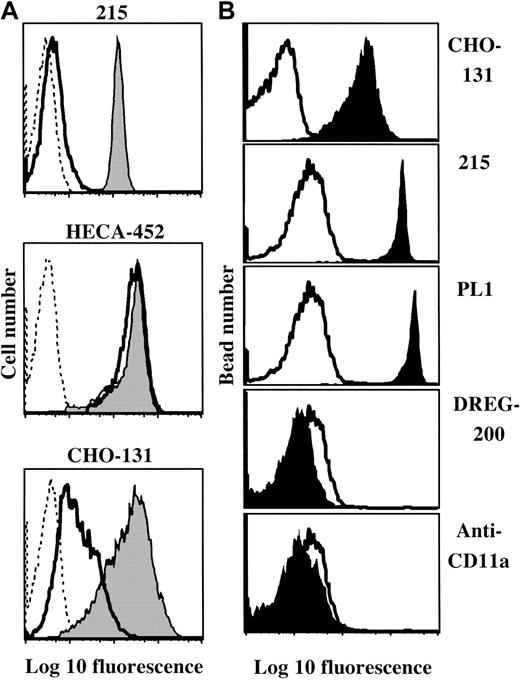
Core 2 O-glycans terminated with sialyl-Lewis x (sLeX) are functionally important oligosaccharides that endow particular macromolecules with high-affinity glycan ligands for the selectin family. To date, antibodies that recognize these structures on leukocytes have not been described. We characterize such a monoclonal antibody (mAb) here (CHO-131). The binding specificity of CHO-131 was directly examined by means of synthetic glycopeptides containing precise O-glycan structures. CHO-131 bound to sLeX extended from a core 2 branch (C2-O-sLeX), but CHO-131 demonstrated no reactivity if this oligosaccharide lacked fucose or if sLeX was extended from a core 1 branch. Using transfected cell lines, we found that CHO-131 binding required the functional activity of the glycosyltransferases α2,3-sialyltransferase, α1,3-fucosyltransferase-VII, and core 2 β1,6 N-acetylglucosaminyltransferase (C2GnT). The C2-O-sLeX motif occurs primarily on sialomucins and has been directly shown to contribute to high-affinity P-selectin glycoprotein ligand–1 binding by P-selectin. Indeed, CHO-131 staining of neutrophils was diminished following sialomucin removal by O-glycoprotease, and its reactivity with transfected hematopoietic cell lines correlated with the expression of P-selectin ligands. CHO-131 also stained a small population of lymphocytes that were primarily CD3+, CD4+, and CD45RO+ and represented a subset (37.8% ± 18.3%) of cutaneous lymphocyte-associated antigen (CLA) T cells, distinguished by the mAb HECA-452, which detects sLeX-related glycans. Unlike anti-sLeX mAbs, CHO-131 binding also indicates C2GnT activity and demonstrates that CLA T cells are heterogeneous based on the glycan structures they synthesize. These findings support evidence that differential C2GnT activity results in T-cell subsets that express ligands for E-selectin, P-selectin, or both.
Introduction
The 3 members of the selectin family facilitate accumulation and rolling on the blood vessel wall of lymphocytes in secondary lymphoid organs and of leukocytes at sites of inflammation. L-selectin (CD62L) is expressed by leukocytes; E-selectin (CD62E) is expressed by activated endothelial cells; and P-selectin (CD62P) is expressed by activated endothelial cells and activated platelets.1 The selectins are Ca+2-dependent lectins that bind discrete glycan structures on particular macromolecules.1,2 The leukocyte-expressed sialomucin, P-selectin glycoprotein ligand-1 (PSGL-1) (CD162) is one such macromolecular ligand that is recognized by P-selectin,3E-selectin,4-7 and L-selectin.8 9
The glycan modifications of PSGL-1 that are responsible for high-affinity binding by P-selectin have been extensively examined; they consist of sialylated and fucosylated, core 2 branched O-glycans.3 In contrast to particular L-selectin glycan ligands expressed by endothelial cells,1 these glycans are not sulfated. Instead, PSGL-1 contains sulfated tyrosine residues that are required for P-selectin binding.10-14 PSGL-1 on myeloid cells possesses 2 major species of sialylated and fucosylated core 2 O-glycans. Extended from the core 2 branch is a 3 N-acetyllactosamine repeat terminated with sialyl-Lewis x (sLeX) or a single N-acetyllactosamine unit terminated with sLeX.15 The latter sLeX motif (C2-O-sLeX) has been demonstrated to directly confer high-affinity P-selectin binding by means of glycosulfopeptides that are modeled after the N-terminus of human PSGL-1 and that contain precise O-glycan structures synthesized on a specific threonine residue.14 16
Although all peripheral blood leukocytes express PSGL-1,3the posttranslational glycan modifications that result in selectin ligands occur differentially and are synthesized by all neutrophils and a subset of lymphocytes, which preferentially home to sites of chronic inflammation in the skin.3,17-20 Properly stimulated and peripheral blood effector/memory T cells in general express up-regulated messenger RNA levels of α2,3-sialyltransferase-IV, α1,3-fucosyltransferase-VII (FucT-VII), and the O-glycan branching enzyme core 2 β1,6N-acetylglucosaminyltransferase (C2GnT).18,21-23 Recent findings indicate a distinct requirement for C2GnT in the synthesis of E- and P-selectin glycan ligands by stimulated T cells. Snapp et al24 have reported that TH1 cells derived from C2GnT−/− mice expressed E-selectin, but not P-selectin, glycan ligands. Moreover, E-selectin–binding TH1 cells derived from C2GnT+/+ mice were heterogeneous for C2GnT activity based on the expression of core-2 O-glycan–modified CD43.24 In the human, T cells that express selectin glycan ligands are distinguished by their expression of the sLeX-related antigen (cutaneous lymphocyte-associated antigen [CLA]), in most cases with the use of the monoclonal antibody (mAb) HECA-452.25,26 A limitation of anti-sLeX mAbs, however, is that their epitope does not indicate the functional activity of C2GnT. Moreover, the sLeX antigen is distributed on numerous leukocyte cell surface macromolecules, including proteins containing N- and O-glycans as well as lipids,27 and its detection does not directly indicate modification of T cell sialomucins with P-selectin glycan ligands.17
We report here the structural elucidation and leukocyte distribution of the epitope for the anticarbohydrate mAb CHO-131. The antigenic specificity of CHO-131 was assessed by means of synthetic glycopeptides with precise glycan structures as well as transfected cell lines expressing specific glycosyltransferases. The CHO-131 epitope is shown to comprise C2-O-sLeX and indicates the functional activity of α2,3-sialyltransferase, FucT-VII, and C2GnT. The CHO-131 epitope primarily occurs on leukocyte sialomucins, including PSGL-1, and was detected on neutrophils as well as on a subset of CLA+ T cells.
Materials and methods
Antibodies
The mAbs CSLEX-1 (mouse IgM) and HECA-452 (rat IgM), specific to the sLeX carbohydrate antigen, have been previously described.28,29 The mAbs PL1 (mouse IgG1), specific to human PSGL-1,30 and DREG-200 (mouse IgG1), specific to human L-selectin,31 have been previously described. The mAb 215 is a mouse IgG2a mAb specific to human PSGL-1 (Figures 1, 5, and 6, and data not shown). Anti-CD11a and the phycoerythrin (PE)–conjugated mAbs anti-CD4, anti-CD8, anti-CD19, and anti-CD45RO were purchased from Becton Dickinson (San Jose, CA). A PE-conjugated, anti-CD3 mAb was purchased from Ancell (Bayport, MN). PE- or fluorescein isothiocyanate (FITC)–conjugated F(ab′)2 goat anti–mouse IgG or IgM was purchased from Jackson Immunoresearch (West Grove, PA). FITC-conjugated, F(ab′)2 goat anti–rat IgM secondary antibody was purchased from Jackson Immunoresearch. Allophycocyanin (APC)–conjugated streptavidin was purchased from Biogenesis (Kingston, NH). The horseradish peroxidase–conjugated antibodies goat anti–mouse IgM and IgG and goat anti–rat IgM were purchased from Pierce (Rockford, IL).
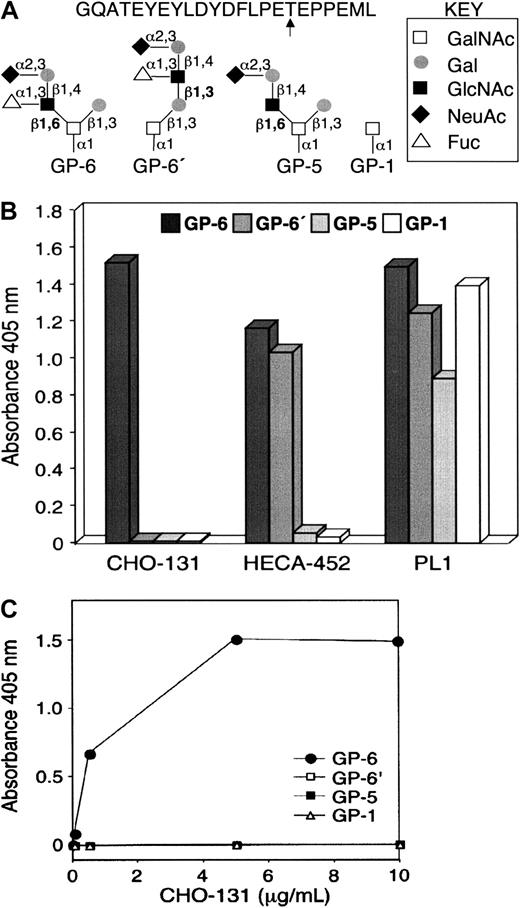
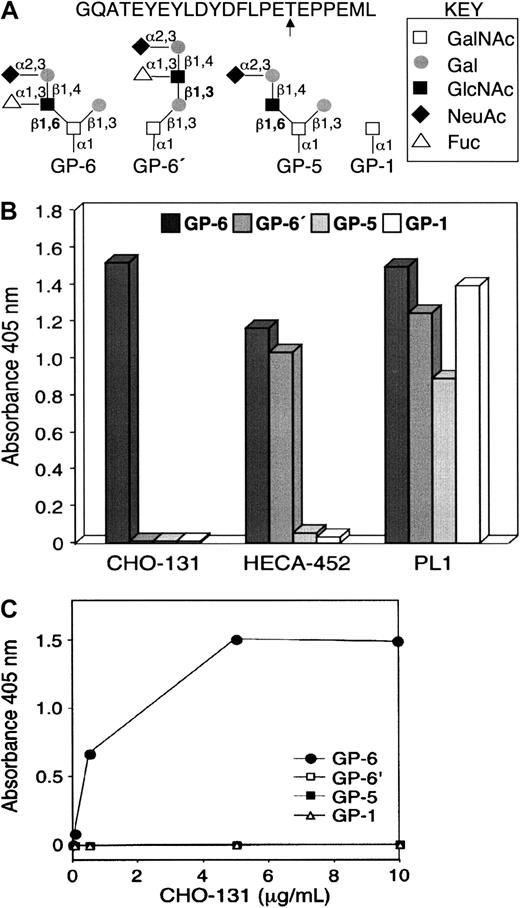
CHO-131 recognition of the glycan structure C2-O-sLeX.
(A) Structures of glycopeptides used in the enzyme-linked immunosorbent assay (ELISA). Glycopeptides corresponding to the N-terminal peptide sequence of human PSGL-1 and the O-glycan structures C2-O-sLeX (GP-6), C1-O-sLeX (GP-6′), C2-O-sLN (GP-5), or GalNAc (GP-1) at threonine residue 57 (arrow) were generated by synthetic methods. (B) Glycopeptide ELISA. Microtiter wells were coated with individual glycopeptides as indicated, blocked with BSA, and incubated with the mAbs CHO-131, HECA-452 (anti-sLeX), or PL1 (anti–PSGL-1 peptide). Bound antibodies were detected by incubation with horseradish peroxidase–conjugated, goat anti–mouse IgM (CHO-131) or IgG (PL1), or goat anti–rat IgM (HECA-452) followed by incubation with ABTS/peroxidase substrate and absorbance measurements at 405 nm. (C) CHO-131 specificity at different antibody concentrations. ELISA assay was performed as in panel B with the use of different concentrations of CHO-131 (0, 0.05, 0.5, 5, 10 μg/mL).
CHO-131 recognition of the glycan structure C2-O-sLeX.
(A) Structures of glycopeptides used in the enzyme-linked immunosorbent assay (ELISA). Glycopeptides corresponding to the N-terminal peptide sequence of human PSGL-1 and the O-glycan structures C2-O-sLeX (GP-6), C1-O-sLeX (GP-6′), C2-O-sLN (GP-5), or GalNAc (GP-1) at threonine residue 57 (arrow) were generated by synthetic methods. (B) Glycopeptide ELISA. Microtiter wells were coated with individual glycopeptides as indicated, blocked with BSA, and incubated with the mAbs CHO-131, HECA-452 (anti-sLeX), or PL1 (anti–PSGL-1 peptide). Bound antibodies were detected by incubation with horseradish peroxidase–conjugated, goat anti–mouse IgM (CHO-131) or IgG (PL1), or goat anti–rat IgM (HECA-452) followed by incubation with ABTS/peroxidase substrate and absorbance measurements at 405 nm. (C) CHO-131 specificity at different antibody concentrations. ELISA assay was performed as in panel B with the use of different concentrations of CHO-131 (0, 0.05, 0.5, 5, 10 μg/mL).
Cells and transfectants
Peripheral blood was collected from normal healthy donors. The procedures were performed in accordance with a protocol approved by the Institutional Review Board: Human Subjects Committee at the University of Minnesota. Leukocytes were isolated by red blood cell lysis or dextran sedimentation, plus ficoll-hypaque centrifugation to separate mononuclear and polymorphonuclear cells, as previously described.8,32 The CHO–dihydrofolate reductase–negative (CHO-dhfr−) (ATCC, Manassas, VA) stable transfectants CHO/PSGL-1 and CHO/PSGL-1/FucT-VII/C2GnT were constructed as previously described12,33 with minor modifications. Briefly, the human wild-type complementary DNAs (cDNAs) encoding PSGL-1, C2GnT, and FucT-VII were ligated into the expression vectors pcDNA3.1/Zeocin (Invitrogen, Carlsbad, CA), pcDNA3 (Invitrogen), and pCDM8/puromycin, respectively. The CHO-dhfr− stable transfectants CHO/FucT-VII and CHO/FucT-VII/C2GnT were constructed as previously described.12,33 The Jurkat/FucT-VII and Molt-4/FucT-VII stable transfectants were constructed as previously described.34,35 Cells were treated with neuraminidase (from Clostridium perfringens, which hydrolyzes α(2-3), α(2-6), or α(2-8) linkages; Roche, Mannheim, Germany) and O-glycoprotease (O-sialoglycoprotein endopeptidase; Accurate Chemical and Scientific, Westbury, NY) to remove terminal sialic acid moieties and sialomucins, respectively, as previously described.36-38
Immunization and mAb generation
These procedures were performed in accordance with a protocol approved by the Institutional Animal Care and Use Committee at the University of Minnesota. CHO/PSGL-1/FucT-VII/C2GnT transfectants were used as immunogen for generation of mAb. CHO/PSGL-1/FucT-VII/C2GnT cells (5 × 107) were injected intraperitoneally into Balb/c mice at biweekly intervals. The initial injection contained the adjuvant Gerbu (GERBU Biotechnik, Heidelberg, Germany), whereas subsequent injections did not contain adjuvant. The SP2/0 myeloma cell line was used as a fusion partner, and previously described procedures were followed in the generation of monoclonal hydridomas.38 39 All mAbs produced were expanded by in vitro tissue-culture procedures. Biotinylation of mAbs was performed by means of NHS-SS-LC-biotin (Pierce), as per manufacturer's instructions. The generated mAb CHO-131 (IgM, kappa light chain) is described in this report.
Preparation of protein-adsorbed 4-μm latex microspheres
Sulfate polystyrene latex microspheres (Interfacial Dynamics, Portland, OR) were adsorbed with human PSGL-1 that was immunoprecipitated from neutrophils. The anti–PSGL-1 mAb PL1 was used to immunoprecipitate PSGL-1; this was done in buffer containing 50 mM n-octyl-β-glucopyranoside. Dilution of this detergent below its critical micelle concentration allowed for PSGL-1 adsorption.8 Approximately 0.1 μg PSGL-1 was adsorbed to 1 × 107 microspheres. The microspheres were then blocked with 5% bovine serum albumin (BSA).
Antibody labeling and flow cytometry
These procedures were performed as previously described.40 Briefly, Fc receptor and nonspecific antibody-binding sites were blocked by an initial incubation of the cells with wash buffer (phosphate-buffered saline [PBS] containing 1% goat serum and 5 mM NaN3). For 1 fluorescent–parameter flow cytometry, cells or microspheres were labeled with a particular unconjugated mAb for 15 minutes followed by incubation with FITC-conjugated, F(ab′)2 goat anti–mouse IgG or IgM or anti–rat IgM. For 2 fluorescent–parameter flow cytometry, cells were stained with CHO-131 for 15 minutes followed by incubation with FITC-conjugated, F(ab′)2 goat anti–mouse IgM for 15 minutes. Cells were then blocked with 10% normal mouse serum for 10 minutes followed by incubation with PE-conjugated anti-CD3, anti-CD4, anti-CD8, anti-CD19, or anti-CD45RO for 15 minutes. For 3 fluorescent–parameter flow cytometry, cells were stained in 6 steps: (1) incubation with HECA-452 for 15 minutes; (2) incubation with FITC-conjugated, F(ab′)2 goat anti–rat IgM for 15 minutes; (3) blocking with 10% normal mouse serum for 10 minutes; (4) incubation with biotinylated CHO-131 for 15 minutes; (5) incubation with APC-conjugated streptavidin for 15 minutes; and (6) incubation with PE-conjugated anti-CD3 for 15 minutes. All steps were performed at 4°C. Cells were washed with wash buffer between steps and finally fixed with 0.5% paraformaldehyde. Isotype-matched negative control mAbs were used to evaluate levels of background staining. For each sample, 5000 to 10 000 antibody-labeled cells or microspheres were analyzed by flow cytometry on a FACSCalibur instrument (Becton Dickinson).
Glycopeptide enzyme-linked immunosorbent assay
Glycopeptides (GPs) (GP-1, GP-5, GP-6, and GP-6′) were prepared as described.16 Microtiter wells (Immulon 2, 96-well plates, Dynatech Laboratories, Chantilly, VA) were coated for 1.5 hours with 5 ng individual glycopeptide in 50 μL PBS, blocked by incubating for 30 minutes with 5% BSA in PBS, and incubated for 1 hour with 50 μL mAb solutions (5 μg/mL mAb CHO-131, HECA-452, or PL1, in PBS containing 0.05% Tween-20 and 1% BSA). The wells were subsequently incubated for 1 hour with 50 μL of 1:5000 dilution of peroxidase-conjugated goat anti–mouse IgM or IgG, or with 50 μL of 1:100 dilution of peroxidase-conjugated goat anti–rat IgM, followed by incubation with 100 μL 2,2′-azino-di(3-ethyl-benzthiazoline-6-sulfonate) (ABTS)/peroxidase substrate (Kirkegaard and Perry Laboratories, Gaithersburg, MD). After a 10-minute incubation with substrate, absorbance at 405 nm was measured by means of a microtiter plate reader (Molecular Devices, Sunnylane, CA). Experiments with mAb CHO-131 were carried out with the use of antibody concentrations of 0, 0.05, 0.5, 5, and 10 μg/mL in PBS containing 0.05% Tween-20 and 1% BSA. Antigen coating and all incubations were performed at room temperature, and the wells were washed 6 times after each incubation on a microtiter plate washer (Dynatech) with PBS containing 0.05% Tween-20. The assays were performed in duplicate, and the results represent averages of 2 determinations.
Results
The CHO-131 epitope includes a core-2 O-glycan when terminated with sLeX. CHO cells expressing transfected human PSGL-1, FucT-VII, and C2GnT (CHO/PSGL-1/FucT-VII/C2GnT) bind P-selectin with high affinity.12,33,41 Mice were immunized with the stable transfectant CHO/PSGL-1/FucT-VII/C2GnT and the subsequently generated hybridoma antibodies initially screened for reactivity with the transfectants CHO/PSGL-1/FucT-VII/C2GnT and CHO/PSGL-1 to identify antibodies possibly requiring the expression of FucT-VII and C2GnT. The mAb CHO-131 was found to stain the transfectant CHO/PSGL-1/FucT-VII/C2GnT, but not CHO/PSGL-1, as determined by flow cytometry (data not shown; also see below). Synthetic peptides corresponding to the N-terminus of human PSGL-1 have been previously used to determine the contributions of posttranslational modifications, such as precise O-glycan structures attached to a particular threonine residue, on binding to P-selectin.14 16 Using different O-glycan structures modeled on the N-terminal peptide sequence from PSGL-1, we examined the binding specificity of CHO-131. The glycopeptides used are shown in Figure1A, and included GP-6, which contains sLeX on a core 2 O-glycan (C2-O-sLeX); GP-6′, which contains sLeX on an extended core 1 O-glycan (C1-O-sLeX); GP-5, which contains sialylated but nonfucosylated N-acetyllactosamine on a core 2 O-glycan (C2-O-sLN); and GP-1, which contains an O-linked GalNAc. The glycopeptides were adsorbed to the wells of a 96-well plate, and antibody reactivity was determined by enzyme-linked immunosorbent assay (ELISA). The anti-sLeX mAb HECA-452 bound to the carbohydrate structures C2-O-sLeX (GP-6) and C1-O-sLeX (GP-6′), but not to C2-O-sLN (GP-5) and GalNAc (GP-1) (Figure 1B). In contrast, CHO-131 bound only to C2-O-sLeX (GP-6), indicating the importance of the core 2 branch for reactivity. However, other determinants, including fucose, also appear to compose the CHO-131 epitope since the structure C2-O-sLN was not recognized (Figure 1B). To demonstrate that the observed reactivities were not due to differences in glycopeptide coating, the anti–PSGL-1 peptide mAb PL1 was used in the ELISA. PL-1 detected high levels of all 4 glycopeptides (Figure 1B). The specificity of CHO-131 was confirmed by the observation that there was no binding to GP-6′, GP-5, and GP-1 even at the highly saturating concentration of 10 μg/mL (Figure 1C).
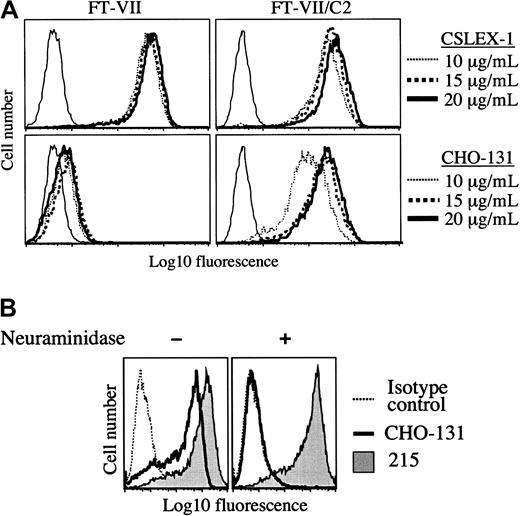
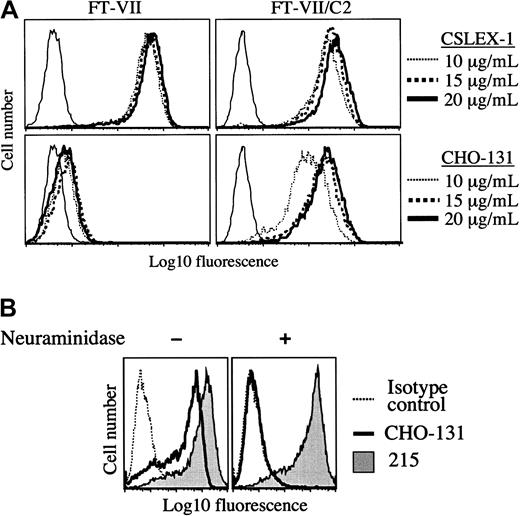
CHO-131 recognizes a sialylated and fucosylated core 2 O-glycan when expressed by cells. Particular glycosyltransferases expressed by transfected cells were examined for their requirement in the synthesis of the CHO-131 antigen. CHO cells have well characterized N- and O-glycan structures and express α1,3-fucosyltransferases and C2GnT at very low levels.42 43 CHO cells transfected with the cDNA of FucT-VII can efficiently synthesize a sLeXmotif on N-glycans, but not O-glycans. Using flow cytometry, we observed high levels of staining of the CHO/FucT-VII transfectant by the anti-sLeX mAb CSLEX-1, but at very low levels of staining by CHO-131 (Figure 2A). Transfected CHO cells expressing FucT-VII and C2GnT can synthesize a sLeX motif on both N- and O-glycans. CHO/FucT-VII/C2GnT transfectants were stained at high levels by both CHO-131 and CSLEX-1 (Figure 2A). Since these transfectants do not express PSGL-1, the results demonstrate that antigen recognition by CHO-131 is independent of the PSGL-1 polypeptide. Mock-transfected CHO cells were not stained by either CSLEX-1 or CHO-131 (data not shown).
Expression of the CHO-131 epitope by transfected CHO cells.
(A) CHO-131 stains at a high level the transfectant CHO/FucT-VII/C2GnT (FT-VII/C2), but not CHO/FucT-VII (FT-VII). The transfectants CHO/FucT-VII/C2GnT and CHO/FucT-VII were labeled with the indicated concentrations of CHO-131 or the anti-sLeX mAb CSLEX-1. Nonspecific antibody labeling was determined by the use of an isotype negative control mAb (thin solid lines). Cell staining levels were examined by flow cytometry. Representative data from multiple repetitions are shown. (B) CHO-131 reactivity requires sialic acid. The transfectant CHO/PSGL-1/FucT-VII/C2GnT was subjected to neuraminidase (0.25 U) or sham treatment and then labeled with CHO-131 or 215 (anti–PSGL-1) as indicated. Nonspecific antibody labeling was determined with the use of the appropriate isotype negative control mAbs (dotted line; mouse IgM control is shown). Cell staining levels were examined by flow cytometry, and 10 000 cells were examined per sample. Representative data from multiple repetitions are shown.
Expression of the CHO-131 epitope by transfected CHO cells.
(A) CHO-131 stains at a high level the transfectant CHO/FucT-VII/C2GnT (FT-VII/C2), but not CHO/FucT-VII (FT-VII). The transfectants CHO/FucT-VII/C2GnT and CHO/FucT-VII were labeled with the indicated concentrations of CHO-131 or the anti-sLeX mAb CSLEX-1. Nonspecific antibody labeling was determined by the use of an isotype negative control mAb (thin solid lines). Cell staining levels were examined by flow cytometry. Representative data from multiple repetitions are shown. (B) CHO-131 reactivity requires sialic acid. The transfectant CHO/PSGL-1/FucT-VII/C2GnT was subjected to neuraminidase (0.25 U) or sham treatment and then labeled with CHO-131 or 215 (anti–PSGL-1) as indicated. Nonspecific antibody labeling was determined with the use of the appropriate isotype negative control mAbs (dotted line; mouse IgM control is shown). Cell staining levels were examined by flow cytometry, and 10 000 cells were examined per sample. Representative data from multiple repetitions are shown.
Terminal sialylation of glycans expressed by CHO cells occurs exclusively in an α2,3-linkage.44 A requirement of sialic acid for CHO-131 reactivity was examined by treating transfected CHO cells expressing recombinant PSGL-1, FucT-VII, and C2GnT with neuraminidase. This treatment was found to abolish staining by CHO-131 (Figure 2B). The glycosidase activity of neuraminidase was confirmed by its disruption of staining by the anti-sLeX mAb CSLEX-1 (data not shown) and its lack of effect on PSGL-1 staining (Figure 2B), which is highly sensitive to protease activity.45
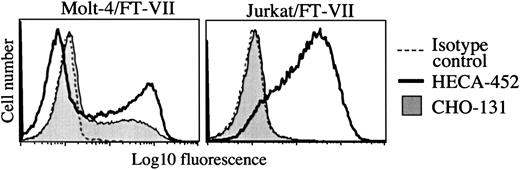
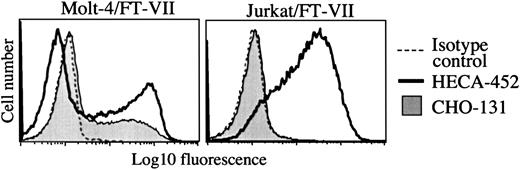
Glycosyltransferase requirements for CHO-131 reactivity were also examined with the use of transfected human hematopoietic cell lines. Molt-4 cells transfected with FucT-VII express P-selectin ligands.35 In contrast, Jurkat cells, which are deficient in core 2 and core 1 O-glycan synthesis,46 when transfected with FucT-VII do not express detectable P-selectin ligands (R.N.K., unpublished data, December 1998 and Knibbs et al35). Using flow cytometry, we observed that CHO-131 and the anti-sLeX mAb HECA-452 stained the Molt-4/FucT-VII transfectants (Figure 3). CHO-131, however, did not bind to Molt-4 parent cells (data not shown), which express endogenous FucT-IV and bind poorly to P-selectin.35 CHO-131 also did not stain Jurkat/FucT-VII transfectants at a level greater than that of an isotype negative control mAb, whereas HECA-452 stained this transfectant at high levels (Figure 3). Together, the data above indicate that cell staining by CHO-131 requires, in part, α2,3-sialyltransferase, FucT-VII, and C2GnT. Moreover, CHO-131 staining correlated with the expression of P-selectin glycan ligands, whereas the reactivity of anti-sLeX mAbs did not.
CHO-131 staining of the transfectant Molt-4/FucT-VII but not of Jurkat/FucT-VII.
The transfected cell lines Molt-4/FucT-VII and Jurkat/FucT-VII were labeled with CHO-131 (15 μg/mL) or the anti-sLeX mAb HECA-452 (15 μg/mL), as indicated. Nonspecific antibody labeling was determined with the use of the appropriate isotype negative control mAbs (dashed line; mouse IgM control is shown). Cell staining levels were examined by flow cytometry, and 10 000 cells were examined per sample. Representative data from multiple repetitions are shown.
CHO-131 staining of the transfectant Molt-4/FucT-VII but not of Jurkat/FucT-VII.
The transfected cell lines Molt-4/FucT-VII and Jurkat/FucT-VII were labeled with CHO-131 (15 μg/mL) or the anti-sLeX mAb HECA-452 (15 μg/mL), as indicated. Nonspecific antibody labeling was determined with the use of the appropriate isotype negative control mAbs (dashed line; mouse IgM control is shown). Cell staining levels were examined by flow cytometry, and 10 000 cells were examined per sample. Representative data from multiple repetitions are shown.
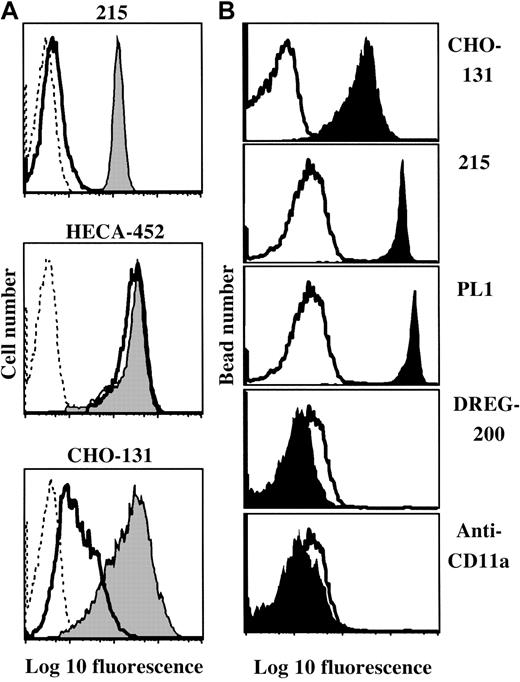
CHO-131 primarily binds to sialomucins. C2-O-sLeX–like structures are common components of sialomucins.15,47,48Thus, consistent with the specificity of CHO-131, it would be predicted that staining would be affected by the selective removal of cell surface sialomucins using O-glycoprotease. In contrast, the sLeX oligosaccharide is broadly distributed on various cell surface macromolecules, and O-glycoprotease treatment has only a modest affect on the overall reactivity by anti-sLeXmAbs.17,49 Using flow cytometry, we observed that both CHO-131 and HECA-452 stained isolated neutrophils at uniformly high levels (Figure 4A). Neutrophils were then treated with O-glycoprotease and stained with HECA-452 or CHO-131. Neutrophils were also stained with an anti–PSGL-1 mAb to assess endoprotease efficiency. We found that staining by CHO-131 was greatly reduced by O-glycoprotease treatment (Figure 4A). As previously described, O-glycoprotease treatment also diminished PSGL-1 staining, but had little effect on sLeX antigen levels (Figure 4A; Alon et al17 and Norgard et al49). Staining of class 1, a nonsialomucin glycoprotein, was not changed by O-glycoprotease treatment, indicating insignificant levels of contaminating proteases (data not shown). These findings show that CHO-131 selectivity binds O-glycoprotease–sensitive sialomucins, which include PSGL-1 immunopurified from neutrophils (Figure 4B).
Preferential binding of CHO-131 to sialomucins.
(A) Neutrophil staining by CHO-131 is diminished following O-glycoprotease treatment. Peripheral blood neutrophils were subjected to O-glycoprotease (solid line; 48 μg/mL, 5 × 106neutrophils/mL) or sham treatment (filled histograms), and then labeled with the mAbs CHO-131, HECA-452 (anti-sLeX), or 215 (anti–PSGL-1) at 15 μg/mL. Nonspecific antibody labeling was determined with the use of the appropriate isotype negative control mAbs (dashed lines). Cell staining levels were examined by flow cytometry, and 10 000 cells were examined per sample. Representative data from multiple repetitions are shown. (B) CHO-131 binds immunoprecipitated PSGL-1. Human neutrophil PSGL-1 was immunoprecipitated with the anti–PSGL-1 mAb PL1 and adsorbed to 4-μm latex microspheres, as described in “Materials and methods.” The microspheres were labeled with CHO-131, the anti–PSGL-1 mAbs 215 and PL1, the anti–L-selectin mAb DREG-200, or an anti-CD11a mAb at 5 μg/mL, as indicated (filled histograms). Nonspecific antibody labeling was determined with the use of the appropriate isotype negative control mAbs (solid line). Microsphere staining was assessed by flow cytometry, and 5000 microspheres were examined per sample. Representative data from multiple repetitions are shown.
Preferential binding of CHO-131 to sialomucins.
(A) Neutrophil staining by CHO-131 is diminished following O-glycoprotease treatment. Peripheral blood neutrophils were subjected to O-glycoprotease (solid line; 48 μg/mL, 5 × 106neutrophils/mL) or sham treatment (filled histograms), and then labeled with the mAbs CHO-131, HECA-452 (anti-sLeX), or 215 (anti–PSGL-1) at 15 μg/mL. Nonspecific antibody labeling was determined with the use of the appropriate isotype negative control mAbs (dashed lines). Cell staining levels were examined by flow cytometry, and 10 000 cells were examined per sample. Representative data from multiple repetitions are shown. (B) CHO-131 binds immunoprecipitated PSGL-1. Human neutrophil PSGL-1 was immunoprecipitated with the anti–PSGL-1 mAb PL1 and adsorbed to 4-μm latex microspheres, as described in “Materials and methods.” The microspheres were labeled with CHO-131, the anti–PSGL-1 mAbs 215 and PL1, the anti–L-selectin mAb DREG-200, or an anti-CD11a mAb at 5 μg/mL, as indicated (filled histograms). Nonspecific antibody labeling was determined with the use of the appropriate isotype negative control mAbs (solid line). Microsphere staining was assessed by flow cytometry, and 5000 microspheres were examined per sample. Representative data from multiple repetitions are shown.
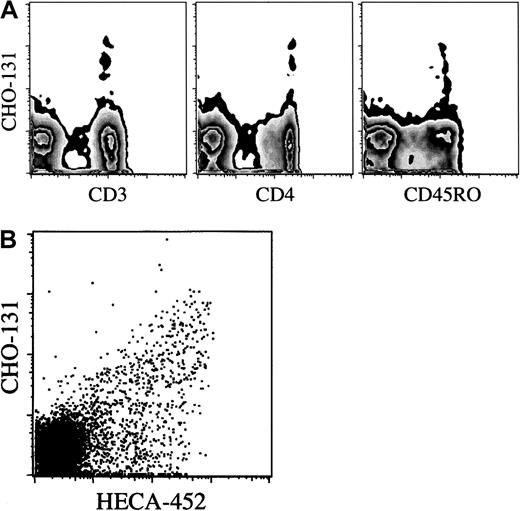
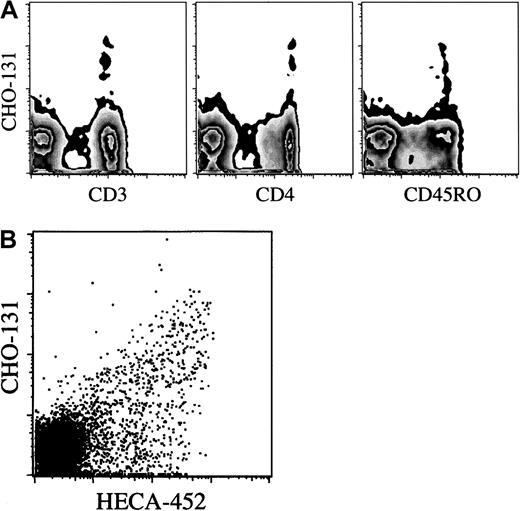
The CHO-131 epitope is distributed on a subset of CLA+ T cells. Using flow cytometry to assess 1-color fluorescence and side-scatter characteristics, we observed that CHO-131 uniformly stained human peripheral blood neutrophils and monocytes (Figure 4A and data not shown). In addition, a small population of lymphocytes were stained. To identify the lymphocyte subsets expressing the CHO-131 epitope, an electronic scatter gate was set for lymphocytes, and 2-color flow cytometry was performed with the use of various lineage markers. The CHO-131 epitope was detected primarily on CD3+T cells (Figure 5A). CD19+ B cells represented fewer than 1% of the CHO-131+lymphocytes. Furthermore, the CHO-131 epitope occurred primarily on CD4+ T cells (Figure 5A). We consistently found that fewer than 10% of CHO-131+ lymphocytes were CD8+. CHO-131+ lymphocytes would also appear to be effector/memory T cells, as indicated by their essentially uniform expression of CD45RO (Figure 5A). Next, we simultaneously examined the distribution of the C2-O-sLeX and sLeX (CLA) antigens on T cells. By means of 3-color flow cytometry, CD3+ lymphocytes were stained by HECA-452 and CHO-131. CHO-131 was found to stain HECA-452+ T cells, but only a subset (Figure 5B). Similar results were observed with the anti-sLeX mAb CSLEX (data not shown). Thus, 3 subsets of T cells were revealed: (1) CLA−/CHO-131−; (2) CLA+/CHO-131−; and (3) CLA+/CHO-131+. From healthy donors, 37.8% ± 18.3% (n = 4) of HECA-452+ lymphocytes were found to contain the CHO-131 epitope.
Distribution of the CHO-131 epitope on lymphocyte subsets.
(A) CHO-131 stains CD4+ effector/memory T cells. Human peripheral blood, gated on lymphocytes (10 000 cells), were dual analyzed for their expression of the CHO-131 epitope and CD3, CD4, or CD45RO, as indicated. (B) CHO-131 stains a subset of CLA+lymphocytes. Peripheral blood lymphocytes, gated on CD3+lymphocytes (10 000 cells), were dual analyzed for their expression of CLA (HECA-452) and C2-O-sLeX (CHO-131), as indicated. Nonspecific antibody labeling was determined with the use of the appropriate isotype negative control mAbs (data not shown). Cell-staining levels were examined by flow cytometry. Representative data from multiple repetitions are shown.
Distribution of the CHO-131 epitope on lymphocyte subsets.
(A) CHO-131 stains CD4+ effector/memory T cells. Human peripheral blood, gated on lymphocytes (10 000 cells), were dual analyzed for their expression of the CHO-131 epitope and CD3, CD4, or CD45RO, as indicated. (B) CHO-131 stains a subset of CLA+lymphocytes. Peripheral blood lymphocytes, gated on CD3+lymphocytes (10 000 cells), were dual analyzed for their expression of CLA (HECA-452) and C2-O-sLeX (CHO-131), as indicated. Nonspecific antibody labeling was determined with the use of the appropriate isotype negative control mAbs (data not shown). Cell-staining levels were examined by flow cytometry. Representative data from multiple repetitions are shown.
Discussion
The novel mAb CHO-131 is demonstrated here to specifically bind the glycan structure C2-O-sLeX, which is a functional sLeX motif on PSGL-1. Other mAbs have been described that recognize antigens on human cells that involve a core 2 O-glycan. The mAbs T305 and 1D4 are specific to CD43 modified by core 2 O-glycans.50,51 The mAb J28 recognizes a fucosylated, core 2 O-glycan–containing antigen; however, its reactivity was shown to be abrogated by FucT-VII activity.52 The mAb NCC-ST-439 also binds a fucosylated, core 2 O-glycan–containing antigen; however, the effects of the leukocyte-specific glycosyltransferases FucT-IV and FucT-VII on NCC-ST-439 reactivity were not examined.53 Moreover, a direct correlation between the expression of P-selectin glycan ligands and the epitopes recognized by these mAbs was not established.
To assess whether CHO-131 also recognized a sialylated and fucosylated core 2–based O-glycan synthesized by cells, transfected cell lines that express well-described glycan structures were examined. The expression of FucT-VII and C2GnT by transfected CHO cells or the expression of FucT-VII by transfected Molt-4 cells resulted in high levels of staining by CHO-131, which was abolished by neuraminidase treatment. In contrast to anti-sLeX mAbs, CHO-131 stained at very low levels CHO cells expressing only transfected FucT-VII, which was abolished by neuraminidase treatment as well, indicating that it was specific (data not shown). CHO-131, however, did not stain Jurkat/FucT-VII transfectants. One explanation for the difference in reactivity of CHO-131 with the Jurkat/FucT-VII and CHO/FucT-VII transfectants may be that CHO cells, which synthesize core 1 O-glycans and have been shown to express low levels of endogenous C2GnT,43 expressed some sialylated and fucosylated core 2 O-glycans when transfected with the cDNA for FucT-VII. Jurkat cells, however, are inefficient at synthesizing core 1 O-glycans, and thus the Jurkat/FucT-VII transfectants would be unlikely to synthesize sialylated and fucosylated core 2 O-glycans.34,46 These data together indicate that CHO-131 reactivity requires the functional activity of α2,3-sialyltransferase, FucT-VII, and C2GnT. At this time, it cannot be ruled out that CHO-131 also recognizes N-glycans containing β1-6 branches (I-type) terminated with sLeX.27 However, such a structure would not contribute to CHO-131 staining of human leukocytes since these cells do not express N-glycans with I-type branching.54 55
Expression of the CHO-131 epitope corresponds with the synthesis of P-selectin glycan ligands. For instance, the human hematopoietic cell line Molt-4, but not Jurkat, expresses P-selectin ligands when transfected with the cDNA for FucT-VII.35 Similarly, Molt-4/FucT-VII transfectants, but not Jurkat/FucT-VII transfectants, were stained by CHO-131, whereas the anti-sLeX mAb HECA-452 stained both transfectants. The CHO-131 epitope is also distributed primarily on sialomucins, which are relevant macromolecular ligands for P-selectin. In contrast, O-glycoprotease digestion of sialomucins has little effect on sLeX antigen levels on neutrophils and CLA+ T cells (Figure 4A; Alon et al17 and Norgard et al49). Despite the functional importance of sLeX-modified, core 2 O-glycans, CHO-131 at a concentration as high as 100 μg/mL did not significantly block neutrophil rolling on platelet-derived P-selectin in an in vitro shear-flow assay, whereas an anti–P-selectin mAb demonstrated complete blocking at 1 μg/mL (data not shown). The lack of blocking function by CHO-131 may be the result of antibody affinity and/or epitope position on the oligosaccharide. Certain mAbs that bind sLeX-containing glycans have been shown to be function blocking, though this is inconsistent. HECA-452 has been reported to block E-selectin binding to purified CLA under shear flow,26 but has also been used to immunopurify CLA T cells with no blocking effects in assays involving E- and P-selectin.20,56 CSLEX-1 has been reported to block neutrophil binding to E-selectin in static assays,57,58whereas HECA-452 and CSLEX-1 ineffectively blocked the binding of T lymphoblasts to E- and P-selectin under shear flow.35NCC-ST-439 has been described as blocking the binding of certain breast cancer cell lines to E-selectin.53
CHO-131 stained all peripheral blood neutrophils and monocytes, as well as a subset of lymphocytes. Lymphocytes expressing the CHO-131 epitope were primarily CD4+ effector/memory T cells and consistently represented a subset of HECA-452–distinguished, CLA+ lymphocytes (approximately equal to 38%). The glycosyltransferases α2,3-sialyltransferase-IV, FucT-VII, and C2GnT are up-regulated in in vitro–stimulated and peripheral blood effector/memory T cells, resulting in the synthesis of selectin glycan ligands.18,21-23 Consistent with our data, CHO-131 may distinguish a subset of T cells that bear the C2-O-sLeXstructure resulting from the activity of α2,3-sialyltransferase, FucT-VII, and C2GnT. Detection of the CHO-131 epitope on a subset of CLA+ lymphocytes could be the result of differential regulation of C2GnT. Indeed, E-selectin–binding T cells have been shown to be heterogeneous for C2GnT activity.24 The differential regulation of particular glycosyltransferases by effector/memory T cells may result in ligands for E-selectin, P-selectin, or both. Based on functional assays, T-cell subsets that bind E-selectin or P-selectin have been described.17 59
In summary, the CHO-131 epitope is directly relevant to the expression of high-affinity glycan ligands for P-selectin. CHO-131 may then be an important tool for detecting expression of these ligands on specific cells: for instance, particular leukocyte subsets or neoplastic cells. Interestingly, it has been reported that the expression level of sLeX–modified O-glycans on particular carcinomas may be indicative of metastasis.60 CHO-131 may also reveal novel sialomucins that serve as selectin ligands.
The authors thank Michael McDaniel and Dr Erik Matala for flow cytometry, Sue Anderson for drawing blood, and Lisa Adwan for proofreading the manuscript.
Prepublished online as Blood First Edition Paper, April 17, 2002; DOI 10.1182/blood-2001-12-0265.
Supported in part by University of Minnesota institutional funds and National Institutes of Health grants AI 48075 (R.D.C.) and HL 65631 (R.P.M.).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Bruce Walcheck, University of Minnesota, 295j AS/VM Bldg, 1988 Fitch Ave, St Paul, MN 55108; e-mail:walch003@umn.edu.