In the paper by Casonato et al1 on reduced von Willebrand factor (VWF) survival in type 2M Vicenza von Willebrand disease (VWD), the authors reported 4 additional families who had the same peculiar phenotype and candidate mutations (2470G>A in exon 17 and 3864G>A in exon 27 of the VWF gene) previously identified by our group in the original families2 and other families from the same geographical area.3 However, Casonato et al1 reported 2 important phenotypic differences. First, ristocetin-induced platelet agglutination (RIPA) was normal, whereas it was invariably reduced in the original cases.4 This led the authors to speculate that VWF Vicenza interacts normally with platelets, even though they did not perform binding studies. Second, their patients showed an aberrant multimeric pattern of plasma VWF on high-resolution agarose gels, characterized by the presence of doublets instead of the typical triplet pattern. Barring methodological differences, these findings support the views that some of the patients reported by Casonato et al do not have typical VWD Vicenza.
The authors state that the “pathognomonic” aspect of VWD Vicenza is the very low level of plasma VWF contrasting with normal levels of platelet VWF. There are cases of VWD other than VWD Vicenza with this discrepant pattern. For instance, heterozygotes with type 1 VWD as a result of the Cys1130Phe mutation also have low plasma levels of VWF but normal platelet levels.5 As the original Vicenza patients, these type 1 patients are responsive to desmopressin treatment with brisk transient normalization of VWF measurements, shortening of the bleeding time (not shown), and postinfusion disappearance rate of FVIII/VWF measurements (Figure1). We believe that the appropriate controls for the studies done by Casonato et al should be patients with type 1 with similar baseline levels of VWF, nothealthy individuals. Furthermore, at variance with them, we believe that the fact that VWF is normal in platelets does not necessarily demonstrate that this protein is secreted normally. If synthesis is normal but there is a dominant-negative intracellular retention due to impaired production and secretion, the platelet VWF content would be expected to be normal as in the example demonstrated in type 1 VWD for the Cys1149Arg mutation and the very similar Cys1130Phe mutation.6 Should the constitutive pathway be mainly affected in VWD Vicenza, as suggested by Schneppenheim et al,7 then studies on the survival of desmopressin-released VWF, which originates from the secretory pathway, are largely irrelevant.
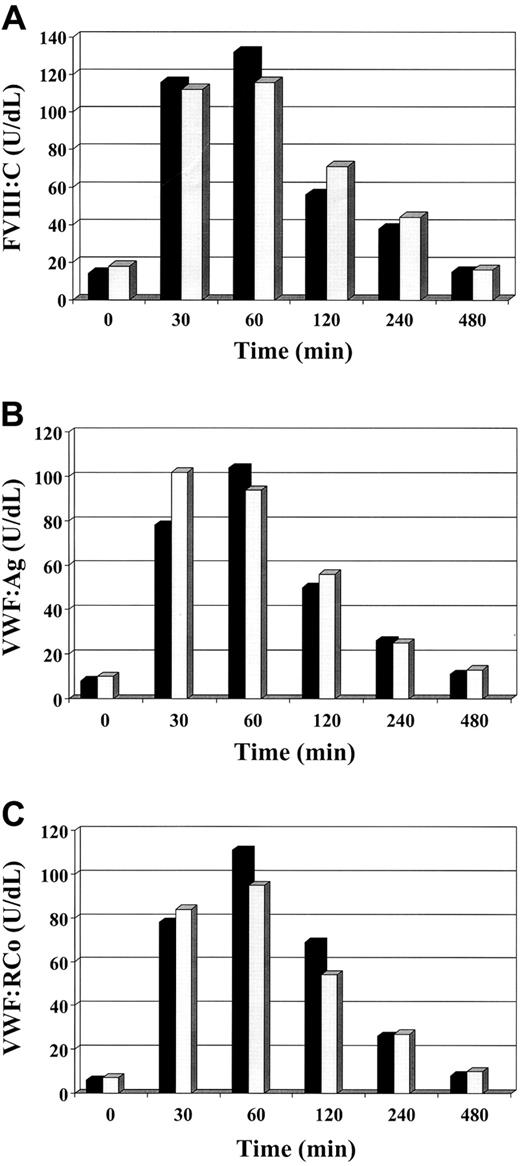
Factor VIII/von Willebrand factor measurements after desmopressin in type 2M Vicenza and Cys1130Phe mutation.
The figure shows the mean of the results of FVIII:C (A), VWF:Ag (B), and VWF:RCo (C) in 2 patients for each group infused intravenously with 0.3 μg/kg of desmopressin. ▪ indicates Vicenza; ░, Cys1130Phe.
Factor VIII/von Willebrand factor measurements after desmopressin in type 2M Vicenza and Cys1130Phe mutation.
The figure shows the mean of the results of FVIII:C (A), VWF:Ag (B), and VWF:RCo (C) in 2 patients for each group infused intravenously with 0.3 μg/kg of desmopressin. ▪ indicates Vicenza; ░, Cys1130Phe.
In conclusion, Casonato et al1 describe 4 additional families of VWD Vicenza with an unusual phenotype without providing definite experimental evidence as to the possible pathogenetic mechanism of the disease. Only expression studies and cotransfection of the mutant gene and wild-type gene could perhaps explain the basis of this subtype of VWD. Further studies are needed prior to dismissal of this subtype from type 2M VWD, which includes cases with defective VWF-platelet interaction accompanied by intact VWF multimers in plasma.
The reduced survival of type Vicenza von Willebrand factor
Type Vicenza von Willebrand disease (VWD) was first described by Mannucci's group in patients coming from Northeast Italy1-1; its phenotype was characterized by significantly reduced plasma levels of von Willebrand factor (VWF), a normal platelet VWF content, and the presence of unusually large VWF multimers. The original type Vicenza patients were also characterized by a normal response to DDAVP infusion with normal T1/2 survival, as compared with type 1 VWD.1-1 Recently, 2 candidate mutations were described; 2470G>A in exon 17 and 3864G>A in exon 27 of VWF gene.1-2,1-3 Why plasma VWF levels are significantly reduced despite the normal platelet VWF content is a matter of discussion. Schneppenheim et al1-2 suggest a defect in the constitutive release of Vicenza VWF, in contrast with the normal acute one. We instead advance that a reduced survival of a normally synthesized VWF underlies the significant reduction in plasma VWF.1-4
Castaman et al suggest in their letter that our patients may not have a typical type Vicenza phenotype. Indeed, they have all the peculiar haemostatic findings and the 2 candidate mutations associated with type Vicenza VWD, but differ in that they have normal or borderline ristocetin-induced platelet agglutination (RIPA) values and a multimer pattern characterized by the presence of doublets instead of the typical triplet pattern. As far as RIPA is concerned, we know that this test may be affected by variations in the plasma VWF concentrations during physiologic or pathologic conditions. Even though we did not perform binding studies, this observation prompted us to speculate about the normality of platelet glycoprotein Ib (GPIb) and type Vicenza VWF. Regarding the multimer pattern, it appears to be evident only under electrophoretic conditions that require high resolution gel. Should our patients not be type Vicenza VWD, we must presume that the 2 candidate Vicenza mutations are not involved in determining the Vicenza phenotype or, alternatively, that our patients also have other mutation(s) affecting their phenotype. However, this seems unlikely as our patients belonged to 4 unrelated families. Regardless of the usual and unusual nature of our type Vicenza VWD patients, we think that the novelty of our report is the demonstration of a reduced VWF survival in patients having the genotype/phenotype of type Vicenza VWD. Our results show that the acutely released type Vicenza VWF has a significantly reduced time of persistence in circulation compared to normal, thereby offering a plausible explanation for its reduced plasma VWF levels. Moreover, Castaman et al suggest that our survival results should have been compared with those of type 1 VWD, having similar baseline VWF levels, rather than a normal counterpart. Since there are many type 1 VWD with different VWF gene mutations, which type should we choose in order to evaluate whether the survival of Vicenza VWF is reduced or normal? Furthermore, when we investigate a laboratory result to decide whether it is normal or pathologic, we have to compare it with findings in a normal population not a pathologic one. Finally, the finding that Vicenza and Cys1130Phe VWF have a similar T1/2 elimination as documented by Castaman's results is not surprising. It is likely that other VWD variants will be identified in the future due to reduced VWF survival. Instead the question should be are Vicenza and Cys1130Phe VWF survivals similar to or different from the normal counterpart!
Castaman et al state that we concluded that VWF is normally secreted because platelet VWF is normal. This is not so; we only said that VWF is normally synthesized because platelet VWF is normal, as is the VWF release because of the normal VWF response to DDAVP administration. That type Vicenza VWF acutely released by DDAVP has a shortened survival remains an incontrovertible, and we suspect not an irrelevant finding, as suggested by Castaman et al. Alternatively, we should presume that the fates of constitutively and acutely secreted VWF are different. Whether or not the constitutive release of Vicenza VWF might also be defective as advanced by Schneppenheim will be clarified by our ongoing expression studies. In the mean time, we have no objection to classifying type Vicenza in the heterogeneous type 2M VWD group.