96 had no influence on platelet activation induced by saturating or lower concentrations of thrombin. It cannot be excluded that a weak activating effect of Gp96 was not visible in our experiments because of preactivation during cell preparation. However, this seems unlikely because no significant staining with anti-CD40L antibody compared to the isotypic control was observed. Thus, the influence of platelet preactivation was negligible.
Gp96 does not interfere with platelet function.
(A) Freshly isolated platelets from 3 healthy donors were preincubated with 2.5 μM ADP, 100 μg/mL Gp96, or ovalbumin at 37°C for 15 minutes. Thereafter, thrombin at different concentrations was added. After an additional 5-minute incubation at 37°C, platelets were fixed and activation was analyzed by flow cytometry after staining with PE-labeled anti-CD40L antibody. Because of varying levels of CD40L expression between different donors, the obtained fluorescence intensities were converted to relative activation values. The median of fluorescence after maximal stimulation with 500 mU/mL thrombin was set as 100% activation (donor 1, 23.71; donor 2, 28.13; donor 3, 18.11). The value for nonstimulated platelets was set as 0% (2.94 ± 0.24). (B) The influence of Gp96 on the aggregation of platelet-rich plasma (2.5 × 105 cells/μL) was measured in an aggregometer. Ten seconds after the start of measurement, Gp96 was added to a final concentration of 50 μg/mL. Control samples were treated with buffer only. After 3 minutes, 2.5 μM or 10 μM ADP, 10 μg/mL collagen, or 50 μM adrenaline were added, and aggregation was followed for an additional 320 seconds. Experiments were performed in triplicate and were repeated for 3 different donors.
Gp96 does not interfere with platelet function.
(A) Freshly isolated platelets from 3 healthy donors were preincubated with 2.5 μM ADP, 100 μg/mL Gp96, or ovalbumin at 37°C for 15 minutes. Thereafter, thrombin at different concentrations was added. After an additional 5-minute incubation at 37°C, platelets were fixed and activation was analyzed by flow cytometry after staining with PE-labeled anti-CD40L antibody. Because of varying levels of CD40L expression between different donors, the obtained fluorescence intensities were converted to relative activation values. The median of fluorescence after maximal stimulation with 500 mU/mL thrombin was set as 100% activation (donor 1, 23.71; donor 2, 28.13; donor 3, 18.11). The value for nonstimulated platelets was set as 0% (2.94 ± 0.24). (B) The influence of Gp96 on the aggregation of platelet-rich plasma (2.5 × 105 cells/μL) was measured in an aggregometer. Ten seconds after the start of measurement, Gp96 was added to a final concentration of 50 μg/mL. Control samples were treated with buffer only. After 3 minutes, 2.5 μM or 10 μM ADP, 10 μg/mL collagen, or 50 μM adrenaline were added, and aggregation was followed for an additional 320 seconds. Experiments were performed in triplicate and were repeated for 3 different donors.
The second important component of platelet function is aggregation after injury to stop bleeding. To analyze the effect of Gp96 on thrombocyte aggregation, freshly prepared PRP was incubated at 37°C with 50 μg/mL Gp96 under continuous stirring in an aggregometer. No formation of aggregates could be observed (Figure 3B; horizontal parts of the curves before addition of other stimuli). Even after 15 minutes of incubation, there was no induction of aggregation by Gp96 alone (data not shown). After 3 minutes, platelet aggregation was induced either by 2.5 or 10 μM ADP, 10 μg/mL collagen, or 50 μM adrenaline. Independent of the effector used, no differences in the kinetics or the final degree of aggregation could be observed between Gp96-pretreated and control samples.
Platelets inhibit Gp96-induced DC maturation
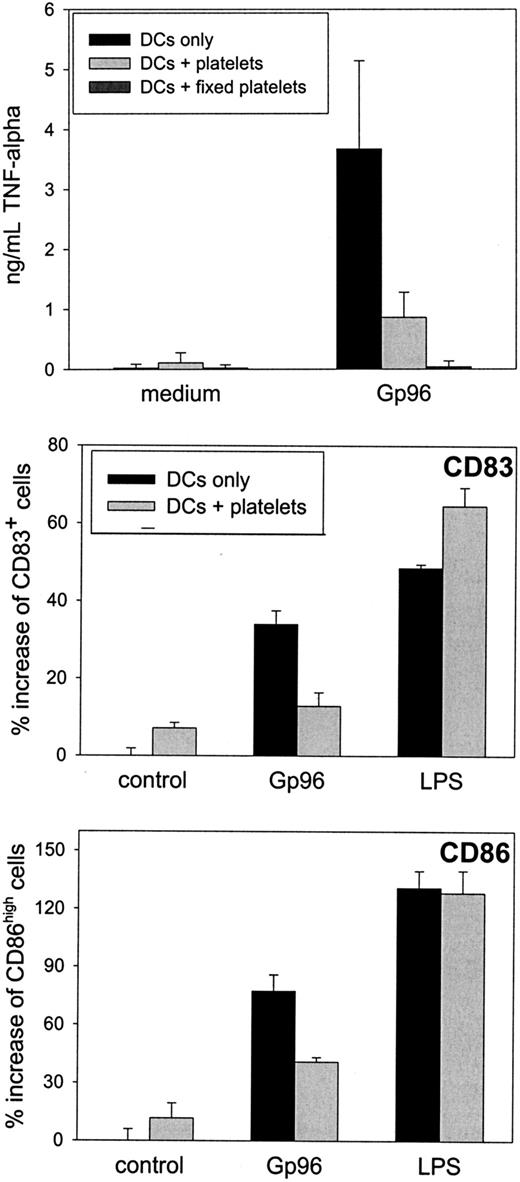
Although no interference of Gp96 with platelet function was apparent in our experiments, we addressed whether the binding of Gp96 to platelets might interfere with the immunostimulatory effect of Gp96. We concentrated on dendritic cells as the key cell type in the initiation of an immune response. Immature DCs were cultured with Gp96 for 2 days in the presence or absence of 2 × 104thrombin-activated autologous platelets per microliter (concentration in blood, 1.5 × 105/μL to 4 × 105/μL).
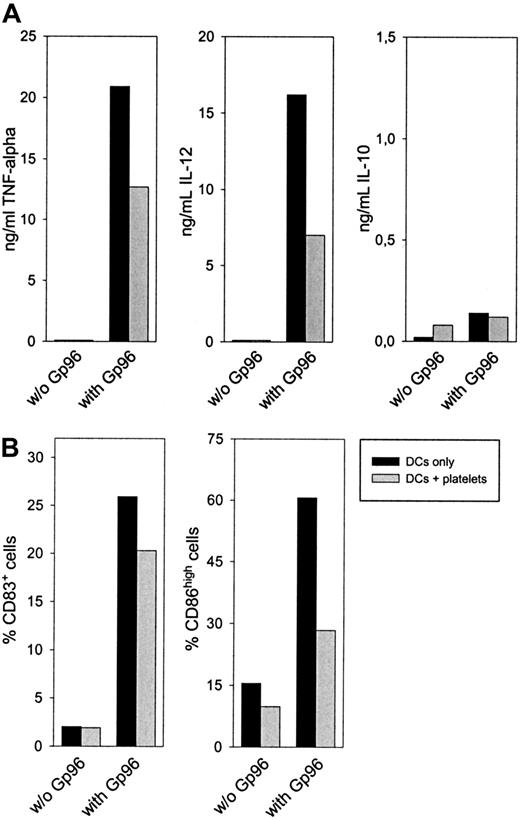
After 24 hours, a reduced concentration of the proinflammatory cytokines TNF-α and IL-12 in the supernatant could be observed when platelets were present in the cell culture (Figure4A). No difference was visible in the secretion of the anti-inflammatory and TH2-promoting cytokine IL-10. A similar cytokine pattern was observed after 48-hour culture and, to a lower extent, when nonstimulated platelets were used (data not shown). Additionally, DCs showed reduced up-regulation of the activation markers CD83 and CD86 after 48-hour activation with Gp96 in the presence of platelets (Figure 4B). Although CD83 expression was only reduced by approximately 20% in all experiments performed, the reduction in CD86 expression was greater than 50%. DC-activating capacities of Gp96 were not caused by low amounts of endotoxin in the Gp96 preparations because boiled Gp96 showed no stimulating effect on DCs (data not shown). In contrast, LPS-induced activation is heat resistant, as we have shown previously.13 When PBMCs from the same donor were used instead of platelets, Gp96-mediated DC activation was not influenced (data not shown). Thus, the anti-inflammatory effect of platelet preparations was not caused by contamination by other blood cells. Therefore, we conclude that platelets themselves reduce Gp96-induced DC activation, resulting in lower secretion of proinflammatory cytokines and in less pronounced differentiation to mature DCs. To analyze further the mechanism of this effect, we performed transwell experiments to avoid cell-to-cell contacts between DCs and platelets. This method also enabled us to increase the platelet–DC ratio without affecting DC viability (1 × 105 platelets/μL, which is closer to the physiological thrombocyte concentration in blood). As shown in Figure5, freshly prepared platelets were able to reduce Gp96-induced TNF-α production, and PFA-fixed platelets, which had been activated with thrombin before fixation, completely abrogated TNF-α production without direct cell-to-cell contact (Figure 5A). The same was observed for the up-regulation of the maturation markers CD83 and CD86 (Figure 5B). In contrast to this, LPS-induced DC maturation was not affected by the presence of platelets (Figure 5B).
Autologous platelets interfere with Gp96-induced DC maturation.
Human monocytes were cultured with granulocyte macrophage–colony-stimulating factor and IL-4 to obtain immature DCs. After 6 days, 4 × 106 thrombin-activated platelets from the same donor were preincubated with 20 μg/mL Gp96 in a 96-well plate for 45 minutes, followed by the addition of 2 × 105 immature DCs per well. (A) Cytokine concentrations of IL-12, TNF-α, and IL-10 in the cell culture supernatant were determined by enzyme-linked immunosorbent assay after 24 hours. (B) The maturation of DCs was measured by determining the number of CD83+ and CD86high cells by flow cytometry. Values for DCs without platelets are shown as filled bars; DC-platelet cocultures are shown as open bars. Data shown are representative for 3 independent experiments with cells from different donors.
Autologous platelets interfere with Gp96-induced DC maturation.
Human monocytes were cultured with granulocyte macrophage–colony-stimulating factor and IL-4 to obtain immature DCs. After 6 days, 4 × 106 thrombin-activated platelets from the same donor were preincubated with 20 μg/mL Gp96 in a 96-well plate for 45 minutes, followed by the addition of 2 × 105 immature DCs per well. (A) Cytokine concentrations of IL-12, TNF-α, and IL-10 in the cell culture supernatant were determined by enzyme-linked immunosorbent assay after 24 hours. (B) The maturation of DCs was measured by determining the number of CD83+ and CD86high cells by flow cytometry. Values for DCs without platelets are shown as filled bars; DC-platelet cocultures are shown as open bars. Data shown are representative for 3 independent experiments with cells from different donors.
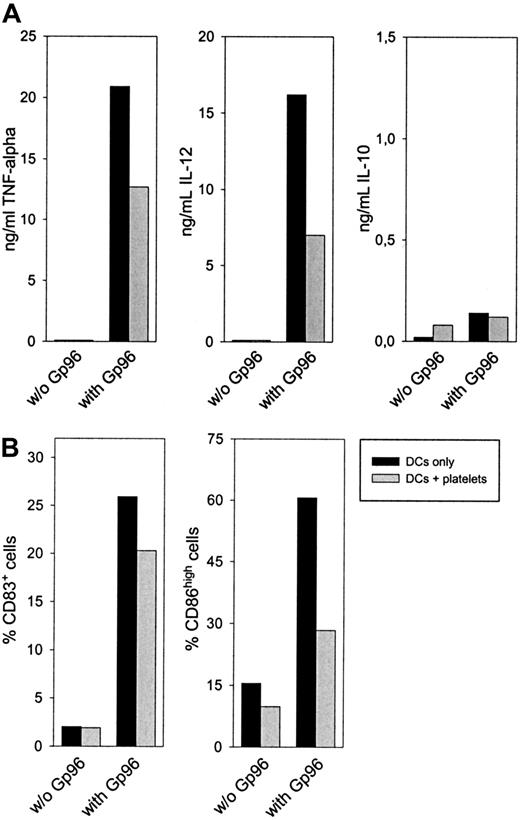
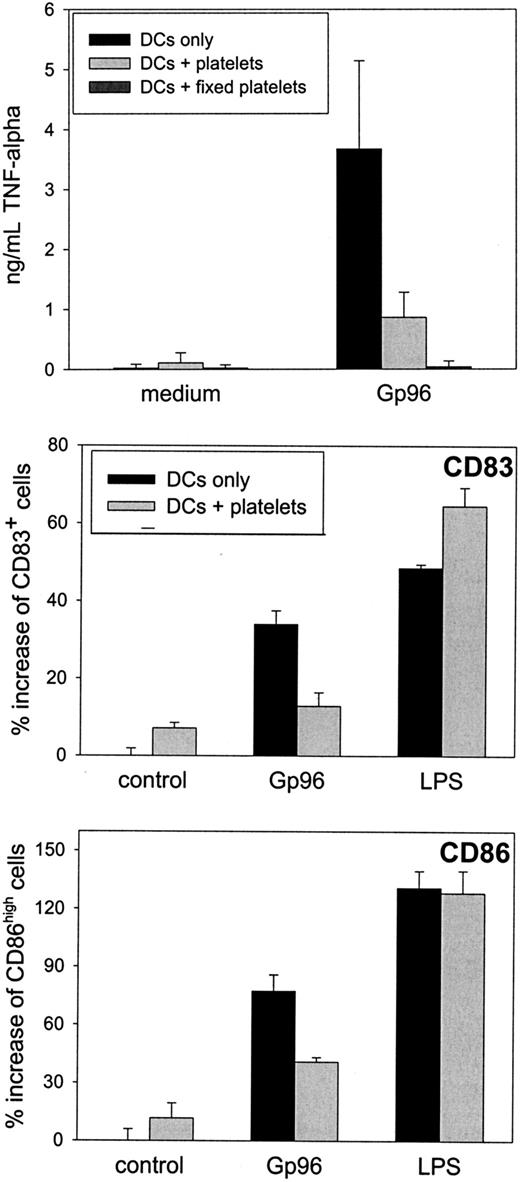
Platelet interference with Gp96-induced DC maturation is dependent on neither cell-to-cell contact nor soluble platelet factors.
Peripheral blood–derived monocytes were cultured to obtain immature human DCs, as described for Figure 4. After 6 days, cells were harvested and 2 × 105 DCs were plated into a 96-well plate. Autologous platelets (1 × 105/μL) were placed in the chamber of a transwell insert. If indicated, 25 μg/mL Gp96 or 1 μg/mL LPS was added to the platelets. (A) Freshly prepared platelets or thrombin-activated platelets, which were fixed with PFA after activation, were used. After 20-hour transwell culture, TNF-α concentration in the supernatant was determined. Mean values of duplicates are shown. The experiment was repeated twice. (B) DCs were left untreated or were stimulated with Gp96 and LPS in the absence or presence of fresh platelets. After 48 hours, CD86 and CD83 expressions were analyzed by flow cytometry. Percentage increase of activated cell numbers compared to nonstimulated DCs in the absence of platelets is shown. Percentages of activated cells in control samples for CD83 and CD86 were 33% and 26%, respectively. Mean values of triplicates are shown with error bars representing SEM.
Platelet interference with Gp96-induced DC maturation is dependent on neither cell-to-cell contact nor soluble platelet factors.
Peripheral blood–derived monocytes were cultured to obtain immature human DCs, as described for Figure 4. After 6 days, cells were harvested and 2 × 105 DCs were plated into a 96-well plate. Autologous platelets (1 × 105/μL) were placed in the chamber of a transwell insert. If indicated, 25 μg/mL Gp96 or 1 μg/mL LPS was added to the platelets. (A) Freshly prepared platelets or thrombin-activated platelets, which were fixed with PFA after activation, were used. After 20-hour transwell culture, TNF-α concentration in the supernatant was determined. Mean values of duplicates are shown. The experiment was repeated twice. (B) DCs were left untreated or were stimulated with Gp96 and LPS in the absence or presence of fresh platelets. After 48 hours, CD86 and CD83 expressions were analyzed by flow cytometry. Percentage increase of activated cell numbers compared to nonstimulated DCs in the absence of platelets is shown. Percentages of activated cells in control samples for CD83 and CD86 were 33% and 26%, respectively. Mean values of triplicates are shown with error bars representing SEM.
The finding that fixed platelets act immunosuppressively strongly suggests that no platelet-derived soluble factor can be involved in the observed effect. This favors a more passive mechanism whereby platelets simply compete with DCs for Gp96 binding. In line with this, the inhibitory effect of platelets was more pronounced when thrombin-activated, fixed platelets were used (Figure 5A), whereas fixed, nonstimulated platelets caused a lower reduction of DC activation (data not shown).
Discussion
The ER-resident HSP Gp96 is released during necrotic cell death and activates dendritic cells. This feature, in combination with its ability to transfer intracellular peptides for their MHC class I–restricted presentation, allows Gp96, together with other HSPs such as HSP70 and HSP90, to be an efficient messaging system alerting the organism to bacterial or viral infection and possibly to injury. Because HSPs are also released during mechanical tissue damage, control mechanisms have to exist that limit the HSP-mediated DC activation locally and prevent the release of pro-inflammatory cytokines in healing wounds. One mechanism has been postulated when CD91 was identified as one of the receptor molecules for Gp96. Interaction of Gp96 with CD91 in the bloodstream is inhibited by the presence of α2-macroglobulin, which binds to CD91 as well.14 Our data reported here indicate a second, more general mechanism: the immunostimulatory capacity of Gp96 is neutralized in the bloodstream by the binding of Gp96 to thrombocytes. This interaction is specific as the binding of Gp96-FITC can be inhibited by unlabeled Gp96 molecules (Figures 1, 2). Through competition experiments with monoclonal antibodies, the 2 known receptor candidates, CD91 and CD36, could be identified as Gp96 receptors on platelets. Competition experiments with both antibodies in combination have been performed (data not shown) but did not show significant additive competition. However, further investigations with more sensitive techniques are needed to reveal whether CD36 and CD91 act together in a receptor complex or whether they form 2 independent receptors. More interestingly, Gp96-FITC binding is enhanced approximately 10-fold after the activation of thrombocytes (Figure 1). This implies an even greater neutralizing effect of platelets on the immunostimulatory capacity of Gp96, when activated during tissue damage accompanied by blood vessel disruption. The presence of physiological amounts of platelets reduces the Gp96-induced secretion of proinflammatory cytokines TNF-α and IL-12 (Figure 4A) and the maturation of DCs, as analyzed by the diminished up-regulation of the activation markers CD83 and CD86 (Figure 4B). Given that the activation status of DCs correlates with their ability to stimulate T cells,13 the induction of immune responses will be impaired. Moreover, the immature DC phenotype and the reduced levels of proinflammatory cytokines in the presence of platelets might even favor the induction of tolerance toward self-antigens released during mechanical tissue damage and possibly presented by immature DCs.28-31 It is important to note that the binding of Gp96 to platelets does not interfere with their function. This is demonstrated by unchanged platelet activation and aggregation in the presence of Gp96 (Figure 3A-B). The functional insensitivity of platelets toward Gp96 binding makes perfect sense because it ensures that though Gp96 is neutralized, wound healing still can take place properly.
The binding of Gp96 on platelets has an impact on the activation of DCs by HSPs when both are present in culture. This inhibition might be the result of competition: the high number of Gp96-binding sites on platelets is likely to reduce the concentration of free heat-shock protein available for binding to APCs. Because 80% of the platelet surface is invaginated building an open canalicular system, a great portion of bound Gp96 is not accessible at all for other cells but is hidden in these invaginations. It cannot be excluded, however, that other mechanisms enhance or diminish the observed immunosuppressive effects of platelets. Thrombocytes themselves may respond to Gp96 binding in a yet unidentified way, leading to an altered DC activation. In our system, secreted platelet factors were not involved in immunosuppression, or fixed platelets would not have been able to reduce DC activation (Figure 5A). Another possible mechanism could be that activated platelets adhere to DCs and modify their responses to external stimuli through direct interaction, as has been shown for other cell types. Isolated monocytes produce various chemokines in the presence of activated platelets.32-34 Autologous platelets enhance the IL-1 and TNF-α responses of PBMCs after LPS stimulation.35 For the latter effect, a direct platelet adhesion to monocytic cells through P-selectin on the platelet surface has proven to be essential, though the activation signal itself is given by other, probably soluble, mediators.32 P-selectin also mediates the specific interaction between neutrophils and activated thrombocytes,36 but the implications on inflammatory events are controversially discussed.37,38Our results suggest that, unlike monocytes, DCs are not activated by platelets themselves at the indicated concentrations (Figure 4B). In contrast, the inflammatory response to Gp96 is diminished. This effect is not dependent on cell-to-cell contact, as shown by transwell experiments (Figure 5). Nevertheless, direct contact between platelets and DCs might contribute to the observed immunosuppressive effect of thrombocytes or might alter the activation state of DCs. Recently, it has been reported that Langerhans cells, a special DC subtype, are activated if they are cocultured with fixed activated platelets from a heterologous source.39 The authors attribute the observed maturation of Langerhans cells to the stimulating capacities of CD40L molecules on activated platelets.20 In our experiments using only low concentrations of autologous thrombocytes, no DC activation or pro-inflammatory cytokine production could be observed without the addition of Gp96 (Figure 4).
Taken together, platelets might influence DC activation on at least 3 different levels—first, neutralization of DC-activating substances (eg Gp96); second, release of cytokines; third, direct interaction through membrane-associated molecules (eg, CD40L/CD40). Another fact supports the relevance of the Gp96-neutralizing effect of platelets in vivo. The platelet–DC ratio used in our experiments is far lower than that in peripheral blood. With 0.6% DCs among all PBMCs and up to 2 × 106 PBMCs per microliter blood, a maximal DC concentration of 12 cells per microliter can be calculated, whereas the platelet concentration varies between 1.5 × 105 and 4 × 105 per microliter. Thus, the platelet–DC ratio in human blood is approximately 104:1. In our experiments we used ratios of 20:1 (coculture) or 115:1 (transwell experiment) and observed a significant influence on Gp96-induced DC maturation. There are no reliable data on the number of DCs and platelets in a scenario of tissue injury with blood vessel disruption, but it seems unlikely that the platelet–DC ratio would be lower than 20:1. In wounds, however, the relevance of the observed DC platelet phenomenon can only be judged when data on the ratio of this cell type in damaged tissue become available.
Recently, other HSPs have been shown to interact with Gp96 receptor CD91,40 41 which is also expressed on thrombocytes. It must be evaluated whether these proteins bind to platelets as Gp96 does. Nevertheless, the interaction of Gp96, and possibly of other HSPs, with thrombocytes can be expected to have important implications in the prevention of systemic inflammation and in reducing the secretion of proinflammatory cytokines in healing wounds.
Acknowledgments
We thank Prof M. Böck (University of Würzburg, Germany) for reading the manuscript and for helpful remarks concerning platelet function analysis, and we thank Dr M. Waidmann and A. Peterfi (Bloodbank, Tübingen) for technical expertise and assistance with platelet aggregation measurements.
Supported by grants of the Deutsche Forschungsgemeinschaft (Sonderforschungsbereich 510, C1 to H.S.) and the European Union (EC Project QLK3-CT-1999-00064).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Hansjörg Schild, Department of Immunology, Institute for Cell Biology, University of Tübingen, Auf der Morgenstelle 15, D-72076 Tübingen, Germany; e-mail:hansjoerg.schild@uni-tuebingen.de.