Von Hippel-Lindau (VHL) disease is a dominantly inherited familial cancer syndrome caused by germline mutations in the VHL tumor-suppressor gene. Central nervous system (CNS) and retinal hemangioblastomas are highly vascular tumors that are hallmarks of the disease. These tumors overexpress vascular endothelial growth factor (VEGF) and represent a potential target for anti-angiogenic drugs. We observed, after 3 to 4 months of treatment, secondary paradoxical polycythemia in 3 VHL patients with CNS or retinal hemangioblastomas treated by the anti-VEGF receptor SU5416. Hematocrit was normal before the beginning of the trial, and no progression of hemangioblastomas was observed. Polycythemia vera and all known causes of secondary polycythemia were also ruled out. Polycythemia has never been reported in current SU5416 trials for advanced malignancies and could express a specific action on red blood cell precursors occurring only in the absence of a functional VHL gene. These findings could also affect the inclusion of VHL patients with pre-existing polycythemia in future anti-VEGF receptor trials.
Introduction
Von Hippel-Lindau (VHL) disease (OMIM 193.300) is a dominantly inherited familial cancer syndrome predisposing to various benign or malignant tumors: central nervous system (CNS) and retinal hemangioblastomas, renal cell carcinoma and renal cysts, pancreatic tumors and cysts, pheochromocytoma, and endolymphatic sac tumors. Renal cell carcinoma is becoming the main cause of death, but hemangioblastomas remain the most prototypic lesion of VHL disease.1 2 Hemangioblastomas are highly vascularized tumors consisting of blood-filled capillaries separated by intervascular stromal cells. CNS hemangioblastomas may cause life-threatening complications, and their treatment remains mainly neurosurgical. Retinal hemangioblastomas are a major cause of visual morbidity and sometimes blindness because treatment by cryotherapy or laser is not always effective.
VHL disease results from germline mutations in the VHL tumor-suppressor gene located on 3p25-26. An important function of the VHL protein (pVHL) is the inhibition of angiogenesis by negative regulation of hypoxia-inducible mRNAs, such as the mRNAs encoding the vascular endothelial growth factor (VEGF).2 pVHL represents the adaptor unit of a multiprotein complex that targets specific proteinlike transcription factors HIF-1α and HIF-2α for degradation by the proteasome.2-5 Thus, the absence or inactivation of pVHL leads to constitutive expression of HIF by VHL tumors, resulting in the overproduction of VEGF and other hypoxia-inducible factors such erythropoietin (EPO), GLUT-1, and transforming growth factor-α. Indeed, the hallmark of all VHL tumors, and moreover of hemangioblastomas, is a high degree of vascularization.1pVHL has multiple other functions, including cell-cycle regulation and fibronectin metabolism, and there is substantial evidence that pVHL may act as a gatekeeper protein.2
Because of the major role of pVHL in VEGF expression and the key pathway of VEGF in hemangioblastoma development, medical treatment with anti-angiogenic drugs was recently proposed in VHL patients affected by untreatable or multiple CNS or retinal hemangioblastomas.6Among anti-angiogenic drugs, SU5416 (Sugen, San Francisco, CA) is a prototype of a novel class of promising cytostatic agents that function by selectively inhibiting the receptor kinase VEGFR-2 (Flk-1/KDR).7,8 SU5416 also targets the stem cell factor receptor c-kit.9 SU5416 inhibits the growth of colon cancer liver metastasis by inducing tumor and endothelial apoptosis, and it is being tested in several trials in various advanced malignancies and Kaposi sarcoma (see the NCI Database,www.cancertrials.nci.nih.gov).7,8 Finally, the first report of stable remission achieved after administration of the drug was recently reported in a patient with acute myeloid leukemia relapse.10
Study design
Case report
We recently started in our Department of Urology a phase 2 clinical trial in VHL disease with SU5416. After approval by our local Institutional Review Board of a specific protocol in accordance with the Helsinki recommendations, and after written informed consent of each patient, 3 French VHL patients with CNS or retinal hemangioblastomas were included in the study (Table1). They were treated by intravenous administration of 145 mg/m2 SU5416 twice a week after premedication by dexamethasone and histamine antagonists to prevent potential hypersensitivity reactions related to the drug or to excipients in the formulation, especially polyoxythelylated castor oil (Cremophor). The safety of SU5416 was assessed monthly through physical examinations, toxicity assessments of vital signs, and laboratory tests (hematology, coagulation, and clinical chemistry).
Methods
Serum EPO was measured by enzyme-linked immunosorbent assay with a commercial kit (R & D Systems, Abingdon, Oxon, United Kingdom).
Red blood cell volume was measured according to the recommendations of the International Committee for Standardization in Hematology (ICSH 1973) using chromium Cr 51 sodium chromate.11 Plasma volume was estimated from red blood cell volume and corrected venous hematocrit. Normal volumes were estimated from the weights and heights of patients according to the Pearson correlation coefficient.12
To assess the possible formation of endogenous erythroid colonies, peripheral blood mononuclear cells from the 3 patients were plated in duplicate with and without the addition of 3 IU/mL recombinant human EPO in a methylcellulose medium containing 30% fetal calf serum and supplemented or not with interleukin-3. In parallel, one positive control (polycythemia vera) and 3 negative controls (2 healthy subjects and one with secondary polycythemia) were studied.
Results and discussion
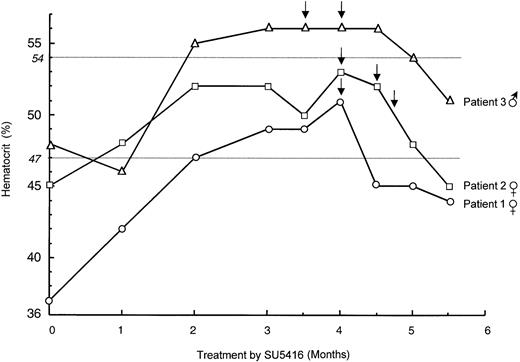
Eight to 10 weeks after the initiation of SU5416 treatment, we surprisingly observed a progressive increase of hematocrit in all 3 enrolled VHL patients. Polycythemia was maximal between 12 and 16 weeks (Table 1; Figure 1), and patients were symptom free except for slight breathlessness in one. Polycythemia was confirmed by isotopic measurement of a red blood cell mass that increased in each patient. Polycythemia vera was eliminated first because there was no evidence of splenomegaly, thrombocytosis, or neutrophil leukocytosis in any patient. Reticulocyte counts and serum B12 levels were also normal. In addition, no erythroid colonies from peripheral blood mononuclear cells were observed in the absence of EPO. Magnetic resonance imaging of the CNS showed stability of the size of CNS hemangioblastomas, and no other tumor cause of polycythemia was present. None of the patients had pheochromocytoma, and only one had a small renal tumor that remained stable for years, as evidenced by computed tomography. Other causes of secondary polycythemia were also ruled out (normal arterial O2 saturation levels, no smoking, no family histories of erythrocytosis).12 Lastly, determination of blood EPO levels demonstrated no change in comparison with the initial measurements performed before the beginning of the SU5416 trial. The administration of SU5416 was not interrupted, and polycythemia was treated by bloodletting with good outcome and without recurrence up to the sixth month of treatment (Figure 1).
Evolution of hematocrit during treatment by SU5416.
The evolution of hematocrit in the 3 enrolled VHL patients is shown: patient 1 (female; open circle), patient 2 (female; open square), patient 3 (male; open triangle). SU5416 administration was not interrupted, and polycythemia was treated by bloodletting (arrows) with good outcome and without recurrence up to the sixth month of treatment. Normal maximal values of hematocrit in males and females, respectively, are indicated by gray horizontal lines.
Evolution of hematocrit during treatment by SU5416.
The evolution of hematocrit in the 3 enrolled VHL patients is shown: patient 1 (female; open circle), patient 2 (female; open square), patient 3 (male; open triangle). SU5416 administration was not interrupted, and polycythemia was treated by bloodletting (arrows) with good outcome and without recurrence up to the sixth month of treatment. Normal maximal values of hematocrit in males and females, respectively, are indicated by gray horizontal lines.
The occurrence of a secondary polycythemia in the 3 VHL patients treated by SU5416 was surprising; it has never been observed in the more than 700 patients with Kaposi sarcoma, advanced cancer, or acute leukemias included in the current SU5416 trials. On the contrary, some of these patients experienced anemia consistent with the negative action of SU516 on c-kit.9 Thus, the occurrence of polycythemia at virtually the same time after the beginning of the treatment in the 3 VHL patients is highly suggestive of a direct action of the drug on this condition. It is well known that polycythemia may occur in approximately 15% to 20% of patients with large cerebellar hemangioblastomas as a consequence of high EPO production by neoplastic stromal cells, as demonstrated by in situ hybridization.1 13-16 In our study only one patient had a large enough cerebellar hemangioblastoma, but he had no initial polycythemia and no change in tumor size was observed after 3 months of treatment.
A trivial hypothesis could be that CNS hemangioblastoma reacted to the privation of VEGF induced by SU5416 in producing increased amounts of hypoxia-inducible peptides. Overexpression of EPO could have been responsible for polycythemia, but, at least in 2 patients, there were only small, quiescent cerebellar hemangioblastomas. In addition, there was no change in serum EPO levels, though this finding does not rule out increased EPO production. Another possibility could be an increased susceptibility of red blood cell precursors to EPO in VHL patients treated with SU5416, and we cannot exclude that SU5416 interacts with other receptors critical for erythropoiesis.
Further in vivo and in vitro investigations are needed to elucidate this unexpected effect. Resolving this problem probably should be of great interest for understanding the action of SU5416 and improvement of anti-angiogenic therapy in VHL patients. Lastly, from a clinical point of view, initial polycythemia could be an exclusion criterion for enrollment of new patients in anti-VEGF receptor therapy trials.
We thank M. G. Allègre and Mrs. P. Rincé for technical assistance. We thank Mrs D. Broneer, Dr C. Béroud and Prof J. P. Grünfeld for their help in the preparation of the manuscript. We also thank Prof G. Tchernia, Prof C. Buffet, Prof G. Benoı̂t, Prof D. Doyon, Prof M. Bléry, Dr J. Langloys, Prof A. Jardin, and the nursing staff of the Department of Urology.
Supported in part by grants from the Ligue Nationale contre le Cancer (Comité du Cher) and the Association Française de Recherche Génétique.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Stéphane Richard, Génétique Oncologique EPHE, CHU, 94276 Le Kremlin-Bicêtre, France; e-mail:stephane.richard@kb.u-psud.fr.