Interleukin-21 (IL-21) is a recently cloned cytokine with homology to IL-2, IL-4, and IL-15. In this study we examined the effects of IL-21 on human myeloma cells. We found that IL-21 induced proliferation and inhibited apoptosis of the IL-6–dependent human myeloma cell lines ANBL-6, IH-1, and OH-2. The potency of IL-21 was close to that of IL-6 in the OH-2 cell line. Neutralizing antibodies to IL-6 or the IL-6 receptor transducer chain (gp130) did not affect IL-21–induced DNA synthesis, indicating that IL-21–induced proliferation was not mediated through these proteins. Tumor necrosis factor (TNF), another stimulator of myeloma cell growth, up-regulated the expression level of IL-21 receptor (IL-21R), and combinations of TNF and IL-21 gave synergistic effects on myeloma cell proliferation. Furthermore, 4 of 9 purified samples of primary myeloma cells showed a significant increase in DNA synthesis on stimulation of the cells by IL-21. By Western blot analysis, we demonstrated that the intracellular signaling pathways of IL-21 in myeloma cells involved phosphorylation of Jak1, Stat3, and Erk1/2 (p44/42 mitogen-activated protein kinase). IL-21 is a novel growth and survival factor in multiple myeloma and may represent a target for future therapy.
Introduction
Multiple myeloma (MM) is a neoplasia of terminally differentiated B lymphocytes. The disease is usually confined to the bone marrow. MM is still incurable, with a median patient survival time of 3 to 4 years.1 2
Refractory late-stage MM is characterized by an increase in plasma cell labeling index and often involves cells that are responsive to cytokine stimulation.3 Several cytokines have been implicated in the pathogenesis of MM. Interleukin-6 (IL-6) is considered the major growth and antiapoptotic factor for myeloma cells.4-6However, other cytokines may substitute for IL-6 as a growth factor in vitro, including tumor necrosis factor (TNF), IL-10, insulinlike growth factor-1 (IGF-1), interferon alpha, and IL-15.7-14 Freshly isolated cells from patients with MM generally grow poorly in vitro despite the presence of known growth-promoting factors. This may imply that myeloma cells depend on still unknown growth factors in vivo. Finding these factors is important for identification of new therapeutic targets.
IL-21 and its receptor (IL-21R) were recently described.15,16 Mature IL-21 is a 131-amino acid residue 4–helix-bundle cytokine with significant sequence homology to IL-2, IL-4, and, in particular, IL-15. IL-21R has highest homology to the IL-2R beta chain and the IL-4R alpha chain.15,16Upon ligand binding, IL-21R has been reported to associate with the common γ chain, a property it shares with the receptors for IL-2, IL-4, IL-7, IL-9, and IL-15.17 The intracellular signaling pathway of IL-21R involves Janus kinase 1 (Jak1), Jak3, signal transducer and activator of transcription 1 (Stat1), Stat3, and Stat5.16 17
The biologic functions of IL-21 have so far not been extensively studied. The main source of IL-21 seems to be activated T lymphocytes.15 IL-21 apparently influences the function of B, T, and natural killer (NK) lymphocytes at concentrations in the range of 2 to 50 ng/mL. In vitro experiments suggest that IL-21 has divergent effects on B cells, depending on the nature of the costimulus. IL-21 stimulates proliferation of CD40-activated B cells but inhibits proliferation induced by anti-IgM antibodies and IL-4.15 Furthermore, IL-21 induces proliferation and maturation of NK cell populations from the bone marrow, especially in synergy with IL-15. IL-21 and anti-CD3 costimulate proliferation of naive, but not memory, T cells.15 These findings indicate an important regulatory role for IL-21 in the immune system.
So far, nothing has been published on the involvement of IL-21 in pathophysiological processes. In this study we examined the effects of IL-21 on myeloma cells.
Patients and methods
Cell lines and culture conditions
We used the human myeloma cell lines ANBL-6 (kind gift from D. Jelinek, Mayo Clinic, Rochester, MN), JJN-3,18OH-2,7 RPMI 8226, and U-266 (the latter 2 from American Type Culture Collection, Rockville, MD).
An IL-6–dependent human myeloma cell line, IH-1, was established in our laboratory from the pleural effusion of a myeloma patient.19 It secretes IgAλ, which is the same as the M-component of the patient. By flow cytometry it is strongly positive for CD138, CD45, and CD38, weakly positive for CD56, and negative for CD11a, CD19, and CD20. This cell marker profile fits that of a human myeloma cell line.20 The IH-1 cell line is Epstein-Barr virus (EBV) negative, as determined by polymerase chain reaction (PCR) (amplification of LMP1).21
Cells were grown in RPMI 1640 (Life Technologies, Paisley, United Kingdom) supplemented with 100 μg/mL L-glutamine and 20 μg/mL gentamicin (referred to as RPMI). ANBL-6, JJN-3, and U-266 were maintained in RPMI supplemented with 10% heat-inactivated fetal calf serum (FCS; Hyclone, Logan, UT) and RPMI 8226 with 15% FCS. OH-2 and IH-1 cells were maintained in RPMI supplemented with 10% human serum (Blood Bank, Trondheim University Hospital, Norway) because these cell lines proliferate and thrive far better in human than in fetal calf serum. The IL-6–dependent cell lines ANBL-6, IH-1, and OH-2 were maintained in media containing 2 ng/mL IL-6. Media were replenished twice weekly. Cells were cultured at 37°C in a humidified atmosphere containing 5% CO2. We washed the IL-6–dependent cell lines and primary myeloma cells 4 times in Hanks balanced salt solution (Life Technologies) to deplete the cells of cytokines before the assays were performed.
Patients and isolation of myeloma cells
After obtaining approval from the regional ethics committee and informed consent from patients, we used myeloma cells from 9 patients admitted to the Section of Hematology, University Hospital of Trondheim. Patient characteristics are given in Table1. Myeloma cells from bone marrow aspirates were purified by immunomagnetic separation using Dynabeads (Dynal, Oslo, Norway) coated with the monoclonal antibody B-B4 (Serotec, Oxford, United Kingdom), which recognizes syndecan-1 (CD138).23 The purity of myeloma cells obtained by this method is 97% or greater, as determined by cytospin preparations. Selected cells were cultured overnight in RPMI supplemented with 10% human serum and 2 ng/mL IL-6, and spontaneously detached cells were used for experiments.
Reagents
IL-21, soluble IL-21R, and antibodies against IL-21R were kind gifts from R. Holly (ZymoGenetics, Seattle, WA). IL-6 was from BioSource (Camarillo, CA), TNF was from Genentech (South San Francisco, CA), and neutralizing monoclonal antibodies to IL-6 and gp130 were from R&D Systems (Abingdon, United Kingdom). All cytokines used were recombinant human.
mRNA analysis
RNA isolation, cDNA synthesis, and reverse transcription (RT)–PCR were performed essentially as described.24RT-PCR reactions were run 40 cycles with an annealing temperature of 64°C. IL-21R sense (5′CCAGGAGTGTGGCAGCTTTC) and antisense (5′GCTTGCCCTTCAGCATGTAGA) primers yielded a 143-base pair amplicon.
Proliferation assays
To measure DNA synthesis, cells were seeded in 96-well plastic culture plates (Corning Costar, Corning, NY) at a density of 2.5 × 104 cells per well in 200 μL RPMI supplemented with 10% FCS, cytokines and antibodies as indicated in each experiment. After 54 hours, they were pulsed with 0.037 MBq methyl-3H-thymidine (NEN Life Science Products, Boston, MA) per well and harvested 18 hours later with a Micromate 96-well harvester (Packard, Meriden, CT). Beta radiation was measured with a Matrix 96 counter (Packard).
For cell number experiments, we seeded 3 × 105 cells in 5 mL RPMI with 10% FCS in 6-well plates or 1 × 106cells in 10 mL RPMI with 10% FCS in culture bottles, and we supplemented the media with 1 ng/mL IL-6 or 10 ng/mL IL-21. The cells were counted 3 times a week. Media were not replenished. For evaluating IL-21 as a long-term growth factor, media were replenished once a week.
Apoptosis assay
Cellular viability and apoptosis were determined by flow cytometric analysis of annexin V binding25 and propidium iodide uptake (APOPTEST-FITC kit; Nexins Research, Hoeven, The Netherlands) and were performed as described.19 The cells were incubated with cytokines as indicated for 72 hours in RPMI containing 10% FCS and 1% human serum.
Surface IL-21R detection
Flow cytometry was used to determine the expression of IL-21R. We incubated 4 × 105 cells with 5 μg/mL biotinylated mouse monoclonal antibody against IL-21R for 30 minutes on ice in phosphate-buffered saline (PBS) containing 0.1% bovine serum albumin. As a negative control we used irrelevant, isotype-matched, biotinylated monoclonal antibodies (anti-FLAG BioM2 biotin conjugate; Sigma, St Louis, MO). The cells were washed, and phycoerythrin-conjugated streptavidin (Becton Dickinson, San Jose, CA) was added for an additional 30 minutes. The cells were washed again and resuspended in PBS before they were analyzed on a flow cytometer (Beckman Coulter). Dead cells and debris were excluded from analysis on the basis of forward- and side-scatter signals.
Western blot analysis
Cells (2 × 106) were incubated in RPMI supplemented with 10% FCS and cytokines as indicated. They were starved overnight before Jak1 and Erk1/2 assays. After centrifugation and solubilization in 100 μL sodium dodecyl sulfate (SDS) sample buffer (100 mM Tris/HCl, pH 6.8, 10% SDS, 40% glycerol, 0.005% bromophenol blue, 0.7 M 2-mercaptoethanol, and 1 mM Na3VO4), DNA was sheared by repeated pipetting. Samples were then heated to 100°C for 2 minutes, and aliquots of 30 μL were loaded onto SDS polyacrylamide gels. Proteins were transferred to nitrocellulose filters (Bio-Rad, Hercules, CA). Filters were blocked in 5% skimmed milk with 0.05% Tween 20 in Tris-buffered saline, pH 7.4, before probing with antibodies against phosphorylated tyrosine residue (pY) 705 of Stat3, total Stat3 (Cell Signaling Technology, Beverly, MA), pY701 of Stat1 (Upstate Biotechnology, Lake Placid, NY), pYpY1022/1023 of Jak1 (BioSource), and phosphorylated threonine residue (pT) 202/pY204 of Erk1/2 (New England Biolabs, Beverly, MA). Antibodies against pY694/pY699 Stat5a/b, pY641 Stat6, and pY1007/pY1008 Jak2 were from Upstate Biotechnology. Bound antibodies were visualized by chemiluminescence (Amersham Pharmacia Biotech, Uppsala, Sweden). The pY705 Stat3-probed filters were stripped of antibodies and reprobed for total Stat3 using stripping buffer (100 mM 2-mercaptoethanol, 2% SDS, 62.5 mM Tris-HCl, pH 6.7).
Nuclear transcription factor-κB assay
Nuclear transcription factor (NF)-κB activation was determined with a Trans-am Transcription Factor Assay Kit (Active Motif, Carlsbad, CA). This is an enzyme-linked immunosorbent assay (ELISA) with a 96-well plate coated with an oligonucleotide containing the NF-κB consensus binding site (5′-GGGACTTTCC-3′). The assay was performed essentially as described by the manufacturer. Briefly, after 4 washes in Hanks balanced salt solution, cells were seeded on a 24-well plate (106 cells and 0.5 mL medium per well) and were stimulated for 3 hours with cytokines as indicated in the legend to Figure 7. Each condition was run in triplicate. Cells were then washed twice in PBS at 4°C and were resuspended in 50 μL lysis buffer provided in the kit. Lysates were diluted 1:10 and added to the ELISA plate. After incubation and 3 washes, primary antibody against NF-κB was added. Detection was accomplished with a horseradish peroxidase–conjugated secondary antibody and a chromogenic substrate. Optical density at 450 nm was read and used as a relative measure of NF-κB activation.
Statistical analysis
Statistical significance was determined by using a 2-tailed, unpaired Student t test. The minimal level of significance was P = .05.
Results
IL-21 induced proliferation of IL-6–dependent human myeloma cell lines and primary myeloma cells
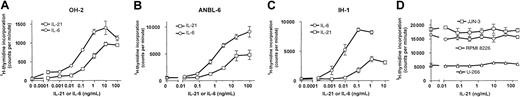
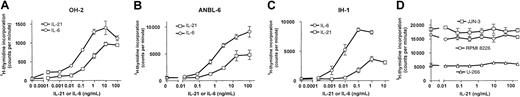
IL-21 increased the proliferation of 3 different IL-6–dependent cell lines in a dose-dependent manner, as determined by3H-thymidine incorporation (Figure 1A-C). Significant effects could be demonstrated at concentrations as low as 10 pg/mL in the cell line OH-2. Half-maximal proliferation was achieved at approximately 10-fold higher concentrations of IL-21 than of IL-6 (Figure 1A-C). DNA synthesis was unchanged in the IL-6–independent cell lines U-266, JJN-3, and RPMI 8226 after IL-21 stimulation (Figure 1D). We repeated the latter experiments under low serum conditions (0.5% FCS) but were still unable to find any effect of IL-21 (data not shown).
IL-21 induced DNA synthesis in IL-6–dependent human myeloma cell lines.
Cell lines IH-1, OH-2, and ANBL-6 were stimulated with IL-21 and IL-6 in concentrations as indicated for 72 hours, and cell lines U-266, JJN-3, and RMPI 8226 were stimulated with IL-21 in the same manner. DNA synthesis was measured by 3H-thymidine incorporation. Data shown are representative of at least 2 different experiments, and error bars represent ± 1 SD of triplicate data.
IL-21 induced DNA synthesis in IL-6–dependent human myeloma cell lines.
Cell lines IH-1, OH-2, and ANBL-6 were stimulated with IL-21 and IL-6 in concentrations as indicated for 72 hours, and cell lines U-266, JJN-3, and RMPI 8226 were stimulated with IL-21 in the same manner. DNA synthesis was measured by 3H-thymidine incorporation. Data shown are representative of at least 2 different experiments, and error bars represent ± 1 SD of triplicate data.
Four of 9 primary myeloma samples showed an increase in DNA synthesis after stimulation with IL-21 (Table 1). A 183% increase in DNA synthesis after IL-21 stimulation was noted in one sample, whereas in 3 other samples the increase was 34% to 82%. Six of 9 samples were responsive to IL-6 (increase range, 38%-793%). All samples that were responsive to IL-21 were also responsive to IL-6. Absolute3H counts (Table 1) and response magnitude were lower for primary cells than for cell lines. This probably reflects the low labeling index of primary cells.26
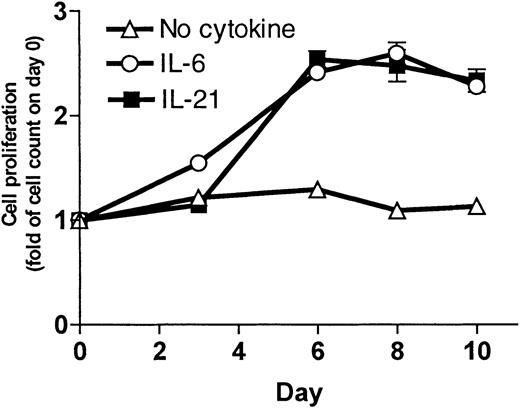
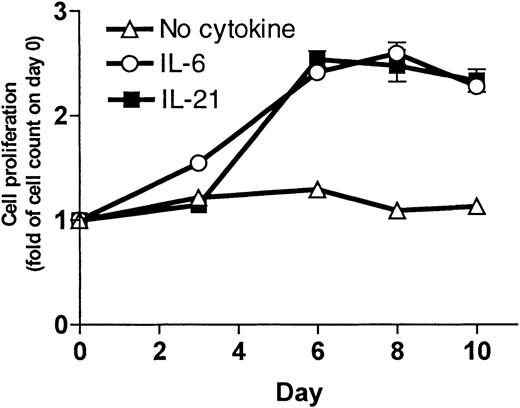
To measure the increase in cell number, cells were grown in culture and counted 3 times a week. Results for the OH-2 cell line are shown in Figure 2. IL-21 and IL-6 stimulation led to an increase in cell counts. For the IH-1 cell line, the increase in cell counts on day 10 following IL-21 stimulation was 2-fold compared with 6-fold following IL-6 stimulation (data not shown).
IL-21 induced cell proliferation in the cell line OH-2.
Cells (1 × 106) were grown in RPMI–10% FCS with supplements of IL-21 (10 ng/mL) or IL-6 (1 ng/mL) or without supplement. We counted the cells 3 times a week. Cell number on day 0 was normalized to 1. Data shown are representative of 3 different experiments, and error bars represent ± 1 SD of duplicate data.
IL-21 induced cell proliferation in the cell line OH-2.
Cells (1 × 106) were grown in RPMI–10% FCS with supplements of IL-21 (10 ng/mL) or IL-6 (1 ng/mL) or without supplement. We counted the cells 3 times a week. Cell number on day 0 was normalized to 1. Data shown are representative of 3 different experiments, and error bars represent ± 1 SD of duplicate data.
We also replaced IL-6 with 2 ng/mL IL-21 as a supplement to the growth medium for long-time culture of the cell line OH-2. IL-21–supplemented cells continued to proliferate (observation time more than 4 months), indicating that IL-21 can replace IL-6 as a long-term growth factor in a myeloma cell line. Cells cultured in media without IL-21 died within 3 weeks, as determined by morphology and by cell number.
IL-21 was an antiapoptotic factor for human myeloma cell lines
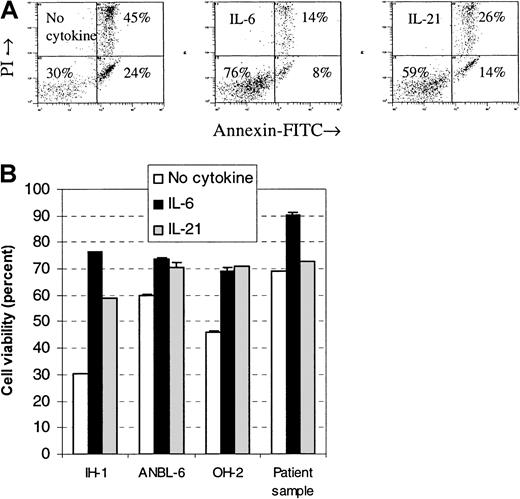
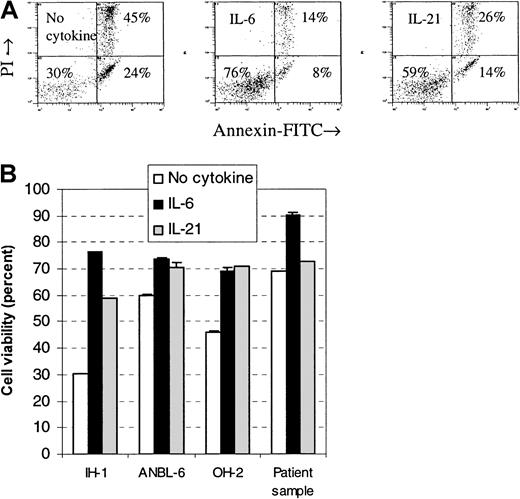
Cells were stimulated with IL-21 or IL-6, and the percentage of viable cells after a 3-day incubation period was measured by flow cytometry. The fraction of viable cells in all examined cell lines increased after stimulation with IL-21 or IL-6 (Figure3). Differences in viability between IL-21– and IL-6–stimulated cells were small in the cell lines OH-2 and ANBL-6, whereas in the cell line IH-1, IL-6 gave a higher proportion of viable cells than IL-21. We examined 3 primary myeloma cell samples and found few or no differences in viability after stimulation with IL-21. In one of the samples, there was a 3% increase in viability following IL-21 stimulation and a 21% increase following IL-6 stimulation (Figure 3). No increase was found in the other 2 samples to either IL-21 or IL-6 (data not shown).
IL-21 protected myeloma cells from apoptosis.
Annexin V–FITC and propidium iodide binding were measured by flow cytometry in cell lines IH-1, OH-2, and ANBL-6 after incubation for 72 hours with IL-21 (20 ng/mL) or IL-6 (1 ng/mL). Typical dot plots for the cell line IH-1 are shown in the upper panel. Percentages of viable cells, located in the lower left quadrant of dot plots, are shown in the lower panel. A patient sample (patient 5 in Table 1) is included in the lower panel. Data shown are representative of 3 different experiments, and error bars represent ± 1 SD of duplicate data.
IL-21 protected myeloma cells from apoptosis.
Annexin V–FITC and propidium iodide binding were measured by flow cytometry in cell lines IH-1, OH-2, and ANBL-6 after incubation for 72 hours with IL-21 (20 ng/mL) or IL-6 (1 ng/mL). Typical dot plots for the cell line IH-1 are shown in the upper panel. Percentages of viable cells, located in the lower left quadrant of dot plots, are shown in the lower panel. A patient sample (patient 5 in Table 1) is included in the lower panel. Data shown are representative of 3 different experiments, and error bars represent ± 1 SD of duplicate data.
Myeloma cell lines expressed IL-21R
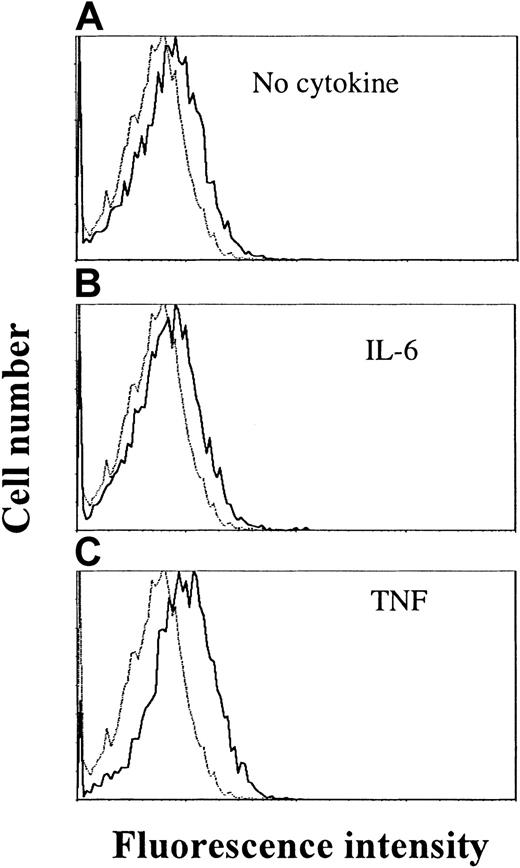
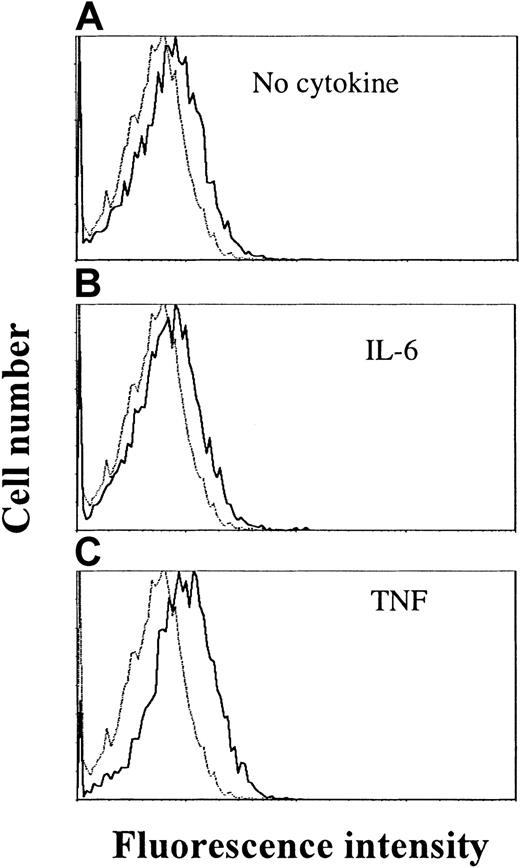
By RT-PCR, the IL-21R transcript was detected in all examined cell lines (OH-2, IH-1, ANBL-6, JJN-3, U-266 and RPMI 8226) (data not shown). Cell surface expression of IL-21R was detected by flow cytometry in ANBL-6 and OH-2 cells, as exemplified in Figure4 for the OH-2 cell line (upper histogram).
TNF stimulation increases IL-21 receptor expression in OH-2 cells.
Cells were stimulated with TNF (10 ng/mL) or IL-6 (1 ng/mL) for 18 hours before the addition of streptavidin–PE-conjugated antibodies against IL-21R. Mean fluorescence intensities were 6.3 (control), 8.4 (no cytokine), 8.2 (IL-6), and 10.9 (TNF). Similar patterns were obtained in the cell line ANBL-6, and data shown are representative of 3 different experiments.
TNF stimulation increases IL-21 receptor expression in OH-2 cells.
Cells were stimulated with TNF (10 ng/mL) or IL-6 (1 ng/mL) for 18 hours before the addition of streptavidin–PE-conjugated antibodies against IL-21R. Mean fluorescence intensities were 6.3 (control), 8.4 (no cytokine), 8.2 (IL-6), and 10.9 (TNF). Similar patterns were obtained in the cell line ANBL-6, and data shown are representative of 3 different experiments.
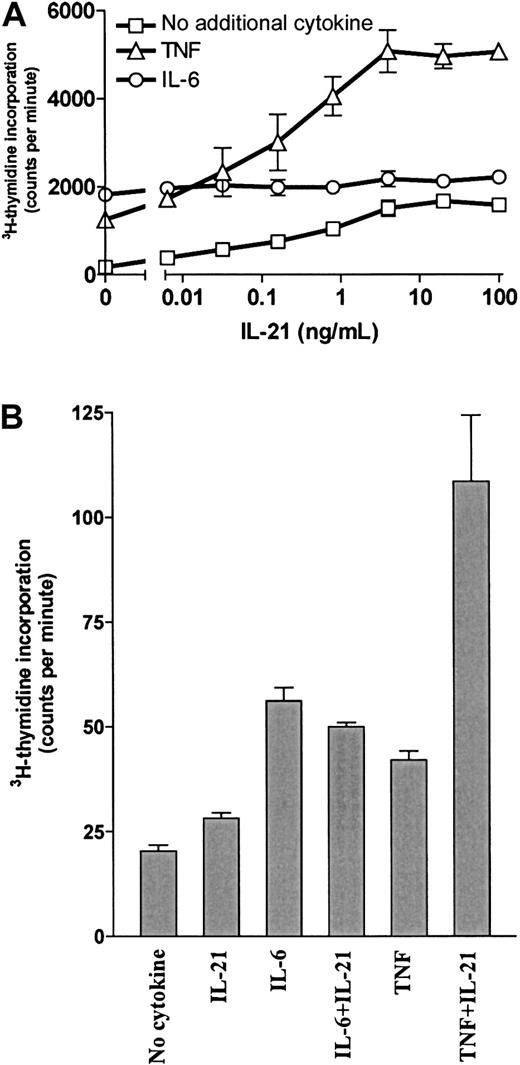
IL-21 and TNF had synergistic effects on myeloma cell proliferation
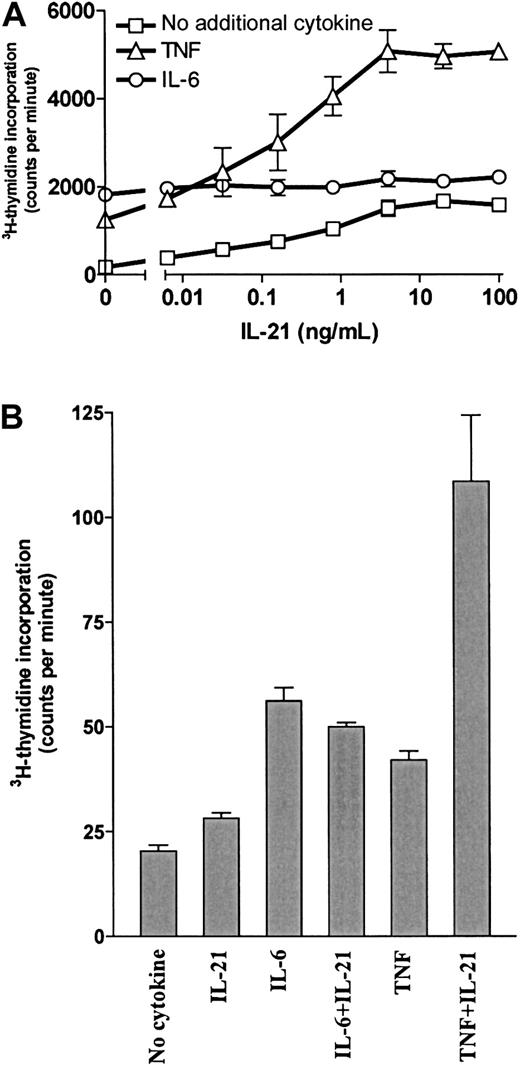
Our group has previously shown that TNF is a growth factor for OH-2 cells and that IL-6 and IL-15 have synergistic effects with TNF.7 10 Therefore, we examined DNA synthesis after combined stimulation with IL-21 and TNF. Results for the cell line OH-2 and one primary myeloma cell sample are shown in Figure5. Similar results were obtained for the cell line IH-1 (data not shown). Combined stimulation with 10 ng/mL TNF and 100 ng/mL IL-21 gave a 29-fold increase in DNA synthesis, compared with a 7-fold and a 10-fold increase with TNF and IL-21 alone, respectively. In contrast, the combination of IL-6 and IL-21 was not synergistic. The synergistic effect of IL-21 and TNF on proliferation was also seen in cell number experiments. In the OH-2 cell line, the combination of TNF and IL-21 increased the cell number more than stimulation with IL-6 alone (data not shown).
IL-21 and TNF had a synergistic effect on DNA synthesis.
(A) OH-2 cells were incubated with increasing concentrations of IL-21 either alone, with IL-6 (1 ng/mL), or with TNF (10 ng/mL) for 72 hours. 3H-thymidine incorporation was measured. Similar results were obtained in the cell line IH-1, and the experiment was repeated twice. (B) A patient sample (patient 5 in Table 1) was incubated for 72 hours with IL-21 (40 ng/mL), IL-6 (1 ng/mL), or TNF (10 ng/mL), and 3H-thymidine incorporation was measured. Error bars in both panels represent ± 1 SD of triplicate data.
IL-21 and TNF had a synergistic effect on DNA synthesis.
(A) OH-2 cells were incubated with increasing concentrations of IL-21 either alone, with IL-6 (1 ng/mL), or with TNF (10 ng/mL) for 72 hours. 3H-thymidine incorporation was measured. Similar results were obtained in the cell line IH-1, and the experiment was repeated twice. (B) A patient sample (patient 5 in Table 1) was incubated for 72 hours with IL-21 (40 ng/mL), IL-6 (1 ng/mL), or TNF (10 ng/mL), and 3H-thymidine incorporation was measured. Error bars in both panels represent ± 1 SD of triplicate data.
We also examined whether IL-6 or TNF had any effect on IL-21R expression after 18 hours of stimulation. TNF stimulation led to an increase in IL-21R expression on the cell surface, in contrast to IL-6, which did not influence IL-21R expression (Figure 4).
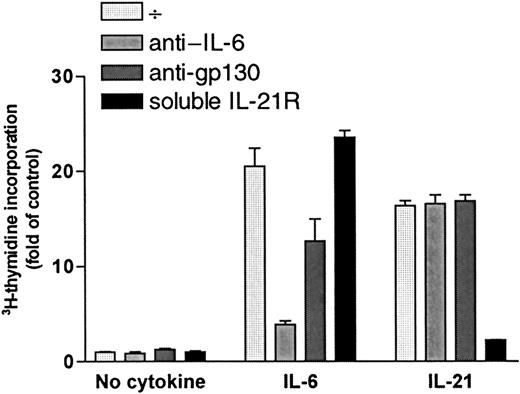
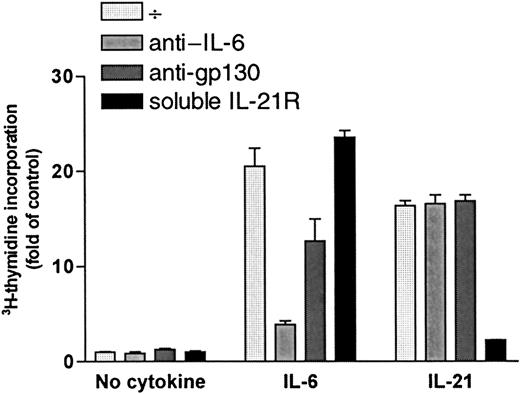
Increase in DNA synthesis after stimulation with IL-21 was not mediated by IL-6 or gp130
DNA synthesis was measured after a 72-hour incubation period with or without cytokines (1 ng/mL IL-6 or 40 ng/mL IL-21) in the presence or absence of specific antibodies against IL-6 (5 μg/mL) or the signal transducer chain of IL-6 receptor, gp130 (5 μg/mL). Results for OH-2 cells are shown in Figure 6, and similar results were obtained in IH-1 cells. IL-21 stimulation was not affected by the addition of anti–IL-6 or anti-gp130, whereas the effects of IL-6 decreased significantly. Similar results were found using equimolar concentrations of IL-21 (5 ng/mL) and IL-6 (2 ng/mL) (data not shown). Soluble IL-21R (5 μg/mL) neutralized the IL-21 effect almost completely. Adding soluble IL-21R to the unstimulated or IL-6–stimulated cells did not influence 3H-thymidine incorporation.
IL-21 signaling was not mediated through IL-6 or gp-130.
OH-2 cells were incubated for 72 hours with IL-6 (1 ng/mL) or with IL-21 (40 ng/mL) in the presence or absence of anti-gp130, anti–IL-6, or soluble IL-21 receptor (sIL-21R). DNA synthesis was measured by3H-thymidine incorporation. Similar results were obtained in the cell line IH-1, and data shown are representative of 3 different experiments. Error bars represent ±1 SD of triplicate data.
IL-21 signaling was not mediated through IL-6 or gp-130.
OH-2 cells were incubated for 72 hours with IL-6 (1 ng/mL) or with IL-21 (40 ng/mL) in the presence or absence of anti-gp130, anti–IL-6, or soluble IL-21 receptor (sIL-21R). DNA synthesis was measured by3H-thymidine incorporation. Similar results were obtained in the cell line IH-1, and data shown are representative of 3 different experiments. Error bars represent ±1 SD of triplicate data.
Thus, there were no indications of IL-21–induced autocrine secretion of IL-6 or IL-21 in myeloma cells. Furthermore, because soluble IL-21R did not influence the effect of IL-6, we have no indication of IL-6–induced IL-21 secretion.
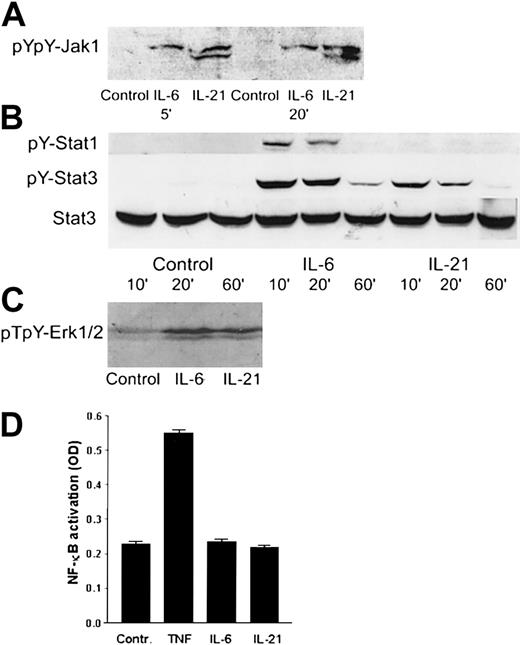
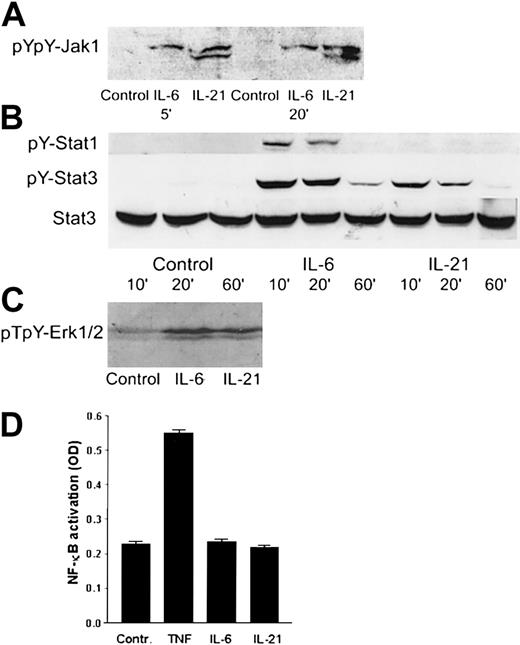
IL-21 mediated its effects through Jak1, Stat3, and Erk1/2
Western blot analysis was used to identify the intracellular signaling pathways of IL-21 in myeloma cells. We found that IL-21 induced tyrosine phosphorylation of Jak1 (Figure7A) and Stat3 (Figure 7B). Stat1 was weakly tyrosine-phosphorylated in IL-6–stimulated cells, but not in IL-21–stimulated cells (Figure 7B). In the mitogen-activated protein kinase (MAPK) pathway, IL-21 and IL-6 led to the phosphorylation of Erk1/2 (p44/42 MAPK) (Figure 7C). TNF, but not IL-21 or IL-6, activated NF-κB (Figure 7D) as determined by ELISA. IL-21 did not affect phosphorylation levels of Jak2, Stat5a/b, or Stat6 proteins (data not shown). In the IL-6–independent cell lines RPMI 8226 and JJN-3, IL-21 did not induce phosphorylation of Stat3. IL-6 induced Stat3 phosphorylation in RPMI 8226, but not in JJN-3 cells. Erk1/2 was phosphorylated in RPMI 8226 and JJN-3 cells after overnight serum starvation, and the level of phosphorylation was not influenced by IL-6 or IL-21 stimulation (data not shown).
IL-21 activated Jak1, Stat3, and Erk1/2, but not Stat1 or NF-κB.
(A) pYpY1022/1023 Jak1 was detected by Western blotting in the cell line OH-2 after stimulation with IL-6 (1 ng/mL) or IL-21 (40 ng/mL) for 5 and 20 minutes. The same pattern was found in IH-1. (B) pY701 Stat1 and pY705 Stat3 were detected by Western blotting in the cell line OH-2 after stimulation with IL-6 or IL-21 for 10, 20, or 60 minutes. The filter was reblotted for total Stat3. The same pattern was found in IH-1 cells. (C) pT202/pY204-Erk1/2 was detected by Western blotting in the cell line IH-1 after stimulation with IL-6 or IL-21 for 20 minutes. (D) NF-κB activity was detected by ELISA in the cell line OH-2 after stimulation with IL-6 (1 ng/mL), IL-21 (50 ng/mL), or TNF (10 ng/mL). Similar results were obtained in the cell line IH-1.
IL-21 activated Jak1, Stat3, and Erk1/2, but not Stat1 or NF-κB.
(A) pYpY1022/1023 Jak1 was detected by Western blotting in the cell line OH-2 after stimulation with IL-6 (1 ng/mL) or IL-21 (40 ng/mL) for 5 and 20 minutes. The same pattern was found in IH-1. (B) pY701 Stat1 and pY705 Stat3 were detected by Western blotting in the cell line OH-2 after stimulation with IL-6 or IL-21 for 10, 20, or 60 minutes. The filter was reblotted for total Stat3. The same pattern was found in IH-1 cells. (C) pT202/pY204-Erk1/2 was detected by Western blotting in the cell line IH-1 after stimulation with IL-6 or IL-21 for 20 minutes. (D) NF-κB activity was detected by ELISA in the cell line OH-2 after stimulation with IL-6 (1 ng/mL), IL-21 (50 ng/mL), or TNF (10 ng/mL). Similar results were obtained in the cell line IH-1.
Discussion
This paper adds a novel factor, IL-21, to the list of cytokines that are able to support myeloma cell growth. We show that IL-21 had potent growth-promoting and antiapoptotic effects on the cytokine-dependent myeloma cell lines ANBL-6, OH-2, and IH-1. Increased DNA synthesis was observed in 4 of 9 patient samples, indicating that responsiveness to IL-21 is not merely a phenomenon seen in the cell lines.
The effect of IL-21 in cellular proliferation was weaker than that of IL-6 in all 3 IL-6–dependent cell lines. In inhibiting apoptosis, IL-21 had effects similar to those of IL-6 in the OH-2 and ANBL-6 cell lines. In the IH-1 cell line, IL-21 was a weaker antiapoptotic factor than IL-6. IL-6 is considered the most important growth factor for myeloma cells.4-6 Importantly, IL-21 was able to replace IL-6 as a growth factor for long-term proliferation of the OH-2 cell line. Cytokine-dependent cell lines were sensitive to stimulation by IL-21, as increased DNA synthesis was seen with concentrations as low as 10 pg/mL in the OH-2 cell lines. In contrast to the results from Parrish-Novak et al15 in normal B and T cells, IL-21 was able to induce the growth of myeloma cells without the presence of a costimulus. Furthermore, IL-21 was a more potent growth and antiapoptotic factor than other known myeloma cell growth factors such as IL-10, IL-15, TNF, and IGF-1 in the 3 IL-6–dependent cell lines examined (A.-T.B., T.B.R., A.W., et al, unpublished observations, May 2001). These results indicate a role for IL-21 in myeloma biology.
Because myeloma cell growth factors may induce autocrine secretion of gp130 family cytokines, it was important to investigate whether this was the case for IL-21.9 11 However, antibodies blocking IL-6 or gp130 did not affect the IL-21–induced growth, arguing against induction of an IL-6 autocrine growth loop. Given that soluble IL-21R did not reduce the proliferation of MM cells under basal or IL-6–stimulated conditions, we have no indication of autocrine secretion of IL-21 from MM cells.
Interestingly, we found a clear synergism between IL-21 and TNF. In fact, a stronger proliferative response could be obtained with the combination of IL-21 and TNF than with an optimal dose of IL-6 alone in OH-2 cells and in one of our patient samples. Combinations of IL-21 and IL-6 did not produce synergistic effects on proliferation. One of the reasons for the IL-21–TNF synergism could be TNF-mediated up-regulation of IL-21R, as demonstrated in the OH-2 and ANBL-6 cell lines. Our group has earlier described synergism between TNF and IL-6 and between TNF and IL-15 in the OH-2 cell line.7 10 These findings indicate that TNF might be more important to myeloma growth as a synergistic stimulant than its effects as a single agent suggest.
To better understand the effect of IL-21 on myeloma cells, we examined some of the intracellular signaling pathways. IL-6 is known to mediate its proliferative and antiapoptotic signal in myeloma cells through the Jak/Stat pathway and the Ras/MAPK pathway.27,28 Stat3 is an important molecule in the proliferation and survival of myeloma cells.29 We show that IL-21 activates Jak1, Stat3, and Erk1/2 in myeloma cells, but apparently not Stat1, Stat5, or NF-κB. In contrast to our findings, Ozaki et al16 reported IL-21R–mediated activation of Jak1 and Stat5, but not of Stat3, in their transfected human cell lines. Asao et al17 found activation of Stat1 in the common γ chain–transfected T-cell line, EDγ-16, after stimulation with IL-21. Our results show that in myeloma cells, IL-6 and IL-21 make use of several of the same downstream signaling molecules, in particular Stat3 and Erk1/2. These observations can explain the similarities in effects of these 2 cytokines and the lack of synergistic effects when the 2 cytokines were combined. The differential use of intracellular signaling pathways by TNF on one side (NF-κB) and IL-6/IL-21 on the other (Stat3 and Erk1/2) may thus be another explanation of the synergistic effect between TNF and the 2 other cytokines.30
Other myeloma growth factors include IGF-1, IL-10, IL-15, IFN-α, vascular endothelial growth factor (VEGF), and hepatocyte growth factor (HGF) (A.-T.B., T.B.R., A.W., et al, unpublished observation, August 2001), among others.8-14 31 This tells us that though myeloma cells might be dependent on extracellular growth support in the marrow, there is likely to be redundancy in this support in the individual patient. Thus, treatment targeted against the growth-supporting microenvironment of myeloma cells should probably include inhibitors of more than one growth factor or inhibitors of intracellular signaling pathways where the signals from several upstream factors converge. In the case of IL-6 and IL-21, for example, inhibitors of Jak1, Stat3, or MAPK members may inhibit growth induced by both cytokines.
The observation that IL-21 and IL-15 induce the growth of myeloma cells opens up the possibility of a pathophysiologic role for the common γ chain or its ligands in MM.10,13 Tinhofer et al13 have shown that the common γ chain, IL-15/IL-2Rβ chain, and IL-15Rα were all expressed in cell lines and in freshly isolated patient cells. Cytokines of the common γ chain family (IL-2, -4, -7, -9, -15, and -21) have a role in B-lymphocyte development and differentiation.32-34 Whether normal bone marrow plasma cells respond to IL-15 or IL-21 is unknown, but the responsiveness of myeloma cells to IL-15 and IL-21 suggests an improperly sustained cytokine responsiveness, as has been postulated for IL-6 and other members of the gp130 cytokine family.35 If the common γ chain is an important element in growth support of myeloma cells, inhibition of this molecule appears to be a logical approach to the treatment of multiple myeloma. However, because persons with inborn nonfunctioning γ chains have severe combined immunodeficiency,36 prolonged inhibition of the common γ chain may cause immunosuppression with subsequent increased risk for infection. With this caution in mind, substances blocking the common γ chain may in the future be tested in myeloma animal models and possibly in patients.
Little is known about cytokine levels in the bone marrow microenvironment of myeloma cells. Minute amounts of IL-6 and TNF and higher amounts of the angiogenic factors HGF, basic fibroblast growth factor, and VEGF have been detected in the bone marrow.37-39 Bone marrow levels of cytokines that use the common γ chain are unknown, and it will be of particular interest to study whether IL-15 and IL-21 are present in the bone marrow of myeloma patients. Sensitive assays for the detection of IL-21 in biologic fluids are currently unavailable.
In conclusion, we demonstrate that IL-21 had growth-promoting and antiapoptotic effects on myeloma cells in vitro. Furthermore, IL-21 could substitute for IL-6 as a long-term growth factor, and there was synergism between IL-21 and TNF.
We thank Rick Holly (ZymoGenetics, Seattle, WA) for kindly providing the IL-21 reagents. We also thank Hanne Hella, Berit F. Stordal, and Mari Sorensen for their excellent technical assistance and Sidsel Krokstad for helping us with the EBV analysis.
Supported by grants from The Norwegian Cancer Society; Rakel og Otto Kr. Bruuns legat; Blix legat; The Cancer Fund of Trondheim University Hospital; and The Norwegian Research Council.
A.-T.B. and T.B.R. contributed equally to this work.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Torstein Baade Ro, Dept of Cancer Research and Molecular Biology, Norwegian University of Science and Technology, MTFS, N-7489, Trondheim, Norway; e-mail:torstein.ro@medisin.ntnu.no.