Constitutive nuclear factor kappaB (NF-κB) activity protects quiescent mature immune cells from spontaneous apoptosis. Here, we examined whether NF-κB exerts its antiapoptotic function in these cells through the control of Bcl-2 family proteins. Specific pharmacologic inhibitors of NF-κB were used to achieve total NF-κB inactivation in quiescent human blood lymphocytes, granulocytes, and monocytes. NF-κB inhibition induced drastic lymphocyte and granulocyte apoptosis, but only moderate monocyte apoptosis. T- and B-cell apoptosis was slow and associated with a gradual down-regulation of the prosurvival Bcl-2 family proteins Bcl-xL and Bcl-2, respectively. By contrast, granulocyte apoptosis was fast and accompanied by a rapid cellular accumulation of Bcl-xS, the proapoptotic Bcl-x isoform that is generated from alternative splicing of the bcl-x pre-mRNA. Finally, antisense bcl-xL and bcl-2knockdown in T and B cells, respectively, and induction of Bcl-xS expression in granulocytes through antisense oligonucleotide-mediated redirection of bcl-x pre-mRNA splicing were sufficient to induce significant apoptosis in these cells. Taken together, these results reveal that basal NF-κB activity preserves homeostasis of quiescent mature lymphocytes and granulocytes through regulation of distinct members of the Bcl-2 family. This study sheds light on the constitutive mechanisms by which NF-κB maintains defense integrity.
Introduction
Quiescent mature immune cells (QMICs) form a reservoir that may be rapidly engaged in innate or adaptive immune responses. In normal conditions, the number of QMICs remains constant,1 thus ensuring the maintenance of an optimal defense potential. This suggests that homeostatic mechanisms tightly control both the emergence of newly formed QMICs and the further survival of these cells. Although crucial, the molecular basis of QMIC survival remains elusive. However, convergent studies have shown that the constitutive presence of transcriptionally active nuclear factor kappaB (NF-κB) complexes in resting lymphocytes and phagocytes allows these cells to escape spontaneous apoptosis, thus contributing to the maintenance of the size of the QMIC pool.2-5
NF-κB is present in an inducible form in virtually all cell types6 and in a constitutively activated form in most immune cells and certain neurons.2,4,7,8 The NF-κB family is composed of 5 structurally related DNA-binding proteins, designated NF-κB1/p50, NF-κB2/p52, RelA/p65, Rel/c-Rel, and RelB.9 RelA, Rel, and RelB contain a C-terminal transactivation domain, whereas p50 and p52 lack a transactivating domain and are not transcriptionally active. Although the most common NF-κB complex is a heterodimer of the RelA and p50 subunits, the different family members can associate in various homo- or heterodimer combinations. Inactive NF-κB complexes are sequestered in the cytosol by inhibitory proteins of the IκB family. Following various stimuli, IκB proteins are phosphorylated, ubiquinated, and degraded by the proteasome, allowing NF-κB nuclear translocation and transcriptional initiation of NF-κB–dependent genes.
NF-κB–dependent genes are involved in development, immunity, and cell proliferation and survival.10 NF-κB target genes implicated in cell death prevention include those encoding the tumor necrosis factor (TNF) receptor-associated factors TRAF1 and TRAF2, the inhibitor of apoptosis proteins c-IAP1, c-IAP2, and X-IAP, the zinc finger protein A20, the immediate-early response protein IEX-1L, and the manganese superoxide dismutase.11 Recently, the genes encoding Bcl-xL and Bfl-1/A1, which are antiapoptotic proteins of the Bcl-2 family, have also been identified as NF-κB–dependent transcriptional targets.12-15 The Bcl-2 family members are essential regulators of cell survival that exhibit either antiapoptotic (Bcl-2, Bcl-xL, Bfl-1/A1, etc) or proapoptotic (Bax, Bcl-xS, Bad, Bid, etc) activities.16 17
To date, only few studies have been devoted to the role of NF-κB–dependent expression of antiapoptotic Bcl-2 family proteins in promoting survival of primary immune cells. However, Grossmann and colleagues18 have recently established that NF-κB activation serves a key antiapoptotic function during the later stages of B-cell maturation through the induction of prosurvival Bcl-2 homologues. In addition, NF-κB activation and subsequent Bfl-1 expression protect mature B cells from antigen receptor ligation-induced apoptosis,13 and T-cell activation via the T-cell receptor (TCR) enhances survival via a pathway involving the serine/threonine kinase protein kinase Bα, NF-κB, and Bcl-xL.19 Although these observations reveal an essential role for NF-κB–induced expression of prosurvival Bcl-2 homologues in preventing the apoptosis of immune cells during maturation and activation, the question of whether this mechanism is also crucial for QMIC survival has not been addressed, except in resting macrophages, where constitutive NF-κB activity is required to maintain Bfl-1 expression and mitochondrial homeostasis.3
Here, we demonstrate using specific pharmacologic inhibitors of NF-κB that basal NF-κB activity protects quiescent mature lymphocytes and granulocytes from spontaneous apoptosis through the regulation of distinct members of the Bcl-2 family. This study is the first to shed light on the constitutive mechanisms by which NF-κB maintains peripheral lymphocyte and granulocyte homeostasis, thereby preserving defense integrity.
Materials and methods
Cell sorting, culture, and treatment
Human blood lymphocytes, monocytes, and granulocytes were obtained from buffy coats (Transfusion Center, Liege, Belgium). Mononuclear cells were separated from granulocytes by density centrifugation (Histopaque; Sigma, Bornem, Belgium). Contaminating erythrocytes were removed from the granulocyte fraction by hypotonic lysis. Granulocyte purity, as determined by counting of cytospin preparations stained with Diff-Quick (Dade Behring, Dudingen, Germany), was always more than 95%. T cells, B cells, and monocytes were purified by negative magnetic selection using microbeads coated with antibodies directed against unwanted cells (Pan T-cell isolation kit, B-cell isolation kit, and monocyte isolation kit; Miltenyi Biotec, Paris, France). This procedure yielded T-cell, B-cell, and monocyte populations that were more than 98% positive for the CD3, CD19, and CD14 markers, respectively, as determined by flow cytometry analyses (FACStar Plus; Becton Dickinson, San Jose, CA). Cells were cultured at 2 × 106/mL in RPMI 1640 medium supplemented with 1% glutamine, 10% fetal calf serum, 50 μg/mL streptomycin, and 50 IU/mL penicillin (all from Gibco BRL, Merelbeke, Belgium). Cells were cultured in the presence or absence of gliotoxin (GTX; Sigma), its inactive analogue methylthiogliotoxin (mGTX; Sigma), cyclopentenone prostaglandin A1 (PGA1; Cayman Chemical, Ann Arbor, MI), or oligonucleotides (see below) for different times before analysis.
Antibodies
Fluorescein isothiocyanate (FITC)–conjugated anti-CD3 (MCA463F), anti-CD14 (MCA596F), and anti-CD19 (MCA1111F) monoclonal antibodies were purchased from Serotec (Oxford, United Kingdom). The antibodies specific for p50 (sc-114 X), p52 (sc-298 X), RelA (sc-372 X), c-Rel (sc-70 X), RelB (sc-226 X), Bfl-1 (sc-8351), Bcl-2 (sc-7382), and α-tubulin (sc-8035) were obtained from Santa Cruz Biotechnology (Santa Cruz, CA). The monoclonal antibody directed against Bax (Ab-3) was purchased from Oncogene Research Products (Darmstadt, Germany), and the monoclonal antibody recognizing Bcl-xL/S (B61220) was obtained from Transduction Laboratories (Lexington, KY). The specificity of the anti–Bfl-1 and –Bcl-2 antibodies was verified using recombinant Bcl-2, Bcl-xL, Bak, and Bax of human origin, and protein extracts from TNF-α–stimulated A549 cells, which contain high amounts of Bfl-1. Recombinant proteins were obtained from Santa Cruz Biotechnology, except the recombinant Bax, which was produced in our laboratory.
Apoptosis assays
Apoptosis was assessed by staining with annexin V and propidium iodide (PI) using the Annexin-V-FLUOS staining kit (Roche, Mannheim, Germany) following the recommendations of the manufacturer. Flow cytometry analyses were performed with a FACStar Plus (Becton Dickinson).
Nuclear protein extraction
Nuclear protein extracts were prepared as previously described.20 Cytoplasmic buffer contained 10 mM HEPES, pH 7.9, 10 mM KCl, 2 mM MgCl2, 0.1 mM ethylenediaminetetraacetic acid (EDTA), 0.2% (vol/vol) NP-40, 1.6 mg/mL protease inhibitors (Complete, Roche), and 3 mM of the serine protease inhibitor diisopropyl fluorophosphate (DFP; Sigma). Pelleted nuclei were resuspended in 20 mM HEPES, pH 7.9, 1.5 mM MgCl2, 0.2 mM EDTA, 0.63 M NaCl, 25% (vol/vol) glycerol, 1.6 mg/mL protease inhibitors, and 3 mM DFP (nuclear buffer), incubated for 20 minutes at 4°C and centrifuged for 30 minutes at 14 000 rpm. Protein amounts were quantified with the Micro BCA protein assay reagent kit (Pierce, Rockford, IL).
Electrophoretic mobility shift assays
Binding reactions were performed for 30 minutes at room temperature with 5 μg nuclear proteins in 20 mM Hepes, pH 7.9, 10 mM KCl, 0.2 mM EDTA, 20% (vol/vol) glycerol, 1% (wt/vol) acetylated bovine serum albumin, 3 μg poly(dI-dC) (Amersham Pharmacia Biotech, Aylesbury, United Kingdom), 1 mM dithiothreitol, 1 mM phenylmethylsulfonyl fluoride, and 100 000 cpm of [32P]-labeled double-stranded oligonucleotide probes. Probes were prepared by annealing the appropriate single-stranded oligonucleotides (Eurogentec, Liege, Belgium) at 65°C for 10 minutes in 10 mM Tris, 1 mM EDTA, and 10 mM NaCl, followed by slow cooling to room temperature. The probes were then labeled by end-filling with the Klenow fragment of Escherichia coli DNA polymerase I (Roche), with [α-32P]-dATP and [α-32P]-dCTP (Dupont-New England Nuclear [NEN] Life Science Products, Les Ulis, France). Labeled probes were purified by spin chromatography on Sephadex G-25 columns (Roche). DNA-protein complexes were separated from unbound probe on 4% native polyacrylamide gels at 150 V in 0.25 M Tris, 0.25 M sodium borate, and 0.5 mM EDTA, pH 8.0. Gels were vacuum-dried and exposed to Fuji x-ray film at −80°C for 12 hours. To confirm specificity, competition assays were performed with a 50-fold excess of unlabeled wild-type probes and with mutated probes. For supershifting experiments, 1.5 μL of each antibody was incubated with the extracts for 30 minutes before addition of the radiolabeled probe. The sequences of the oligonucleotides used in this work were as follows: wild-type palindromic κB probe, 5′-TTG GCA ACG GCA GGG GAA TTC CCC TCT CCT TAG GTT-3′; mutated palindromic κB probe, 5′-TTG GCA ACG GCA GAT CTA TTC CCC TCT CCT TAG GTT-3′.
Immunoblots
Whole-cell extracts (10 μg) were added to a loading buffer (10 mM Tris-HCl, pH 6.8, 1% [wt/vol] sodium dodecyl sulfate, 25% [vol/vol] glycerol, 0.1 mM β-mercaptoethanol, 0.03% [wt/vol] bromophenol blue), boiled, and run on a 10% sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) gel. After electro-transfer to polyvinylidene difluoride membranes (Roche) and blocking overnight at 4°C with 20 mM Tris, pH 7.5, 500 mM NaCl, 0.2 (vol/vol) Tween 20 (Tris/HCl/Tween) plus 5% (wt/vol) dry milk, the membranes were incubated for 1 hour with the first antibody (1:200 dilution), washed, and then incubated for 45 minutes with peroxidase-conjugated goat anti–rabbit IgG for Bfl-1 (1:5000 dilution; Kirkegaard & Perry Laboratories, Gaithersburg, MD) and peroxidase-conjugated rabbit anti–mouse IgG for Bax, Bcl-2, and Bcl-xL/S (1:1000 dilution; Dako, Glostrup, Denmark). The reaction was revealed using the enhanced chemiluminescence detection method (ECL kit, Amersham Pharmacia Biotech). Equal loading of proteins on the gel was confirmed by probing the blots for α-tubulin (data not shown).
Reverse transcription–polymerase chain reactions
Total RNA was extracted from cells using the Rneasy Mini kit according to manufacturer's instructions (Qiagen, Hilden, Germany). Poly(A) RNA was primed with oligo(dT) (Roche) and reverse transcribed with AMV reverse transcriptase (Roche) for 1 hour at 42°C. cDNA products were amplified by polymerase chain reaction (PCR) using primers specific for bcl-2 (5′ primer GAT GTC CAG CCA GCT GCA CCT G; 3′ primer CAC AAA GGC ATC CCA GCC TCC),bcl-xL/S (5′ primer ATG GCA GCA GTA AAG CAA G; 3′primer GCT GCA TTG TTC CCA TAG A), and glyceraldehyde 3-phosphate dehydrogenase (GAPDH) as a control (5′ primer ACT GGC ATG GCC TTC CGT GT; 3′ primer TTA CTC CTT GGA GGC CAT GT). Thebcl-xL/S primers allowed simultaneous amplification of bcl-xL (353 base pair [bp]) and bcl-xS (164 bp). All primers were purchased from Eurogentec. A 50 μL PCR reaction was set up containing 5 μL cDNA, 10 mM Tris-HCl, 25 pmol of each primer, 1.5 mM MgCl2, 0.2 mM dNTP, and 2.5 U of AmpliTaq DNA polymerase (Perkin Elmer, Boston, MA). Amplification consisted of 30 (bcl-2) or 35 (bcl-xL/S) cycles of denaturation at 94°C for 20 seconds, annealing at 60°C (bcl-2) or 56°C (bcl-xL/S) for 30 seconds, and extension at 72°C for 1 minute. Amplification products were electrophoresed on 1.2% agarose gels, and PCR product quantities were assessed by densitometry (Gel Doc 2000; Bio-Rad, Hercules, CA).
Antisense knockdown of bcl-2 and bcl-x
T and B cells were treated with antisense (AS) oligodeoxyribonucleotides (ODNs) sequences complementary tobcl-x and bcl-2 mRNA, respectively. Phosphorothioate backbone AS ODNs targeting bcl-x andbcl-2 were synthesized by Eurogentec. Their sequences and those of scrambled (SC) ODNs, which were used as negative controls, were as follows: bcl-x AS ODN, 5′-TGT ATC CTT TCT GGG AAA GC-3′; bcl-x SC ODN, 5′-TAA GTT CCG ATG CGA CTT GT-3′;bcl-2 AS ODN, 5′-TCT CCC AGC GTG CGC CAT-3′;bcl-2 SC ODN, 5′-TAC CGC GTG CGA CCC TCT-3′. The ODNs were delivered to the cells in the form of complexes with a liposome formulation of the cationic lipid 1,2-dimyristyloxypropyl-3-dimethyl-hydroxy ethyl ammonium bromide (DMRIE) and cholesterol (DMRIE-C Reagent; Gibco BRL). A quantity of 1 mL OPTI-MEM I Reduced Serum Medium (Gibco BRL) containing 24 μL DMRIE-C and 1 mL OPTI-MEM I containing 8 μg DNA was mixed and allowed to complex for 45 minutes at room temperature. A quantity of 2 × 106 freshly isolated cells in 0.25 mL of serum-free medium were then added to the transfection medium and incubated for 4 hours at 37°C in a CO2 incubator. Afterward, 2 mL of growth medium containing 20% fetal calf serum was added, and the cells were cultured for another 20 hours.
Oligoribonucleotide-mediated redirection of bcl-xpre-mRNA splicing
Expression of Bcl-xS was induced in granulocytes through oligoribonucleotide (ORN)–mediated redirection ofbcl-x pre-mRNA splicing, as recently described by Taylor and coworkers,21 except that a 20-mer phosphorothioate AS ORN containing uniform 2′-O-methyl (2′-OMe) modifications was used. Its sequence was as follows: 5′-CUG GAU CCA AGG CUC UAG GU-3′. An ORN containing 5 mismatched (MM) bp was used as a control (5′-CUG GUU ACA CGA CUC CAG GU-3′). The ORNs were synthesized by Eurogentec. Transfection was performed as described above, except that granulocytes were left in the transfection medium for 8 hours before analysis.
Results
Total inhibition of constitutive NF-κB activity results in late lymphocyte and early granulocyte apoptosis
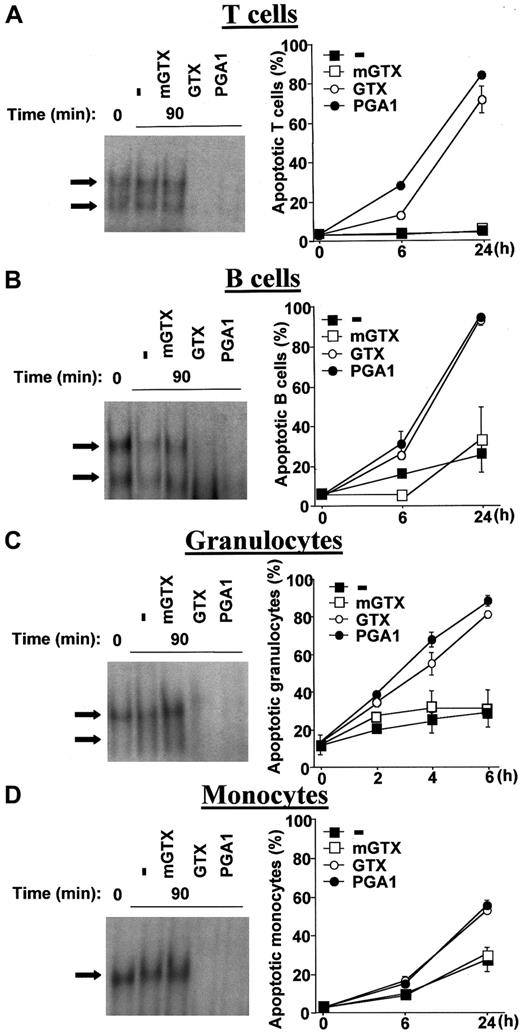
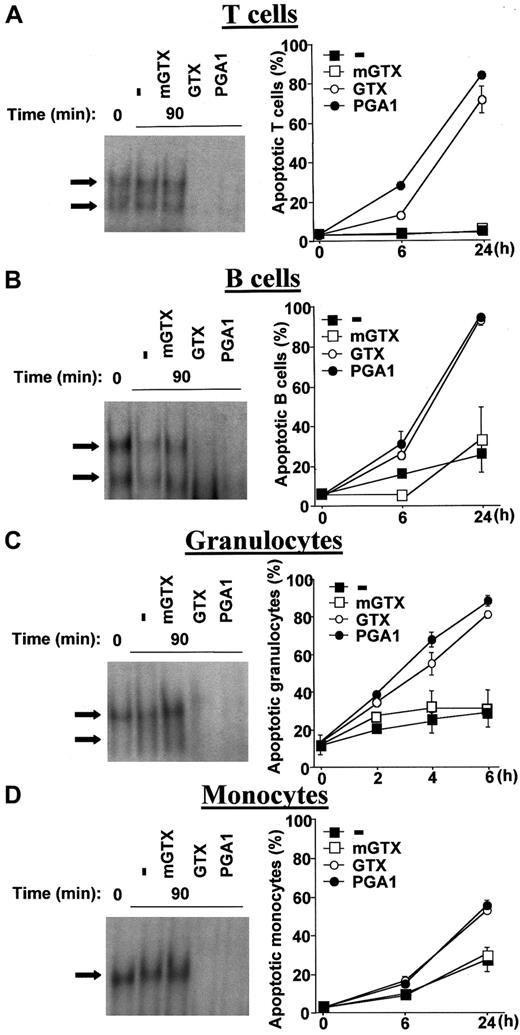
The fungal metabolite gliotoxin (GTX) and the cyclopentenone prostaglandin A1 (PGA1), 2 specific pharmacologic inhibitors of NF-κB,22-24 were used to obtain total NF-κB inactivation in QMICs. Quiescent human blood T and B cells, granulocytes, and monocytes were isolated from buffy coats by density centrifugation and negative magnetic selection and cultured in the presence or absence of various doses of GTX, its inactive analogue methylthiogliotoxin (mGTX), or PGA1 before analysis of NF-κB DNA-binding by electrophoretic mobility shift assays (EMSAs). Quantities of 1.5 μM GTX and 48 μM PGA1 were sufficient to obtain total NF-κB inhibition in lymphocytes and granulocytes, whereas 5 μM GTX and 96 μM PGA1 were required to completely inhibit NF-κB activity in monocytes (Figure 1A-D). These concentrations were used throughout the study. Total inhibition of NF-κB activity was always observed 90 minutes after treatment with GTX or PGA1. mGTX did not affect NF-κB activity in any cell type (Figure 1A-D).
Inhibition of constitutive NF-κB activity by GTX and PGA1 induces QMIC apoptosis.
Human blood T cells (A), B cells (B), granulocytes (C), and monocytes (D) were isolated from buffy coats by density centrifugation and negative magnetic selection and cultured for 90 minutes in the presence or absence of 1.5 μM mGTX, 1.5 μM GTX, or 48 μM PGA1, except for monocytes, which were treated with 5 μM mGTX, 5 μM GTX, or 96 μM PGA1. Nuclear extracts were then prepared and analyzed for NF-κB–binding activity by EMSAs. The arrows indicate specific NF-κB complexes. EMSAs are representative of at least 3 comparable assays. Untreated and treated cells were also cultured for 6 hours and 24 hours (mononuclear cells) or 2 hours, 4 hours, and 6 hours (granulocytes) before conducting apoptosis assays using a dual-color annexin-V–FITC/PI staining and flow cytometry analyses. Data are presented as means ± standard deviations (n = 6).
Inhibition of constitutive NF-κB activity by GTX and PGA1 induces QMIC apoptosis.
Human blood T cells (A), B cells (B), granulocytes (C), and monocytes (D) were isolated from buffy coats by density centrifugation and negative magnetic selection and cultured for 90 minutes in the presence or absence of 1.5 μM mGTX, 1.5 μM GTX, or 48 μM PGA1, except for monocytes, which were treated with 5 μM mGTX, 5 μM GTX, or 96 μM PGA1. Nuclear extracts were then prepared and analyzed for NF-κB–binding activity by EMSAs. The arrows indicate specific NF-κB complexes. EMSAs are representative of at least 3 comparable assays. Untreated and treated cells were also cultured for 6 hours and 24 hours (mononuclear cells) or 2 hours, 4 hours, and 6 hours (granulocytes) before conducting apoptosis assays using a dual-color annexin-V–FITC/PI staining and flow cytometry analyses. Data are presented as means ± standard deviations (n = 6).
To characterize the NF-κB complexes present in QMICs, supershift experiments were performed with antibodies directed against the various members of the NF-κB family. These experiments identified RelA/p50, c-Rel/p50, and p50/p50 dimers in lymphocytes and granulocytes, whereas only p50 homodimers were present in monocytes (data not shown). DNA-binding competition experiments using 50-fold excess of unlabeled wild-type and mutated palindromic κB probes confirmed the specificity of NF-κB binding in all cell types (data not shown).
To determine the effects of total NF-κB inhibition on lymphocyte, granulocyte, and monocyte longevity, purified cells were cultured for different times in the presence or absence of mGTX, GTX, or PGA1 and assayed for apoptosis and necrosis using dual- color annexin-V–FITC/PI staining and flow cytometry analyses. NF-κB inhibition caused a drastic induction of lymphocyte death (Figure 1A-B). Nearly all dead lymphocytes were annexin-V-FITC–positive and PI-negative, demonstrating that loss of viability was mainly due to apoptosis rather than necrosis (data not shown). Although increased lymphocyte apoptosis was already detectable at 6 hours, most cells died later. At 24 hours, when the rate of spontaneous apoptosis was 5.3% ± 0.4% in T cells and 26.2% ± 9.7% in B cells, NF-κB inhibition caused more than 70% and more than 90% apoptosis, respectively. Both GTX and PGA1 induced early and massive granulocyte apoptosis (Figure 1C). Indeed, apoptosis of GTX- and PGA1-treated granulocytes reached 80% at 6 hours, while the rate of spontaneous apoptosis was less than 30%. The effects of complete NF-κB inhibition were less pronounced in monocytes (Figure 1D). After 24 hours of GTX and PGA1 treatment, the number of apoptotic monocytes was only increased by 25% to 30% compared with untreated controls. mGTX had no significant effect on the spontaneous rate of lymphocyte, granulocyte, and monocyte apoptosis (Figure 1A-D). To ascertain that apoptosis of GTX- and PGA1-treated lymphocytes and granulocytes specifically resulted from NF-κB inhibition rather than from nonspecific cytotoxicity of these drugs, A549 cells, which do not display constitutive NF-κB activity, have been treated with 10 μM GTX or 100 μM PGA1. These treatments did not affect A549 cell viability over a period of 24 hours, as determined by dual-color annexin-V–FITC/PI staining (data not shown), confirming that the effects of GTX and PGA1 were specific.
These data (1) demonstrate that constitutive NF-κB activity in QMICs may be totally inhibited by GTX and PGA1; (2) confirm that NF-κB is essential for the survival of these cells, especially lymphocytes and granulocytes; and (3) show that complete NF-κB deactivation is associated with slow lymphocyte and fast granulocyte apoptosis.
Complete NF-κB inactivation induces Bcl-xL or Bcl-2 down-regulation in lymphocytes and Bcl-xS expression in granulocytes
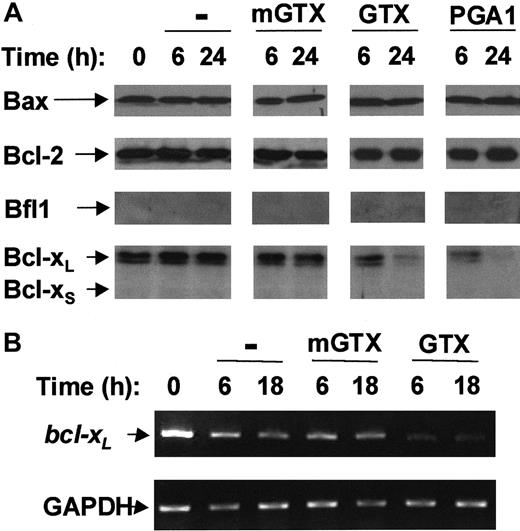
NF-κB has been demonstrated to induce the expression of Bcl-xL and Bfl-1, 2 antiapoptotic proteins of the Bcl-2 family.12-15 To address the question of whether constitutive NF-κB activity protects QMICs from apoptosis through the control of Bcl-2 family proteins, the expression levels of 5 representative members of this family, namely Bax, Bcl-2, Bfl-1, and Bcl-xL/S, were assessed by immunoblots and reverse transcription (RT)–PCRs in untreated and in GTX-, mGTX-, and PGA1-treated QMICs.
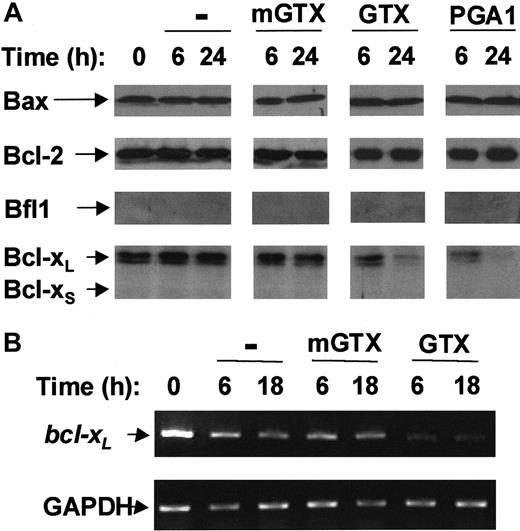
Bfl-1 and Bcl-xS were undetectable in freshly isolated blood T cells. Conversely, T cells contained Bax, Bcl-2, and Bcl-xL proteins (Figure 2A). Bax, Bcl-2, and Bcl-xL protein levels remained constant over a period of at least 24 hours in untreated and mGTX-treated T cells. Neither Bax nor Bcl-2 protein levels were modified following GTX or PGA1 treatment. By contrast, the amount of Bcl-xLprotein in T cells began to decrease 6 hours after NF-κB inactivation reaching nearly undetectable levels at 24 hours (Figure 2A). To confirmbcl-xL down-regulation in GTX- and PGA1-treated T cells, RT-PCR analyses were performed (Figure 2B; data not shown).bcl-xL mRNA levels were dramatically reduced 6 hours after GTX or PGA1 treatment and remained low for at least 18 hours. Conversely, bcl-xL mRNA levels remained constant throughout the procedure in untreated and mGTX-treated T cells.
Complete NF-κB inactivation induces Bcl-xLdown-regulation in quiescent human blood T cells.
(A) Blood T cells were isolated from buffy coats by negative magnetic selection and cultured for 6 hours and 24 hours in the presence or absence of 1.5 μM mGTX, 1.5 μM GTX, or 48 μM PGA1. Whole-cell extracts were then prepared and analyzed by immunoblotting for Bax, Bcl-2, Bfl1, and Bcl-xL/S expression. (B) RNA was prepared from blood T cells cultured for 6 hours and 18 hours in the presence or absence of 1.5 μM mGTX or 1.5 μM GTX and analyzed by RT-PCR for expression of bcl-xL. As a control for quantification, glyceraldehyde phosphate dehydrogenase (GAPDH) was also amplified. These results are representative of at least 3 comparable experiments.
Complete NF-κB inactivation induces Bcl-xLdown-regulation in quiescent human blood T cells.
(A) Blood T cells were isolated from buffy coats by negative magnetic selection and cultured for 6 hours and 24 hours in the presence or absence of 1.5 μM mGTX, 1.5 μM GTX, or 48 μM PGA1. Whole-cell extracts were then prepared and analyzed by immunoblotting for Bax, Bcl-2, Bfl1, and Bcl-xL/S expression. (B) RNA was prepared from blood T cells cultured for 6 hours and 18 hours in the presence or absence of 1.5 μM mGTX or 1.5 μM GTX and analyzed by RT-PCR for expression of bcl-xL. As a control for quantification, glyceraldehyde phosphate dehydrogenase (GAPDH) was also amplified. These results are representative of at least 3 comparable experiments.
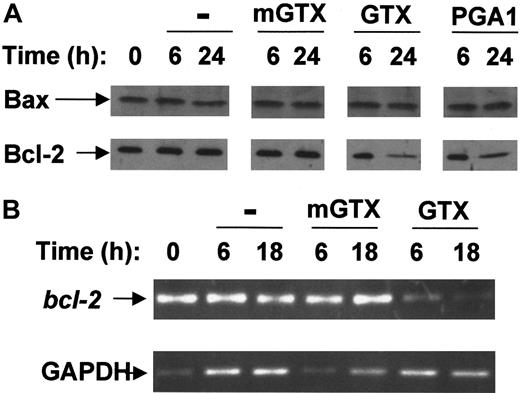
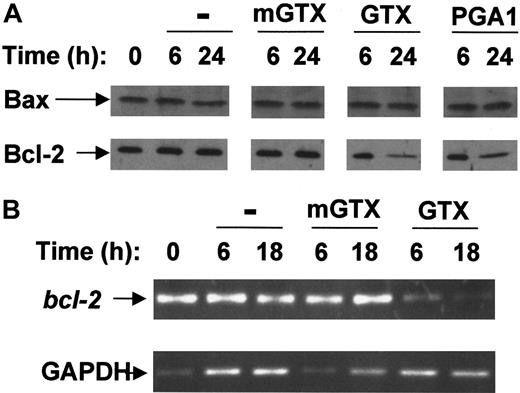
Freshly purified blood B cells expressed only Bax and Bcl-2 proteins (Figure 3A). Untreated and mGTX-treated B cells maintained high levels of Bax and Bcl-2 proteins for at least 24 hours. By contrast, the amount of Bcl-2 protein gradually decreased following NF-κB inhibition reaching very low levels at 24 hours (Figure 3A). At 6 hours and 18 hours,bcl-2 mRNA was drastically reduced in GTX- and PGA1-treated B cells compared with untreated and mGTX-treated controls (Figure 3B; data not shown), confirming bcl-2 down-regulation in NF-κB–inactivated B cells.
Complete NF-κB inhibition results in Bcl-2 down-regulation in resting human blood B cells.
(A) Blood B cells were isolated from buffy coats by negative magnetic selection and cultured for 6 hours and 24 hours in the presence or absence of 1.5 μM mGTX, 1.5 μM GTX, or 48 μM PGA1. Whole-cell extracts were analyzed by immunoblotting for Bax and Bcl-2 expression. (B) RNA prepared from blood B cells cultured for 6 hours and 18 hours in the presence or absence of 1.5 μM mGTX or 1.5 μM GTX was analyzed by RT-PCR for expression of bcl-2. To control for quantification, GAPDH was amplified. These results represent at least 3 comparable experiments.
Complete NF-κB inhibition results in Bcl-2 down-regulation in resting human blood B cells.
(A) Blood B cells were isolated from buffy coats by negative magnetic selection and cultured for 6 hours and 24 hours in the presence or absence of 1.5 μM mGTX, 1.5 μM GTX, or 48 μM PGA1. Whole-cell extracts were analyzed by immunoblotting for Bax and Bcl-2 expression. (B) RNA prepared from blood B cells cultured for 6 hours and 18 hours in the presence or absence of 1.5 μM mGTX or 1.5 μM GTX was analyzed by RT-PCR for expression of bcl-2. To control for quantification, GAPDH was amplified. These results represent at least 3 comparable experiments.
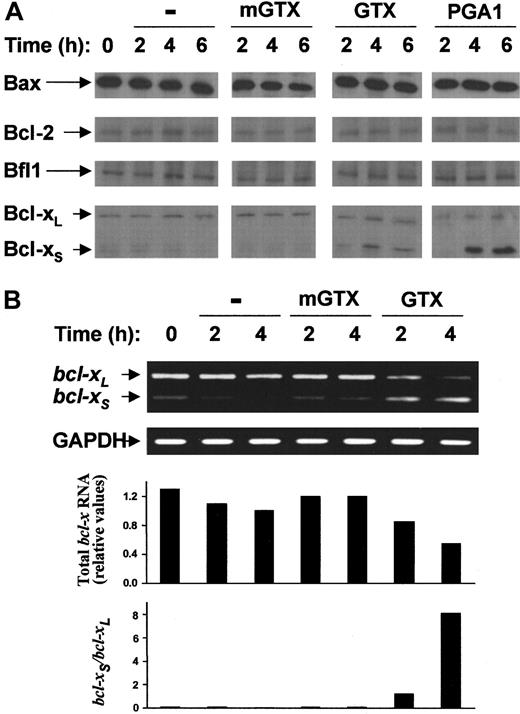
As shown in Figure 4A, blood granulocytes contained large amounts of the proapoptotic protein Bax but weakly expressed the antiapoptotic proteins Bcl-2, Bfl-1, and Bcl-xL. Bcl-xS was barely detectable in freshly isolated granulocytes. The levels of Bax, Bcl-2, Bfl-1, and Bcl-xL proteins remained unmodified for at least 6 hours after treatment with GTX or PGA1. Conversely, a rapid accumulation of Bcl-xS was observed in GTX- and PGA1-treated granulocytes but not in untreated and mGTX-treated controls (Figure 4A). Maximal levels of Bcl-xS protein were recorded as soon as 4 hours after NF-κB inhibition. Since alternate splicing of bcl-xpre-mRNA gives rise to 2 transcripts encoding either Bcl-xLor Bcl-xS,25 the results provided by immunoblots suggested that redirection of bcl-x pre-mRNA splicing from bcl-xL towardbcl-xS occurred in NF-κB–inactivated granulocytes. To confirm increased expression of the variantbcl-xS transcript in GTX- and PGA1-treated granulocytes, bcl-xL andbcl-xS mRNA isoforms were simultaneously amplified by RT-PCRs using primers designed to hybridize to common regions of bcl-xL andbcl-xS. Both bcl-xL andbcl-xS mRNAs were present in freshly purified granulocytes (Figure 4B). However, the levels ofbcl-xL mRNA were much higher than those ofbcl-xS mRNA. NF-κB inhibition led to a concomitant decrease in bcl-xL mRNA and increase in bcl-xS mRNA. This process was rapid: equal amounts of bcl-xL and bcl-xStranscripts were found in granulocytes as early as 2 hours after GTX or PGA1 treatment (Figure 4B; data not shown). At 4 hours, thebcl-xS/bcl-xL mRNA ratio was completely inverted relative to pretreatment conditions, as demonstrated by densitometry analyses (Figure 4B). At 4 hours,bcl-xS mRNA was indeed abundant, whereasbcl-xL mRNA was nearly undetectable. Densitometry analyses also showed that total bcl-x mRNA decreased with time in NF-κB–inactivated granulocytes (Figure 4B). Sequencing of PCR products confirmed that the amplified transcripts were identical to published bcl-xL andbcl-xS mRNA sequences25 (data not shown). To ascertain that the changes in the Bcl-xS/Bcl-xL ratio was NF-κB–dependent, A549 cells have been transiently transfected with the empty pCDNA3 plasmid or with a pCDNA3 plasmid encoding the superinhibitory form of IκB-α (ie, IκB-α with serine 32 and serine 36 mutated to alanine). Twenty-four hours after transfection, the cells were stimulated with TNF-α for 6 hours before being assayed for the expression of Bcl-xL and Bcl-xS using immunoblots. A549 cells transfected with the superinhibitory form of IκB-α before stimulation with TNF-α expressed Bcl-xS, whereas the controls did not (data not shown), demonstrating that Bcl-xS expression may result from specific NF-κB inhibition. To ensure that the superinhibitory form of IκB-α was functionally expressed in transfected cells, immunoblots and EMSAs were performed. These experiments showed that the superinhibitory form was expressed at high levels and strongly inhibited TNF-α–induced NF-κB activation in transfected A549 cells (data not shown).
Complete NF-κB inactivation induces rapid accumulation of Bcl-xS in quiescent human blood granulocytes.
(A) Blood granulocytes were isolated from buffy coats by density centrifugation and cultured for 2 hours, 4 hours, and 6 hours in the presence or absence of 1.5 μM mGTX, 1.5 μM GTX, or 48 μM PGA1. Whole-cell extracts were prepared and analyzed by immunoblotting for Bax, Bcl-2, Bfl-1, and Bcl-xL/S expression (B). RNA was prepared from blood granulocytes cultured for 2 hours and 4 hours in the presence or absence of 1.5 μM mGTX or 1.5 μM GTX and analyzed by RT-PCR for expression of bcl-xL/S. To control for quantification, GAPDH was also amplified. Filled columns show the amount of total bcl-x mRNA and the ratio betweenbcl-xS and bcl-xL mRNAs, as determined by densitometry analyses. These results represent at least 3 comparable experiments.
Complete NF-κB inactivation induces rapid accumulation of Bcl-xS in quiescent human blood granulocytes.
(A) Blood granulocytes were isolated from buffy coats by density centrifugation and cultured for 2 hours, 4 hours, and 6 hours in the presence or absence of 1.5 μM mGTX, 1.5 μM GTX, or 48 μM PGA1. Whole-cell extracts were prepared and analyzed by immunoblotting for Bax, Bcl-2, Bfl-1, and Bcl-xL/S expression (B). RNA was prepared from blood granulocytes cultured for 2 hours and 4 hours in the presence or absence of 1.5 μM mGTX or 1.5 μM GTX and analyzed by RT-PCR for expression of bcl-xL/S. To control for quantification, GAPDH was also amplified. Filled columns show the amount of total bcl-x mRNA and the ratio betweenbcl-xS and bcl-xL mRNAs, as determined by densitometry analyses. These results represent at least 3 comparable experiments.
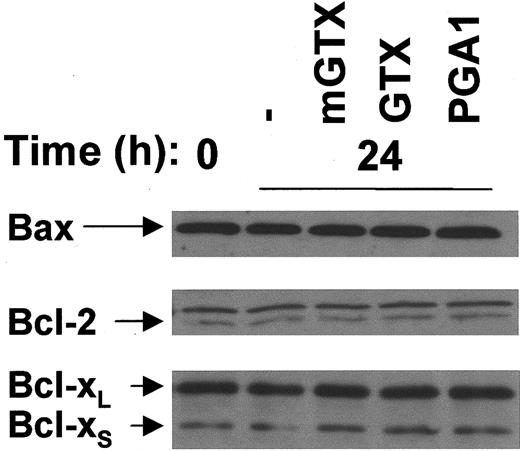
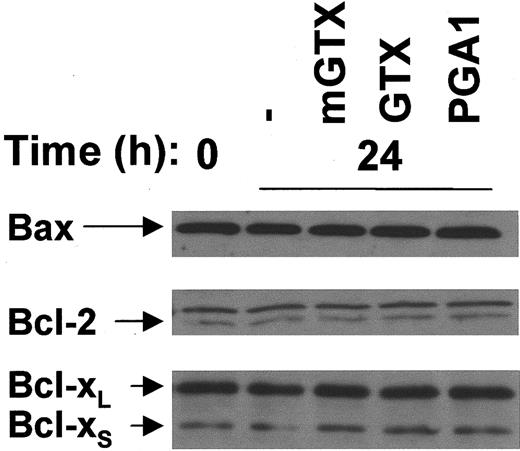
Bfl-1 was not detectable in monocytes (data not shown). The expression of Bax, Bcl-2, and Bcl-xL/S was not modified in monocytes cultured for 24 hours in the presence of GTX or PGA1 when compared with untreated and mGTX-treated controls (Figure5).
Complete inhibition of NF-κB does not affect Bax, Bcl-2, and Bcl-xL/S expression in human monocytes.
(A) Monocytes were isolated from buffy coats by negative magnetic selection and cultured for 24 hours with or without 5 μM mGTX, 5 μM GTX, or 96 μM PGA1. Whole-cell extracts were analyzed by immunoblotting for Bax, Bcl-2, and Bcl-xL/S expression. Comparable results were obtained in at least 3 experiments.
Complete inhibition of NF-κB does not affect Bax, Bcl-2, and Bcl-xL/S expression in human monocytes.
(A) Monocytes were isolated from buffy coats by negative magnetic selection and cultured for 24 hours with or without 5 μM mGTX, 5 μM GTX, or 96 μM PGA1. Whole-cell extracts were analyzed by immunoblotting for Bax, Bcl-2, and Bcl-xL/S expression. Comparable results were obtained in at least 3 experiments.
These results suggest that (1) gradual reduction in Bcl-xLand Bcl-2 expression may be responsible for inducing late apoptosis in NF-κB–inactivated mature T and B cells, respectively, and (2) rapid cellular accumulation of Bcl-xS following NF-κB inhibition may be the cause of the early granulocyte apoptosis.
Changes in Bcl-2 family protein expression are sufficient to explain the drastic induction of lymphocyte and granulocyte apoptosis following NF-κB inhibition
To confirm whether the alterations in Bcl-2 family protein expression observed in NF-κB–inactivated lymphocytes and granulocytes were sufficient to explain apoptosis induction in these cells, we determined (1) the effects of selectively decreasing the levels of Bcl-xL and Bcl-2 expression on T- and B-cell survival, respectively, and (2) the effects of selectively increasing the levels of Bcl-xS expression on granulocyte longevity. In these experiments, we used various oligonucleotides, which were delivered to the cells in the form of complexes with a liposome formulation of cationic DMRIE and DMRIE-C.
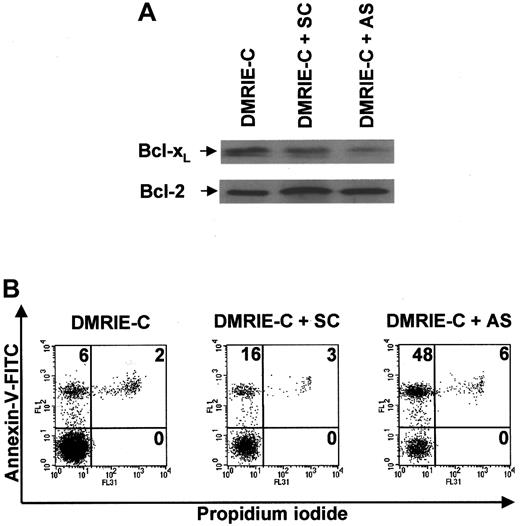
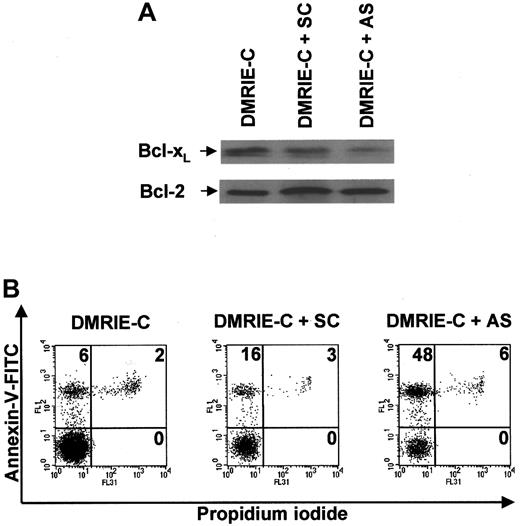
Exposure of freshly purified blood T cells to an optimal dose of DMRIE-C/bcl-x AS ODN complexes for 24 hours induced a significant decrease in Bcl-xL protein expression, whereas DMRIE-C alone had no effect (Figure6A). SC control bcl-x ODNs only weakly decreased the levels of Bcl-xL protein. None of these treatments affected the level of Bcl-2 expression used as a control. The ability of bcl-x AS ODNs to specifically down-regulate Bcl-xL allowed exploration of its role in T-cell survival. Apoptosis assays using dual-color annexin-V–FITC/PI staining and flow cytometry analyses were performed at 24 hours on T cells treated with DMRIE-C alone or in combination withbcl-x SC ODNs or bcl-x AS ODNs (Figure 6B).bcl-x AS ODNs consistently increased T-cell apoptosis by 40% ± 4%, whereas bcl-x SC ODNs increased apoptosis only by 10% ± 5%.
Antisense knockdown ofbcl-xL induces human blood T cell apoptosis.
Blood T cells were isolated from buffy coats by negative magnetic selection, cultured, and treated for 24 hours with DMRIE-C alone (24 μL per 2 × 106 cells) or DMRIE-C combined withbcl-x AS or SC ODNs (8 μg per 2 × 106cells). (A) Whole-cell extracts were prepared and analyzed by immunoblotting for Bcl-xL and Bcl-2. (B) Alternatively, apoptosis assays using dual-color annexin-V–FITC/PI staining and flow cytometry analyses were performed on treated T cells. The percentage of single- or double-positive cells in the individual quadrants is shown. These results are similar to at least 3 comparable experiments.
Antisense knockdown ofbcl-xL induces human blood T cell apoptosis.
Blood T cells were isolated from buffy coats by negative magnetic selection, cultured, and treated for 24 hours with DMRIE-C alone (24 μL per 2 × 106 cells) or DMRIE-C combined withbcl-x AS or SC ODNs (8 μg per 2 × 106cells). (A) Whole-cell extracts were prepared and analyzed by immunoblotting for Bcl-xL and Bcl-2. (B) Alternatively, apoptosis assays using dual-color annexin-V–FITC/PI staining and flow cytometry analyses were performed on treated T cells. The percentage of single- or double-positive cells in the individual quadrants is shown. These results are similar to at least 3 comparable experiments.
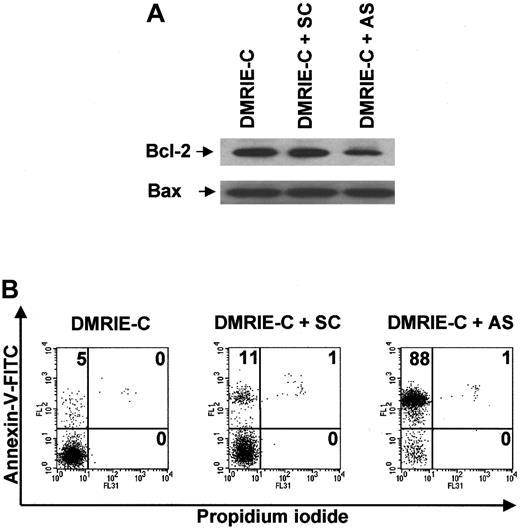
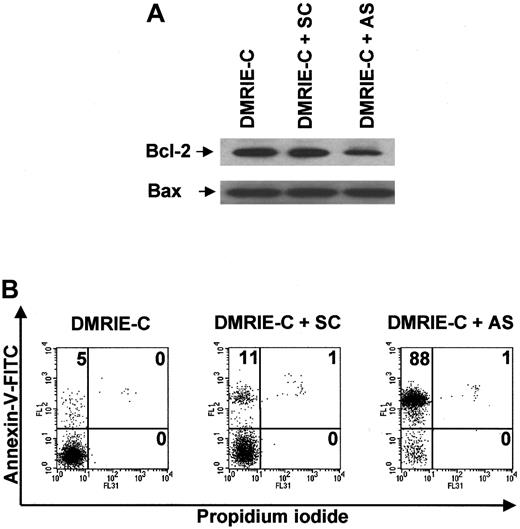
Blood B cells cultured for 24 hours in the presence of bcl-2AS ODNs exhibited reduced levels of Bcl-2 protein, whereas neitherbcl-2 SC ODNs nor DMRIE-C alone was able to alter Bcl-2 protein expression (Figure 7A). None of these treatments affected the level of Bax expression used as a control. Figure 7B shows that decreased Bcl-2 expression was associated with drastic induction of apoptosis in B cells treated withbcl-2 AS ODNs. Indeed, the percentage of apoptotic B cells averaged 85% ± 3% after AS treatment, whereas only 10% ± 2% apoptotic B cells were observed after SC treatment.
Antisense knockdown of bcl-2 leads to drastic human blood B-cell apoptosis.
Blood B cells were isolated from buffy coats by negative magnetic selection, cultured, and treated for 24 hours with DMRIE-C alone (24 μL per 2 × 106 cells) or DMRIE-C combined withbcl-2 AS or SC ODNs (8 μg per 2 × 106cells). (A) Whole-cell extracts were analyzed by immunoblotting for Bcl-2 and Bax. (B) Alternatively, apoptosis assays using dual-color annexin-V–FITC/PI staining and flow cytometry analyses were performed on treated B cells. The percentage of single- or double-positive cells in the individual quadrants is shown. These results were representative of at least 3 comparable experiments.
Antisense knockdown of bcl-2 leads to drastic human blood B-cell apoptosis.
Blood B cells were isolated from buffy coats by negative magnetic selection, cultured, and treated for 24 hours with DMRIE-C alone (24 μL per 2 × 106 cells) or DMRIE-C combined withbcl-2 AS or SC ODNs (8 μg per 2 × 106cells). (A) Whole-cell extracts were analyzed by immunoblotting for Bcl-2 and Bax. (B) Alternatively, apoptosis assays using dual-color annexin-V–FITC/PI staining and flow cytometry analyses were performed on treated B cells. The percentage of single- or double-positive cells in the individual quadrants is shown. These results were representative of at least 3 comparable experiments.
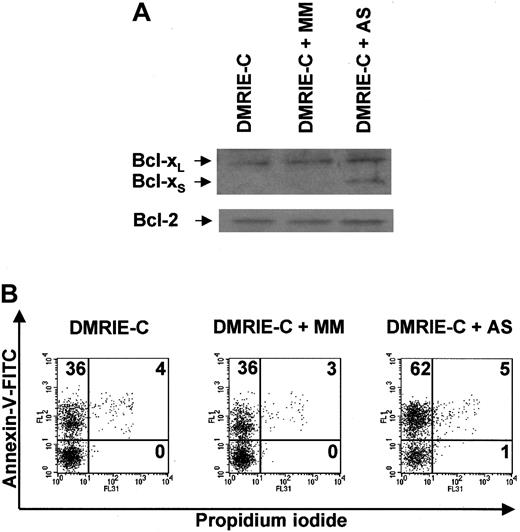
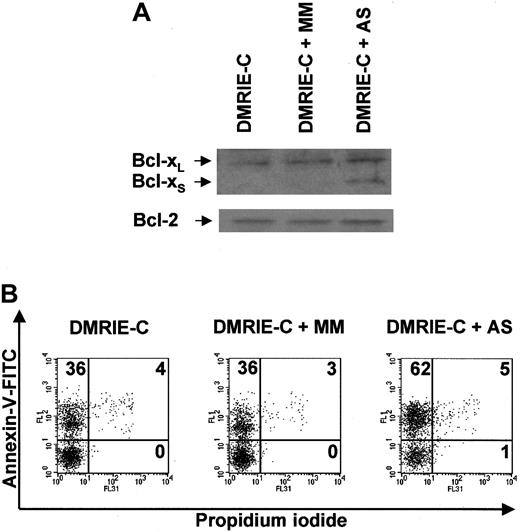
AS ORNs containing uniform 2′-OMe modifications were used to induce Bcl-xS expression in resting blood granulocytes. These AS ORNs were designed to inhibit the use of the 5′ splice site in exon II of bcl-x RNA, thereby redirecting the splicing machinery to the 5′ bcl-xS splice site.21 AS ORNs efficiently induced Bcl-xS expression in granulocytes, whereas 5-bp mismatched MM ORNs and DMRIE-C alone did not affect Bcl-xS protein expression (Figure8A). None of these treatments affected the level of Bcl-2 expression used as a control. Immunoblots revealed that Bcl-xS was clearly present in AS-treated granulocytes 8 hours after treatment (Figure 8A). At this time point, the percentage of apoptotic granulocytes averaged 60% ± 6% after AS treatment, whereas 38% ± 3% and 35% ± 4% apoptotic granulocytes were found after treatment with MM ORNs and with DMRIE-C alone, respectively (Figure 8B).
Antisense ON-mediated induction of Bcl-xSexpression in human granulocytes leads to apoptosis.
Blood granulocytes were isolated from buffy coats by density centrifugation and treated for 8 hours with DMRIE-C alone (24 μL per 2 × 106 cells) or DMRIE-C combined with AS (or MM) ONs designed to redirect bcl-x pre-mRNA splicing towardbcl-xS (8 μg per 2 × 106cells). (A) Whole-cell extracts were analyzed by immunoblotting for Bcl-xL/S and Bcl-2. (B) Apoptosis assays were performed on treated granulocytes using dual-color annexin-V–FITC/PI staining and flow cytometry analyses. The percentage of single- or double-positive cells in the individual quadrants is shown. These results were representative of at least 3 comparable experiments.
Antisense ON-mediated induction of Bcl-xSexpression in human granulocytes leads to apoptosis.
Blood granulocytes were isolated from buffy coats by density centrifugation and treated for 8 hours with DMRIE-C alone (24 μL per 2 × 106 cells) or DMRIE-C combined with AS (or MM) ONs designed to redirect bcl-x pre-mRNA splicing towardbcl-xS (8 μg per 2 × 106cells). (A) Whole-cell extracts were analyzed by immunoblotting for Bcl-xL/S and Bcl-2. (B) Apoptosis assays were performed on treated granulocytes using dual-color annexin-V–FITC/PI staining and flow cytometry analyses. The percentage of single- or double-positive cells in the individual quadrants is shown. These results were representative of at least 3 comparable experiments.
Altogether, these results demonstrate that the alterations in Bcl-2 family protein expression induced by total NF-κB inhibition are sufficient to trigger massive lymphocyte and granulocyte apoptosis.
Discussion
In the present report we have demonstrated that NF-κB exerts its antiapoptotic function in quiescent mature lymphocytes and granulocytes by controlling the expression of distinct Bcl-2 family proteins.
Quiescent blood T cells contained relatively high levels of Bax, Bcl-2, and Bcl-xL proteins. Whether Bcl-xL is expressed in resting mature T cells is a matter of debate. Indeed, Bcl-xL was first reported to be confined to immature DP thymocytes and activated mature T cells,25-28 whereas further study demonstrated substantial amounts of Bcl-xL in resting mature T cells.19 In the present report, we confirm the presence of Bcl-xL in quiescent peripheral T cells and demonstrate that NF-κB–dependent expression of Bcl-xL plays a key role in maintaining the viability of these cells.
Mice deficient for nfκb-1 orrel29,30 and irradiated SCID mice engrafted withrela−/− fetal liver cells31exhibit no intrinsic defect in the establishment of normal mature T-cell populations, findings consistent with the potential for functional redundancy among NF-κB family members. Conversely, lethally irradiated mice engrafted withrela−/−/rel−/− fetal liver hematopoietic progenitors,32 transgenic mice expressingtrans-dominant forms of IκB-α in the T lineage,33-35 and bcl-x double-knockout chimeric mice36 exhibit impaired T-cell maturation. These findings, coupled with the present results, indicate that suppression of NF-κB activity has similar effects as loss ofbcl-xL expression on the T lineage, raising the possibility that these 2 major antiapoptotic factors are involved in a common pathway required for both T-cell maturation and further survival. However, a small number of maturebcl-x−/− and NF-κB–inactivated T cells exhibiting a normal life span may be found in mouse models,32-36 a finding consistent with our observation that total NF-κB inhibition in resting mature T cells induces drastic but incomplete apoptosis. Indeed, approximately 30% NF-κB–inactivated mature T cells were still alive 24 hours after GTX or PGA1 treatment. These findings suggest that a subset of T cells is less dependent on NF-κB–induced Bcl-xL expression for protection against apoptosis. Because trans-dominant IκB-α transgene expression in T cells predominantly blocks NF-κB activity in CD4+ cells but leads to a preferential reduction in CD8+ cell numbers in the thymus and periphery,33-35 it is plausible that NF-κB–mediated Bcl-xL expression is dispensable to maintain survival in a subset of CD4+ cells.
An intriguing question concerns the mechanisms responsible for continuous induction of NF-κB–mediated Bcl-xL expression in quiescent mature T cells. Thymus-positive selection requires the simultaneous recognition of peptides and major histocompatibility complex (MHC) molecules.1 Similarly, survival of resting mature T cells requires continuous TCR engagement by MHC molecules.1 Recent studies devoted to T-cell development have demonstrated that pre-TCR signaling causes NF-κB activation, which selectively protects pre-TCR–positive cells from apoptosis.37 Moreover, CD28 costimulation promotes peripheral T-cell survival through the activation of NF-κB and subsequent Bcl-xL expression.26 38 These findings, combined with our results, support a model in which continuous TCR-MHC interaction, and most likely CD28 costimulation, maintains NF-κB activation and Bcl-xL expression in quiescent mature T cells, which thereby avoid spontaneous apoptosis.
Total NF-κB inhibition in mature B cells was associated with Bcl-2 down-regulation, and loss of Bcl-2 was sufficient to induce massive B-cell death. Although recent studies have clearly shown that the expression of Bcl-xL and Bfl-1 is under NF-κB control,12-15 many investigations failed to demonstrate a role for NF-κB in Bcl-2 expression. However, a few studies provided evidence for a relation between NF-κB activation and induction of Bcl-2 expression in immature B cells, hippocampal cells, and some epithelial cancer cell lines,18,39 40 indicating that regulation of Bcl-2 expression by NF-κB is highly restricted to some cell types, and is of particular importance in the B lineage. In epithelial cancer cell lines, NF-κB likely regulates Bcl-2 expression through both indirect mechanisms and direct binding to the P2bcl-2 promoter (P. Viatour, personal communication, October 2001). However, the question of whether NF-κB directly or indirectly controls bcl-2 expression in B cells remains to be answered.
Lymphoid cells develop normally for a short time after birth inbcl-2−/− mice, indicating that Bcl-2 is dispensable for lymphocyte maturation but is required for maintaining immune homeostasis.41 However, further studies have provided conflicting results. Indeed, transplantation of hematopoietic stem cells from bcl-2−/− mouse bone marrow into irradiated normal recipient mice results in long-term reconstitution of nonlymphoid cells but is associated with both the absence of T lymphopoiesis and severely impaired B-cell development.42 Similarly, mice engrafted withrel−/−/rela−/− fetal liver hematopoietic stem cells lack mature IgMloIgDhiB cells and exhibit increased apoptosis in immature IgMhiIgDlo and IgMhiIgDhi B-cell populations.18Moreover, this decreased survival of double-knockout B cells coincides with reduced expression of bcl-2 andbfl-1 and is abolished by enforced expression of a bcl-2 transgene.18 These observations, coupled with our results, indicate that NF-κB–induced Bcl-2 expression contributes to the maintenance of both immature and mature B-cell survival. However, NF-κB induces coexpression of Bcl-2 and Bfl-1 in immature B cells,18 whereas it induces Bcl-2 expression only in quiescent mature B cells, which require stimulation to express additional prosurvival Bcl-2 homologues, such as Bfl-1 and Bcl-xL.13,14 43 This finding suggests that NF-κB–mediated Bcl-2 expression is particularly crucial for promoting resting mature B-cell survival, a hypothesis that is further supported by our finding that specific bcl-2 knockdown results in drastic induction of apoptosis in these cells.
In the present study, we confirm that constitutive NF-κB activity is essential for resting mature granulocyte survival4 and demonstrate that NF-κB inactivation triggers granulocyte apoptosis through the induction of Bcl-xS expression. Bcl-xL is the most abundant Bcl-x isoform found in vivo.44 However, Bcl-xSmay be overexpressed in some circumstances, such as during thymic selection, mammary gland involution, withdrawal of progesterone from the endometrium, and brain ischemia.44 The mechanisms that regulate alternative bcl-x pre-mRNA splicing are totally unknown. Our study is the first to provide evidence for a role of NF-κB in controlling the Bcl-xS/Bcl-xL ratio through the direct or indirect regulation of bcl-x pre-mRNA splicing.
Complete NF-κB inhibition had only a weak effect on monocyte survival and did not affect Bcl-2 family protein expression. Since monocytes appeared to mainly contain inactive p50 homodimers, a finding consistent with previous reports,45,46 the inability of NF-κB inactivation to alter Bcl-2 family protein expression and to substantially induce monocyte apoptosis is not surprising. Monocytes have a short life span and their transit time in the circulation is between 1 to 2 days, after which time they extravasate and differentiate into macrophages. Monocyte differentiation is associated with RelA expression and subsequent NF-κB–dependent Bfl-1 expression, which are critical events in the generation of long-lived macrophages.3,45 These findings are consistent with increased apoptosis of maturingrel−/−/rela−/−macrophages32 and collectively show that transcriptionnaly active NF-κB complexes are dispensable for monocyte survival until they begin differentiation.
In summary, our study establishes that constitutive NF-κB activity rescues QMICs from spontaneous apoptosis through the regulation of various Bcl-2 family proteins. Indeed, our results unambiguously demonstrate that constitutive NF-κB activity induces directly or indirectly the expression of prosurvival proteins of the Bcl-2 family in lymphocytes and prevents the expression of the proapoptotic Bcl-2 homologue Bcl-xS in granulocytes. Furthermore, we show that the transcriptional control of these Bcl-2 family proteins by NF-κB is required to preserve homeostasis of resting lymphocytes and granulocytes. These findings provide novel insights into the molecular mechanisms responsible for the maintenance of a sufficient defense reserve.
The authors thank Drs Pierre Chatelain, Bruno Fuks, Jacques Gielen, and Roy Massingham for advice, and Muriel Chapelier, Martine Leblond, Miguel Lopez, Ilham Sbaı̈, and Andrée Villers for excellent technical and secretarial assistance.
Supported in part by UCB Pharma (Belgium) and the “Ministère de la Région Wallonne” (Belgium). F.B. is a Research Assistant, M.-P.M. and A.V. are Research Associates, and V.B. is a Senior Research Associate at the National Fund for Scientific Research (FNRS, Belgium).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Fabrice Bureau, Department of Physiology, Faculty of Veterinary Medicine, University of Liège, Boulevard de Colonster, Bâtiment B42, Sart-Tilman, B-4000, Liège, Belgium; e-mail: fabrice.bureau@ulg.ac.be.