The CC chemokine receptor CCR5 is an important coreceptor for human immunodeficiency virus (HIV), and there is a major thrust to develop anti-CCR5–based therapies for HIV-1. However, it is not known whether CCR5 is critical for a normal antiviral T-cell response. This study investigated the immune response to lymphocytic choriomeningitis virus in mice lacking CCR5 (CCR5−/− mice). This infection is a classical model for studying antiviral immunity, and influx of CCR5-expressing CD8+ T cells and macrophages is essential for both virus control and associated immunopathology. Results showed that the virus-induced clonal expansion of antigen-specific T cells was augmented in CCR5−/− mice especially with regard to the CD4+ subset. Despite absence of CCR5, intracerebral infection invariably resulted in lethal T cell-mediated meningitis, and quantitative and qualitative analysis of the inflammatory exudate cells did not reveal any significant differences between gene-targeted mice and wild-type controls. CCR5 was also found to be redundant regarding the ability to eliminate virus from internal organs. Using delayed-type hypersensitivity to evaluate CD8+ T cell-mediated inflammation, no significant influence of CCR5 was found, not even when viral peptide was used as local trigger instead of live virus. Finally, long-term CD8+ T cell-mediated immune surveillance was efficiently sustained in CCR5−/− mice. Taken together, these results indicate that expression of CCR5 is not critical for T cell-mediated antiviral immunity, and this molecule may therefore constitute a logic and safe target for anti-HIV therapies.
Introduction
Chemokines are small inducible proteins that are involved in the normal trafficking of leukocytes to both lymphoid and nonlymphoid organs and in the recruitment of leukocytes to sites of injury and infection.1-3 Moreover, chemokines play an important role in immune regulation; thus, chemokines have been reported to mediate activation, costimulation, and differentiation of T cells and monocytes during innate and adaptive immune responses.1,4-7 The biologic effects of chemokines are mediated via their interaction with a large group of 7 transmembrane-spanning, G protein-coupled receptors.8,9The 2 major families of chemokine receptors are the CXC chemokine receptors and the CC chemokine receptors (CCR) so named for their binding of CXC and CC chemokines, respectively.8,10-12While CXC chemokine receptors traditionally have been associated with acute inflammatory responses, the CCRs are mostly expressed on cell types found in connection with chronic inflammation and T cell-mediated inflammatory reactions: eosinophils, basophils, monocytes, macrophages, dendritic cells, and T cells.8,13 14
T cells play an important role in antiviral immunity and, in particular, CD8+ effector T cells are important in promoting host recovery and virus clearance.15,16 The main effector function of virus-specific CD8+ T cells is contact-dependent lysis,17-20 but production of cytokines such as interferon-γ (IFN-γ) is also important.21 22Both of these effector mechanisms have a short action range, and cell-cell contact is required for virus-specific CD8+ T cells to fulfill their effector function. Moreover, because the replication rate of most viruses is very high, it is important that effector T cells are rapidly focused at sites of viral replication. Consequently, an optimal antiviral defense requires efficient mechanisms for targeting of activated T cells to sites of infection, and insight into the regulation of CD8+ T-cell trafficking is therefore essential when trying to understand how viral infections are controlled.
Influenza-specific CD8+ T cells polarized in vitro into either cytotoxic T cells type 1 (Tc1) or type 2 (Tc2) cells have been shown to express a differential chemokine receptor profile.23 Thus Tc1 cells were shown to express CCR2 and CCR5 messenger RNA (mRNA), whereas Tc2 cells primarily expressed CCR4. Importantly, this study revealed that although both subsets were recruited to the lungs of influenza-infected mice, Tc2 cells did so with delayed kinetics and did not localize near the infected airway epithelium. As a consequence, virus control was significantly delayed.23 Together, these findings strongly suggest that CCR2 and CCR5 expression is required for optimal CD8+effector T-cell function and point toward a role of CCR2 and CCR5 in the recruitment and positioning of virus-specific T cells. The actual importance of CCR2 and CCR5 in this process was, however, not addressed in that study.
Macrophage inflammatory protein-1α (MIP-1α) is a major ligand of CCR5, and an important role of MIP-1α in antiviral host defense has been revealed in studies using MIP-1α–deficient mice.24-26 However, MIP-1α may also bind to CCR1,11,14,27 and it is therefore not possible to dissect the role of individual CC receptors in the antiviral host defense using MIP-1α–deficient mice alone. Recently, the effect of CCR5 deficiency was studied in the murine model of influenza A virus-induced pneumonitis.28 The CCR5-deficient mice (CCR5−/−) displayed an increased mortality rate as a result of an overwhelming early accumulation of macrophages in the lungs.28 In contrast, no significant difference in viral titers was observed among surviving mice of either strain, which could indicate that recruitment of influenza-specific T cells to the infected lungs was unimpaired in the absence of CCR5 expression.28To our knowledge, however, no studies have directly addressed the role of CCR5 in both the afferent and efferent phases of virus-induced T cell-mediated inflammation.
To obtain a better understanding of how effector T cells are targeted to sites of viral infection, we have for several years been using the murine lymphocytic choriomeningitis virus (LCMV) model.29LCMV is a noncytopathogenic virus that induces little or no inflammation unless virus-specific T cells are present.30,31 The presence of specific effector T cells is associated with substantial inflammation in infected organs, and intracerebral infection leads to a fatal T cell-mediated meningitis 6 to 8 days after infection.32 The inflammatory exudate consists predominantly of CD8+ T cells and monocytes/macrophages, whereas virtually no CD4+ T cells are recruited to the inflammatory site.33 By use of this model, we have recently shown that in vivo activated LCMV-specific CD8+ T cells express CCR2 and CCR5 mRNA.34Furthermore, following intracerebral infection, up-regulation of cerebral CCR2 and CCR5 mRNA expression required the presence of T cells and directly correlated with influx of inflammatory cells into the central nervous system (CNS).34 Notably, concurrent analysis of virus-induced cerebral chemokine expression revealed that although maximal chemokine expression and meningeal inflammation coincided, chemokine expression was an early event that preceded inflammation of the CNS.34 Interestingly, MIP-1α, MIP-1β, and RANTES, which are all ligands of CCR5,35,36were among the early expressed chemokines.34 Besides T cells, monocytes/macrophages are also recruited to the inflammatory site following LCMV infection,33 and when recovered from an inflammatory exudate these cells were shown to express CCR1, CCR2, and CCR5.34 It therefore appeared likely that expression of CCR5 could play a significant role in the recruitment of both effector subsets to sites of LCMV infection. The present study was therefore undertaken to directly explore the role of CCR5 in the afferent and efferent phases of the LCMV-induced T cell-mediated immune response. This was done by comparing parameters of the T-cell response in LCMV-infected wild-type mice and in similarly infected CCR5−/− mice.
Materials and methods
Mice
The CCR5−/− mice (B6,129P-CmKbr5 < tm/Kn2 >) were bred locally at the Panum Institute from breeding pairs obtained from the Jackson Laboratory (Bar Harbor, ME). Wild-type (C57BL/6) mice were purchased from Bomholtgaard (Ry, Denmark). The latter mice were always allowed to acclimatize to the local environment for at least 1 week prior to use, and 7- to 10-week-old mice were used in all experiments. Animals were housed under controlled conditions (specific pathogen free) that included testing of sentinels for unwanted infections according to Federation of European Laboratory Animal Science Association (FELASA) standards; no such infections were revealed.
Virus
The LCMV of the Traub strain, produced and stored as previously described,37 was used throughout the study. Mice to be infected systemically with LCMV received an intravenous virus dose of 103 mean lethal dose (LD50) in a volume of 0.3 mL. This route of infection normally results in a transient, immunizing infection.38 Mice to be infected intracerebrally with LCMV Traub received the same virus dose in a volume of 0.03 mL. Intracerebral infection induces a fatal T cell-mediated meningitis from which animals succumb on day 6 to 8 after infection.32
Virus titration
Virus titrations were carried out by intracerebral inoculation of 10-fold dilutions of a 10% organ suspension into young adult Swiss mice. Titration endpoints were calculated by the Kärber method and expressed as mean lethal doses (LD50).
Survival study
Mortality was used to evaluate the clinical severity of acute LCMV-induced meningitis. Mice were checked twice daily until 100% mortality was reached.
Assay of LCMV-specific delayed-type hypersensitivity
Two different approaches were used to assess LCMV-specific delayed-type hypersensitivity (DTH). (1) Mice were infected locally in the right hind footpad with 103 LD50 LCMV Traub in a volume of 0.03 mL, and the local swelling reaction was followed between day 6 and 13 after infection.39 (2) Mice were infected intravenously with the same dose of virus and challenged in the right hind footpad with 0.03 mL of an immunodominant class I-restricted peptide (LCMV GP33-41, 50 μg/mL) on postinfection day 8 (acute phase) or postinfection day 60 (memory phase).40The swelling reaction was followed 16, 24, 48, and 72 hours after the peptide challenge. Footpad thickness was measured with a dial caliper (Mitutoyo 7309), and the virus-specific DTH reaction was determined as the difference in thickness of the infected/challenged right and the uninfected left hind footpad.38
Cell preparations
Mice were killed and their spleens removed. Single-cell suspensions were obtained by pressing the organs through a fine steel mesh. For flow cytometric analysis, erythrocytes were lysed by 0.83% NH4Cl treatment (Gey solution). Cerebrospinal fluid (CSF) was obtained from the fourth ventricle of mice that had been anesthetized with ether and exsanguinated. The total number of CSF cells (cells/μL) was determined by cell counting. The background level of cells in the CSF in uninfected mice is less than 100 cells/μL.31 39
Cytotoxicity assay
The LCMV-specific Tc activity was assayed in a51Cr-release assay37 using histocompatible EL-4 cells pulsed for 1 hour with either LCMV GP33-41 or LCMV NP396-404 peptide as targets. Unpulsed EL-4 cells served as control targets. Assay time was 6 hours, and percent specific release was calculated as described previously.37 39
In vivo bromodeoxyuridine labeling
Mice were given the thymidine analogue bromodeoxyuridine (BrdU; Sigma, St Louis, MO) in their drinking water at a concentration of 0.8 mg/mL for 3 days. BrdU-containing water was protected from light and changed daily.41
Monoclonal antibodies
The following monoclonal antibodies (mAbs) were purchased from Pharmingen (San Diego, CA) as rat antimouse antibody: fluorescein isothiocyanate (FITC)–conjugated anti-CD49d (common α4-chain of LPAM-1 and VLA-4) (R1-2), Cy-chrome (Cy)–conjugated anti-CD8a (53-6.7), Cy-conjugated anti-CD4 (RM4-5), phycoerythrin (PE)–conjugated anti-IFN-γ (XMG1.2). For BrdU staining, FITC-conjugated anti-BrdU (B-44; Becton Dickinson, San Jose, CA) was used.
Flow cytometric analysis
To detect intracellular IFN-γ,42 splenocytes were cultured at 37°C in 96-well round-bottom plates at a concentration of 2 × 106 cells/well in a volume of 200 μL RPMI medium supplemented with 10% fetal calf serum (FCS), 50 U/mL murine recombinant interleukin-2 (IL-2) (R & D Systems, Abingdon, United Kingdom), and 3 μM monensin (Sigma). The cells were cultured with LCMV GP33-41 peptide at a concentration of 0.1 μg/mL or without. After 5 hours of culture, cells were washed once in FACS medium (phosphate-buffered saline [PBS] containing 1% bovine serum albumin [BSA], 0.1% NaN3, and 3 μM monensin) and subsequently incubated with relevant surface antibodies in the dark for 20 minutes at 4°C. Cells were washed twice in PBS with 3 μM monensin and resuspended in 100 μL PBS with monensin, and then 100 μL 2% paraformaldehyde in PBS was added. After 30 minutes of incubation in the dark at 4°C, cells were washed in FACS medium and resuspended in PBS with 0.05% saponin. After 10 minutes of incubation in the dark at 20°C, cells were pelleted and resuspended in PBS with 0.05% saponin and anti-IFN-γ antibody. After incubation for 20 minutes at 4°C, cells were washed twice in PBS/saponin and analyzed by flow cytometry.
The combined intracellular IFN-γ and BrdU staining of splenocytes was performed by using the BrdU Flow Kit from Becton Dickinson. Briefly, the cells were restimulated with peptide and stained for the CD8 or CD4 surface markers as described above. After the surface staining the kit manual from Becton Dickinson was followed. All samples were analyzed using a Becton Dickinson FACS Calibur, and 1 to 5 × 104viable mononuclear cells were gated using a combination of forward angle and side scatter to exclude dead cells and debris. Data analysis was carried out using the Cell Quest program (Becton Dickinson) and results are presented as dot plots. Splenocytes from uninfected controls analyzed in parallel were used to set cut-off for levels of expression.
Preparation of total RNA
Mice were killed at various times after intracerebral infection, and the brain was immediately removed, snap-frozen in liquid nitrogen, and stored in a liquid nitrogen freezer until RNA preparations were to be performed. Total RNA was extracted from homogenized brain by use of the RNeasy midi kit (Qiagen, Hilden, Germany).
RNase protection assay experiments
Chemokine and chemokine receptor mRNA were detected using the RiboQuant multiprobe RNase protection assay (RPA) system (Pharmingen).34 The following template sets (both from Pharmingen) were used. To detect chemokine mRNA a custom-made template set that included IP-10 in addition to lymphotactin, RANTES, eotaxin, MIP-1β, MIP-1α, MIP-2, IFN-γ-inducible protein 10 (IP-10), monocyte chemotactic protein 1 (MCP-1), and T-cell activation gene 3 (TCA-3) mRNA was used. To detect CC chemokine receptor mRNA, the mCR5 template set was used. This template set enables the detection of CCR1, CCR1b, CCR2, CCR3, CCR4, and CCR5 mRNA. Both template sets included templates for the murine housekeeping genes L-32 (a ribosomal protein) and glyceraldehyde-3-phosphate dehydrogenase (GADPH) to serve as loading controls. The RPA was performed according to the manufacturer's instructions. Briefly, α-32P] UTP-labeled antisense RNA transcripts were generated from the template sets using T7 RNA polymerase. RNA from each sample was allowed to hybridize to the labeled probe for 16 to 20 hours at 56°C. Single-stranded RNA was digested with an RNase/T1 mixture, and the hybrids were analyzed on a denaturing urea-polyacrylamide gel. Protected fragments were visualized by autoradiography by placing dried gels on film (Biomax MS-1; Kodak, New Haven, CT) in cassettes with intensifying screens (Biomax MS; Kodak), which were then exposed at −80°C. For quantitative results, gels were subjected to Phosphorimager analysis (Fuji, Tokyo, Japan), and the data were subsequently analyzed using Image Gauge software (Fuji).
Results
Generation of LCMV-specific T cells
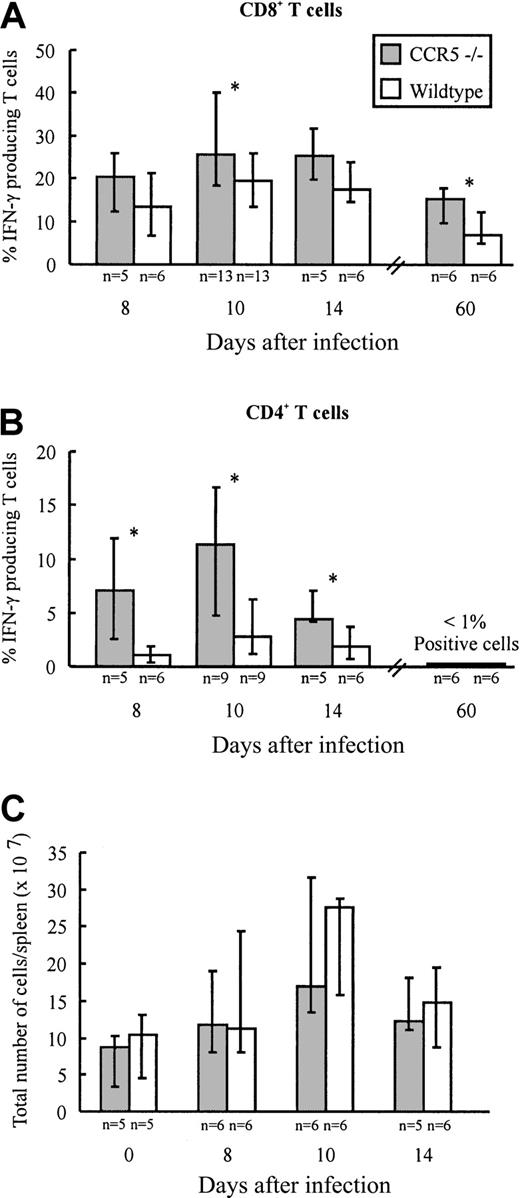
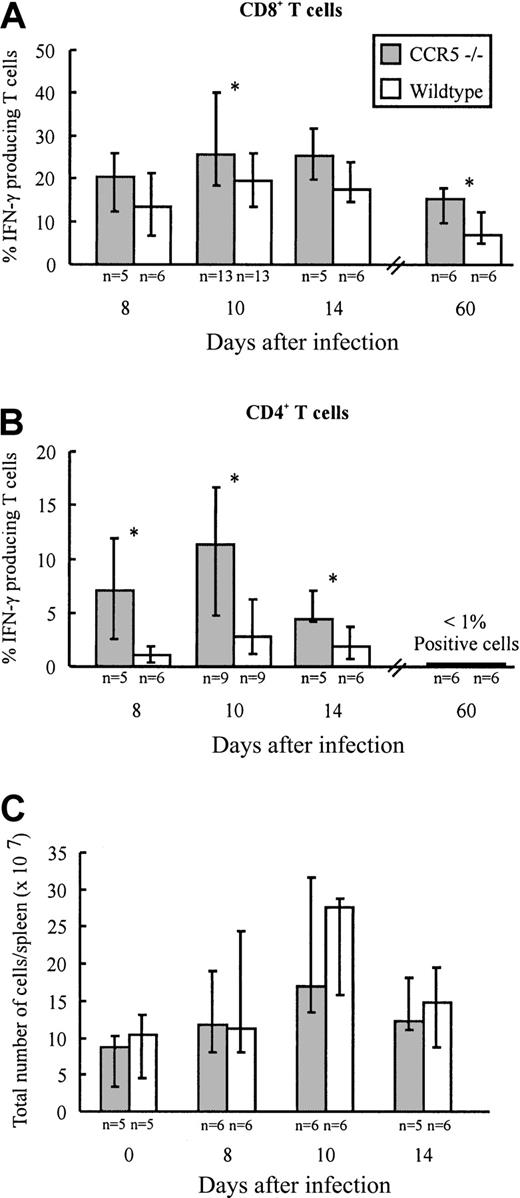
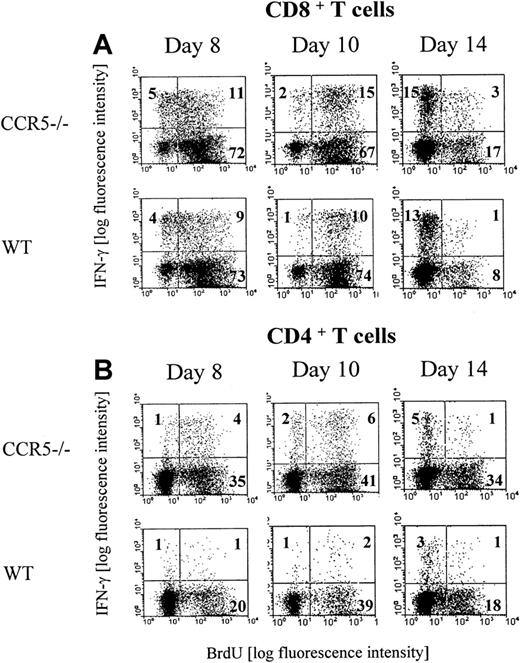
Infection with LCMV induces the generation of a large subset of CD8+ T cells and a smaller subset of CD4+ T cells with an activated phenotype.29 Most of these activated T cells represent virus-specific cells as disclosed through staining with specific peptide/major histocompatibility complex (MHC) class I tetramers (CD8+ T cells) or visualized by intracellular IFN-γ staining (CD8+ and CD4+ T cells).43,44 To investigate whether lack of CCR5 expression would influence the generation of LCMV-specific T cells, the frequency of virus-specific CD4+ and CD8+ T cells present in the spleen of CCR5−/− and wild-type mice was determined on days 8, 10, and 14 after infection (Figure1A,B). On the indicated days, splenocytes were briefly restimulated ex vivo with either an immunodominant class I-restricted LCMV peptide (GP33-41)45 or an immunodominant class II-restricted LCMV peptide (GP61-80).46 As shown in Figure 1A comparable frequencies of GP33-41–specific IFN-γ–producing CD8+ T cells were present in the spleen on days 8 and 14 after LCMV infection in CCR5−/− and wild-type mice, whereas a significantly higher frequency of GP33-41–specific CD8+ T cells was found in CCR5−/− mice on day 10 after infection. In contrast, the frequency of GP61-80–specific IFN-γ–producing CD4+ T cells was significantly higher in CCR5−/− mice on all of the 3 days selected for analysis (Figure 1B). The higher frequencies of virus-specific T cells observed in CCR5−/− mice were not due to differences in the total number of splenocytes, because the number of cells per spleen was of similar magnitude in the 2 strains when compared on day 0 and days 8, 10, and 14 after infection (Figure 1C).
Frequency of LCMV-specific T cells and total spleen cell numbers in CCR5−/− and wild-type (wt) mice after intravenous LCMV Traub infection.
CCR5−/− and wt mice were infected intravenously with 103 LD50 of LCMV Traub. (A,B) On postinfection days 8, 10, 14, and 60, splenocytes were harvested and the frequency of LCMV-specific VLA-4+ IFN-γ + CD8+T cells (A) or CD4+ T cells (B) were evaluated by flow cytometry. (C) On day 0 (uninfected) and postinfection days 8, 10, and 14, spleens were harvested and the total number of cells per spleen was determined. The results are shown as histograms; columns represent group medians and bars represent ranges. Group sizes are shown below each column. The asterisk denotes P < .05, Mann-Whitney rank test versus wild-type mice.
Frequency of LCMV-specific T cells and total spleen cell numbers in CCR5−/− and wild-type (wt) mice after intravenous LCMV Traub infection.
CCR5−/− and wt mice were infected intravenously with 103 LD50 of LCMV Traub. (A,B) On postinfection days 8, 10, 14, and 60, splenocytes were harvested and the frequency of LCMV-specific VLA-4+ IFN-γ + CD8+T cells (A) or CD4+ T cells (B) were evaluated by flow cytometry. (C) On day 0 (uninfected) and postinfection days 8, 10, and 14, spleens were harvested and the total number of cells per spleen was determined. The results are shown as histograms; columns represent group medians and bars represent ranges. Group sizes are shown below each column. The asterisk denotes P < .05, Mann-Whitney rank test versus wild-type mice.
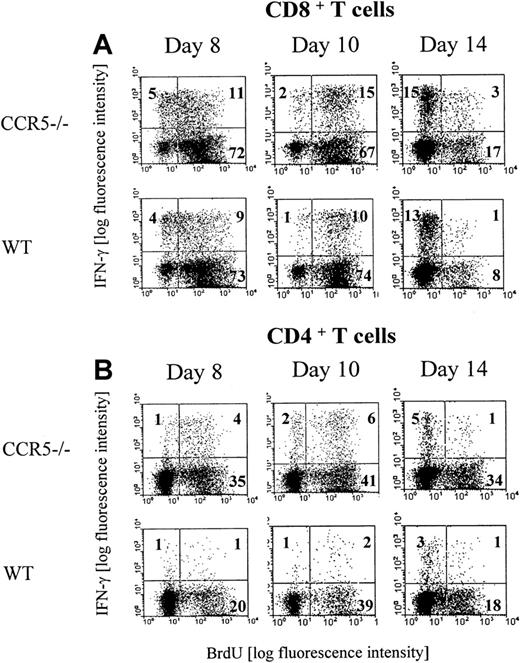
We next investigated the in vivo proliferation of GP33-41–specific CD8+ and GP61-80–specific CD4+ cells in LCMV-infected CCR5−/− and wild-type mice. This was done by combined intracellular IFN-γ and BrdU staining of splenocytes isolated on days 8, 10, and 14 after infection from mice that had received BrdU in their drinking water for the last 3 days before analysis (Figure 2A,B). Regarding the CD8+ subset, our analysis revealed that the majority of the LCMV GP33-41–specific CD8+ T cells had proliferated during the day 5 to 8 and 7 to 10 intervals, whereas virtually none had proliferated during the day 11 to 14 interval. However, in agreement with the data presented above, a higher frequency of LCMV GP33-41–specific CD8+ T cells had proliferated in the day 7 to 10 interval in CCR5−/− mice compared with matched wild-type mice (Figure 2A). Regarding the CD4+ T cells (Figure 2B), our analysis revealed that also the majority of the LCMV GP61-80–specific CD4+ T cells had proliferated during the day 5 to 8 and 7 to 10 interval, whereas virtually none had proliferated in the day 11 to 14 interval. However, compared with wild-type mice, accelerated and augmented CD4+ T-cell proliferation was noted in CCR5−/− mice, and a higher frequency of LCMV GP61-80–specific CD4+ T cells had been proliferating in CCR5−/− mice during the day 5 to 8 and 7 to 10 intervals. In this context, it may be of interest to note that lack of CCR5 has previously been reported to result in an enhanced CD4+ T-cell–mediated DTH reaction, and it has therefore been suggested that signaling through CCR5 might serve as part of negative regulatory feedback loop on T-cell activation.47
Proliferation of LCMV-specific T cells.
Groups of CCR5−/− and wild-type (wt) mice were infected with 103 LD50 of LCMV Traub and given BrdU in their drinking water for 3 days prior to postinfection days 8 (day 5-8), 10 (day 7-10), or 14 (day 11-14). Splenocytes were harvested and the cells were stained with anti-CD8 Cy or anti-CD4 Cy and anti-IFN-γ PE and anti-BrdU FITC. The mice were evaluated by flow cytometry for the presence of (A) CD8+ BrdU+IFN-γ+ T cells or (B) CD4+ BrdU+IFN-γ+ T cells. Representative dot plots from 1 of 2 experiments are presented. Three mice per group were included in each experiment.
Proliferation of LCMV-specific T cells.
Groups of CCR5−/− and wild-type (wt) mice were infected with 103 LD50 of LCMV Traub and given BrdU in their drinking water for 3 days prior to postinfection days 8 (day 5-8), 10 (day 7-10), or 14 (day 11-14). Splenocytes were harvested and the cells were stained with anti-CD8 Cy or anti-CD4 Cy and anti-IFN-γ PE and anti-BrdU FITC. The mice were evaluated by flow cytometry for the presence of (A) CD8+ BrdU+IFN-γ+ T cells or (B) CD4+ BrdU+IFN-γ+ T cells. Representative dot plots from 1 of 2 experiments are presented. Three mice per group were included in each experiment.
Tc effector function and virus clearance
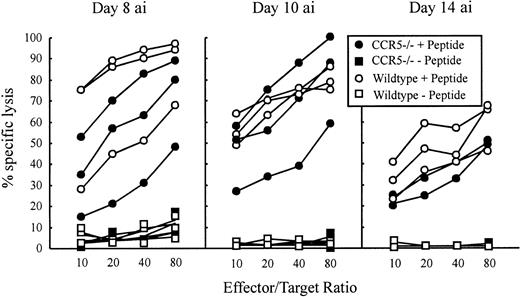
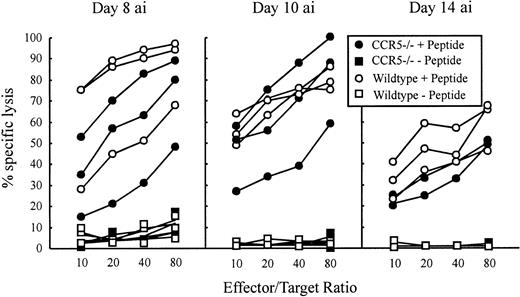
The LCMV-specific CD8+ T cells are required to control the LCMV infection, which they do primarily through perforin-mediated killing of virus-infected cells.17,18 22 To investigate whether absence of CCR5 would influence the Tc effector potential, a functional evaluation of Tc cell activity was performed in CCR5−/− and wild-type mice. LCMV-specific Tc responses were assayed ex vivo on postinfection days 8, 10, and 14 using EL-4 cells pulsed with the immunodominant class I-restricted LCMV peptides GP33-41 or NP396-404. Because we found that T cells from virus-infected CCR5−/− and wild-type mice were about equally cytolytic using both epitopes, only results for GP33-41 are shown (Figure3).
LCMV-specific Tc activity in CCR5−/− and wild-type (wt) C57BL/6 mice 8, 10, and 14 days after intravenous infection.
Mice were infected intravenously with 103 LD50of LCMV, and Tc activity was assayed in a 51Cr-release assay by use of GP33-41 peptide pulsed EL-4 cells as target cells. Unpulsed EL-4 cells served as control target cells. The Tc activity of 3 mice per group was assayed on the indicated days. “ai” denotes after infection.
LCMV-specific Tc activity in CCR5−/− and wild-type (wt) C57BL/6 mice 8, 10, and 14 days after intravenous infection.
Mice were infected intravenously with 103 LD50of LCMV, and Tc activity was assayed in a 51Cr-release assay by use of GP33-41 peptide pulsed EL-4 cells as target cells. Unpulsed EL-4 cells served as control target cells. The Tc activity of 3 mice per group was assayed on the indicated days. “ai” denotes after infection.
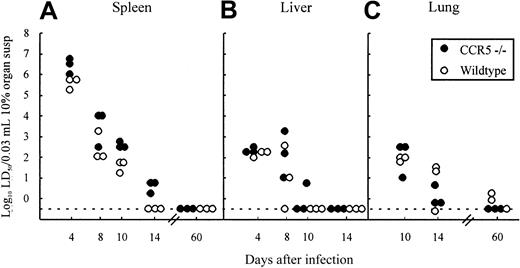
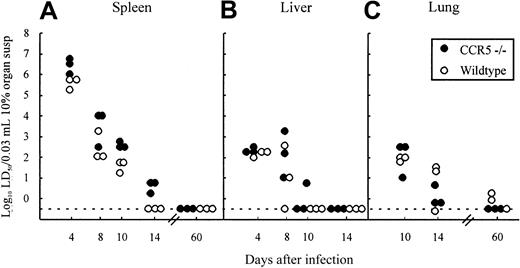
The most relevant measure of the capacity of CD8+ effector T cells to function in vivo is virus clearance in infected animals.17,38 48 To evaluate the possible role of CCR5 in Tc cell-mediated clearance of LCMV, the kinetics of virus clearance were studied in CCR5−/− mice and compared with wild-type controls. Mice were infected intravenously with 103LD50 LCMV Traub and groups of 3 mice were killed at different times after infection (4, 8,10, and 14 days), and their spleens, livers, and lungs were removed for determination of virus content (Figure 4A-C). As shown in Figure4, the kinetics of virus clearance was similar in the 2 strains independent of the organ studied; thus lack of CCR5 does not influence Tc effector function in vivo.
Organ virus titers in CCR5−/− and wild-type (wt) mice infected intravenously with 103LD50 LCMV Traub.
Organs (spleen, liver, and lung) were harvested on the indicated days relative to virus inoculation and virus titers were determined. Points represent individual mice. Susp indicates suspension.
Organ virus titers in CCR5−/− and wild-type (wt) mice infected intravenously with 103LD50 LCMV Traub.
Organs (spleen, liver, and lung) were harvested on the indicated days relative to virus inoculation and virus titers were determined. Points represent individual mice. Susp indicates suspension.
Role of CCR5 in the pathogenesis of LCMV-induced meningitis
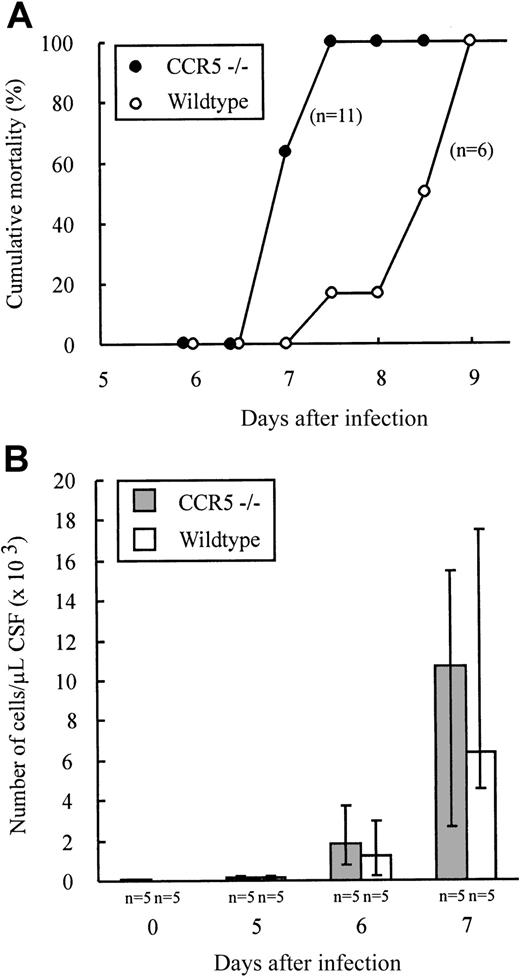
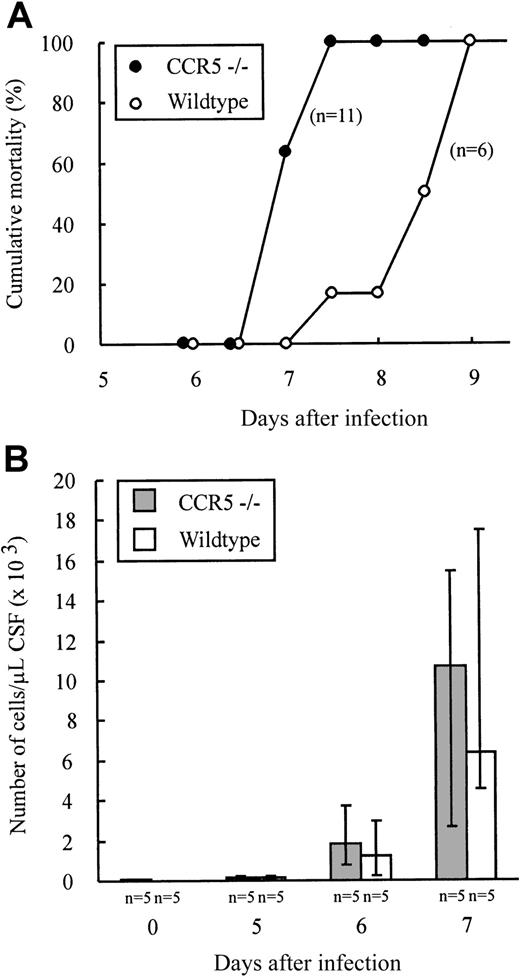
The above results provide indirect evidence that effector T-cell homing to infected organs such as lungs and liver as well as intrasplenic migration do not require CCR5 expression. However, to study the possible role of CCR5 in T-cell homing to a solid organ, we investigated whether lack of CCR5 expression would influence the recruitment of lymphocytes to the LCMV-infected brain. CCR5−/− and wild-type mice were infected intracerebrally with 103 LD50 LCMV Traub and clinical susceptibility to meningitis, measured as mortality, was determined (Figure 5A). As shown in Figure 5A, infected mice of both strains died within 9 days of infection. There was, however, a tendency toward a slightly accelerated disease pattern in CCR5−/− mice. Therefore, although fatal disease was induced in the absence of CCR5, the recruitment of effector cells to the inflammatory site might be quantitatively or qualitatively different in CCR5−/− mice. Consequently, we also performed quantitatively and qualitatively analyses of the cellular exudate in the CSF. As shown in Figure 5B, no significant differences in the number of mononuclear cells contained in the CSF were revealed on either day 5, 6, or 7 after infection. Similarly, the composition of the inflammatory exudate with regard to CD8+ T cells and Mac-1+ monocytes/macrophages was found to be similar in CCR5−/− and wild-type mice (data not shown). Altogether, the above findings indicate that expression of CCR5 on CD8+T cells and monocytes/macrophages is not essential for the recruitment of these cells to the inflamed meninges.
Outcome of intracerebral LCMV infection and kinetics of leukocyte recruitment to the LCMV infected meninges.
CCR5−/− and wild-type (wt) mice were infected intracerebrally with 103 LD50 LCMV Traub and mortality was registered (A) or on the indicated days CSF was harvested and the exudate cells present in the CSF counted (B). The number of mice per group is indicated in the figure.
Outcome of intracerebral LCMV infection and kinetics of leukocyte recruitment to the LCMV infected meninges.
CCR5−/− and wild-type (wt) mice were infected intracerebrally with 103 LD50 LCMV Traub and mortality was registered (A) or on the indicated days CSF was harvested and the exudate cells present in the CSF counted (B). The number of mice per group is indicated in the figure.
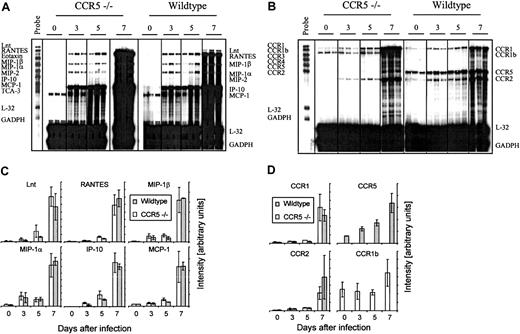
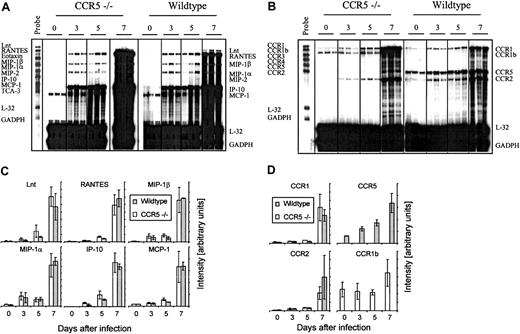
However, it might be argued that absence of CCR5 would result in an altered cerebral chemokine or chemokine receptor profile, which would allow Tc1 cells and monocytes/macrophages to be recruited to the infected meninges despite the lack of CCR5 expression. To test this, we studied cerebral chemokine and CCR gene expression in CCR5−/− and wild-type mice infected intracerebrally with LCMV Traub 3, 5, and 7 days earlier. Control mice of either strain, which had been sham injected with the same volume of PBS intracerebrally, were included to show the basal level of mRNA expression (Figure 6A-D). As shown in Figure 6, panels A and C, neither the composition nor the kinetics of chemokine gene expression was different in CCR5−/− mice. Thus, lack of CCR5 expression did not result in any apparent change in activation of chemokine genes. CCR5−/− mice did, however, express a different CCR profile, because these mice expressed CCR1b, which is an orphan receptor structurally related to CCR149(Figure 6B). This receptor was not expressed in wild-type mice. The function of CCR1b is not well described in the literature, but it is assumed that it, like CCR1, binds the CC chemokines MIP-1α and RANTES.49 However, because the expression of CCR1b only increased moderately compared to CCR1 and CCR2 (Figure 6D), and thus does not correlate with the influx of inflammatory cells, it seems unlikely that expression of CCR1b compensates for the lack of CCR5 expression in CCR5−/− mice.
Comparison of the cerebral chemokine and chemokine receptor mRNA expression in CCR5−/− and wild-type (wt) mice after intracerebral LCMV infection.
Mice were infected intracerebrally with 103LD50 LCMV Traub or sham injected intracerebrally with PBS. On the indicated days after infection total RNA was isolated from the brain, and 20 μg total RNA was subjected to RNA protection assay analysis. (A) Cerebral chemokine mRNA expression and (B) cerebral CCR mRNA expression. Quantitative analysis of chemokine (C) and chemokine receptor (D) gene expression based on a similar experiment; columns represent average ± SD of 3 mice.
Comparison of the cerebral chemokine and chemokine receptor mRNA expression in CCR5−/− and wild-type (wt) mice after intracerebral LCMV infection.
Mice were infected intracerebrally with 103LD50 LCMV Traub or sham injected intracerebrally with PBS. On the indicated days after infection total RNA was isolated from the brain, and 20 μg total RNA was subjected to RNA protection assay analysis. (A) Cerebral chemokine mRNA expression and (B) cerebral CCR mRNA expression. Quantitative analysis of chemokine (C) and chemokine receptor (D) gene expression based on a similar experiment; columns represent average ± SD of 3 mice.
Virus-induced DTH in the absence of CCR5
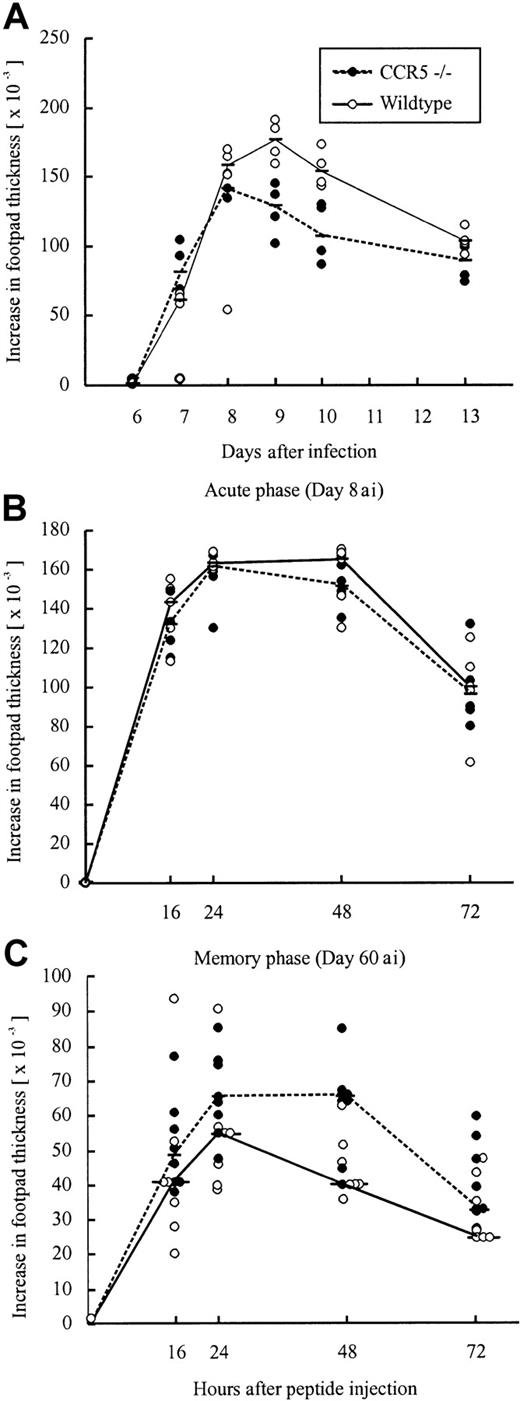
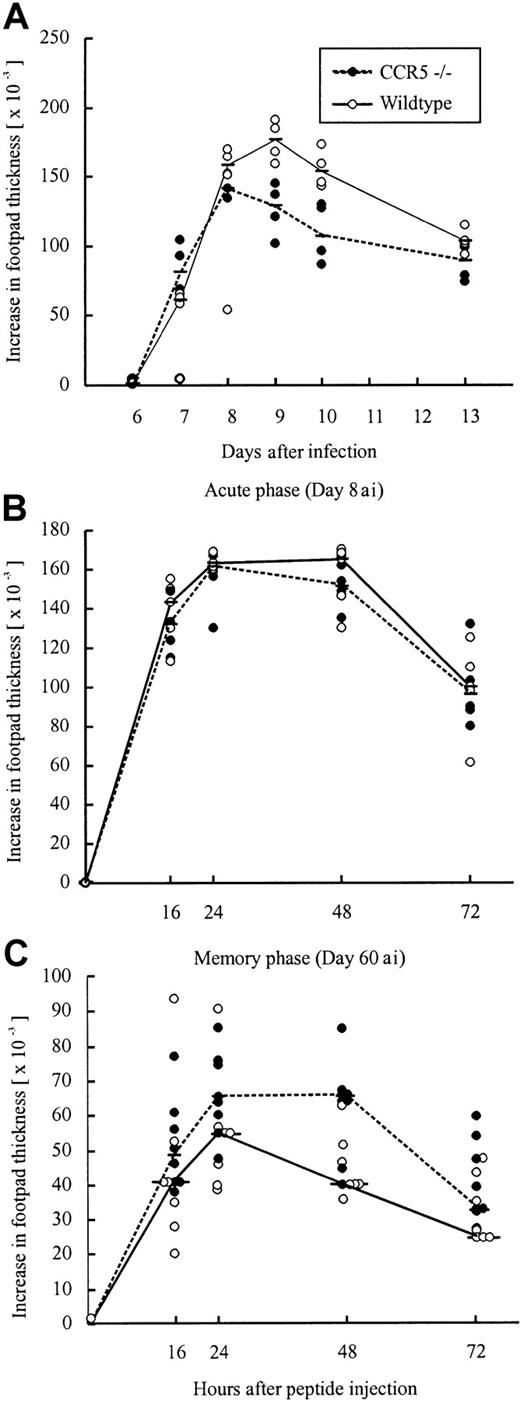
Another classical model for studying CD8+ effector T-cell migration to solid tissue is the primary LCMV-induced footpad swelling reaction that represents the response to subdermal virus challenge.39 50 To study the role of CCR5 in this DTH-like reaction, CCR5−/− mice and wild-type mice were infected with 103 LD50 LCMV Traub in the right hind footpad, and the footpad swelling was measured between postinfection days 6 and 13. No significant difference in the response pattern of CCR5−/− mice and wild-type mice was observed (Figure7A), indicating that also a subdermal inflammatory response can proceed in the absence of CCR5 expression.
Time course of primary or peptide-induced footpad swelling in CCR5−/− and wild-type (wt) mice after LCMV infection.
(A) A primary footpad swelling was elicited by infecting mice with 103 LD50 LCMV Traub in the right hind footpad; the footpad swelling was measured from postinfection days 6 to 13. (B,C) Mice were infected intravenously with 103LD50 LCMV Traub and challenged in the right hind footpad with LCMV GP33-41 (50 μg/mL, 30 μL) on day 8 after infection to examine the acute phase (B) or on postinfection day 60 to examine the memory phase (C). Points represent individual mice. “ai” denotes after infection.
Time course of primary or peptide-induced footpad swelling in CCR5−/− and wild-type (wt) mice after LCMV infection.
(A) A primary footpad swelling was elicited by infecting mice with 103 LD50 LCMV Traub in the right hind footpad; the footpad swelling was measured from postinfection days 6 to 13. (B,C) Mice were infected intravenously with 103LD50 LCMV Traub and challenged in the right hind footpad with LCMV GP33-41 (50 μg/mL, 30 μL) on day 8 after infection to examine the acute phase (B) or on postinfection day 60 to examine the memory phase (C). Points represent individual mice. “ai” denotes after infection.
To test whether the redundancy of CCR5 was related to the use of live virus as a local trigger of inflammation, we also measured the ability of (systemically) infected mice to respond to local challenge (on postinfection day 8) with an immunodominant viral MHC class I-restricted peptide (LCMV GP33-41); previous results have established that this is a valid way of assessing CD8+ T cell-dependent inflammation.40 However, even under these conditions no significant difference was observed between CCR5−/− and wild-type mice (Figure 7B). Thus, a substantial inflammatory response was induced in the absence of CCR5 expression, which appears to be redundant for a Tc1-mediated inflammatory response.
The role of CCR5 in the memory phase of LCMV infection
Although no overt functional impact of CCR5 deficiency was observed in the acute phase of LCMV infection, it could be argued that the redundancy of CCR5 might result from the high number of recently activated effector cells present in mice undergoing acute infection, especially because the number of effector cells was even higher in CCR5−/− mice. We therefore wanted to examine the role of CCR5 in the memory phase of LCMV infection, which we analyzed in CCR5−/− mice and wild-type mice, that had been infected 2 months earlier with 103 LD50 LCMV Traub.
First, we investigated whether lack of CCR5 would result in altered frequencies of IFN-γ–producing LCMV-specific CD8+ and CD4+ T cells in the memory phase. On day 60 after infection, splenocytes were restimulated ex vivo for a short period with either an immunodominant class I-restricted LCMV peptide (GP33-41) or an immunodominant class II-restricted LCMV peptide (GP61-80; Figure1A,B). As shown in Figure 1, a significantly higher frequency of LCMV GP33-41–specific CD8+ T cells was observed in CCR5−/− mice on postinfection day 60 (Figure 1A). With regard to the CD4+ T-cell subset, frequencies in both strains were at or below the level of detection (Figure 1B). The higher frequency of LCMV GP33-41–specific CD8+ T cells in CCR5−/− mice could result from a higher cellular turnover within this subset. This picture is often seen in persistent viral infections, including the LCMV infection, if the balance between the host response and the virus is in favor of the virus and, even a slight reduction in the effectiveness/function of LCMV-specific effector T cells has been reported to change this balance.51-53However, cell cycle analysis of the LCMV GP33-41–specific CD8+ T-cell subset did not reveal any difference between CCR5−/− mice and wild-type mice (data not shown).
In agreement with this finding, determinations of the virus content in spleens and lungs of CCR5−/− mice and wild-type mice infected 2 months earlier with LCMV, did not reveal any signs of an impaired long-term virus control in CCR5−/−, because both strains controlled the infection effectively (Figure 4A,C).
The higher frequency of LCMV GP33-41–specific CD8+ T cells found on postinfection day 60 in CCR5−/− mice could lead to an improved memory response to rechallenge with antigen. To examine this, we measured the footpad swelling reaction (DTH) of systemically LCMV-primed mice to local challenge (on day 60 after infection) with an immunodominant viral MHC class I-restricted peptide (LCMV GP33-41; Figure 7C). However, as shown in Figure 7C, no difference in the inflammatory response was observed between CCR5−/− mice and wild-type mice.
Discussion
CCR5 is a major coreceptor for human immunodeficiency virus (HIV),54-56 and it is known that patients homozygous for a 32bp deletion allele of the CCR5 gene are quite resistant to this infection.57,58 In contrast, relatively little is known about the normal biologic function of this receptor. There are no reports revealing an increased susceptibility to infection in humans with the CCR5 deletion mutation. However, this does not necessarily mean that CCR5 is redundant. Thus, mice deficient in expression of CCR5 are substantially more susceptible to infection with Cryptococcus neoformans59 and Toxoplasma gondii,60 exhibit defects in clearance ofListeria monocytogenes,47 and are impaired in IFN-γ production following infection with Leishmania donovani.61 Whether CCR5 plays a role in antiviral immunity and CD8+ effector T-cell migration has not, however, been investigated before, and understanding the role of CCR5 in antiviral immunity is particularly important because CCR5 is currently in focus as a potential target for anti-HIV-1 therapeutic intervention.62
With regard to the afferent phase of the antiviral immune response, our results using CCR5−/− mice point toward an immune regulatory role of CCR5. Thus, a somewhat higher frequency of LCMV-specific CD8+ (postinfection day 10) and CD4+ (postinfection days 8, 10, and 14) T cells was found in LCMV-infected CCR5−/− mice compared with the frequencies found in similarly infected wild-type mice. Interestingly, cell cycle analysis revealed a higher proliferative response within the LCMV-specific CD8+ T-cell subset between days 7 and 10 after infection and within the LCMV-specific CD4+ T-cell subset between postinfection days 5 to 8 and 7 to10. Thus, it appears that signaling through CCR5 might serve a function as part of a negative regulatory cycle on T-cell proliferation. Similar effects of CCR5 deficiency have previously been reported for CD4+ T cells,47 but to our knowledge such an effect has not previously been found to apply to CD8+ T cells.
To study the effect of CCR5 deficiency on the ability of Tc1 effector cells to extravasate at sites of viral infection, we used LCMV-induced T cell-mediated meningitis as our primary experimental model. Additionally, localized subdermal inflammation (footpad swelling), as well as virus clearance in internal organs, was analyzed. All of these reaction types are known to be critically dependent on the recruitment of Tc1 cells,17,32,39,48 and it has previously been found that ligands of CCR5 are prominent among the chemokines produced in virus-infected tissues.28,34 63
Given that CCR5 is the only receptor for MIP-1α, MIP-1β, and RANTES normally expressed on Tc1 effector cells,23,34 it was somewhat surprising to find that fatal meningitis could be induced equally efficiently in the absence of CCR5. Indeed, if anything, more rapid disease progression was seen in LCMV-infected CCR5−/− mice. Moreover, both quantitative and qualitative analyses of the inflammatory cells present in the CSF of intracerebrally infected animals failed to reveal significant differences between CCR5−/− and wild-type mice. In a recent study on influenza A-induced pneumonitis, CCR5 deficiency was found to result in an overwhelming accumulation of macrophages in the lungs, which correlated with an enhanced early expression of RANTES and MCP-1 mRNA in the lungs.28 In contrast to these findings, our analyses on the cerebral chemokine mRNA expression revealed that both the profile and the kinetics of expression were identical between CCR5−/− mice and wild-type mice. However, besides the expected lack of CCR5 mRNA expression in CCR5−/− mice, our analysis of the cerebral CCR mRNA expression revealed induction of CCR1b mRNA expression in CCR5−/− mice. The function of this receptor is not well described, but it is believed that it is able to bind the CC chemokines MIP-1α and RANTES,49 and it may therefore be speculated that expression of CCR1b serves to compensate for the lack of CCR5 expression in CCR5−/−mice. However, because the expression of this receptor does not increase proportionately to expression of CCR1 and CCR2, it seems unlikely that it should be expressed on the immigrating inflammatory cells. Consistent with this, analysis of mRNA from purified splenic CD8+ T cells from virus-infected mice did not reveal expression of CCR1b on these cells (unpublished observation, 2001). Therefore, it is unlikely that CCR1b is essential for the unimpaired inflammatory response in CCR5−/− mice. Whatever the underlying mechanism, our results strongly indicate that expression of CCR5 is redundant in the recruitment of antiviral effector cells to the brain.
Expression of CCR5 was also found to be redundant regarding the ability of effector cells to eliminate virus in internal organs, including nonlymphoid tissues. Virus clearance in infected organs is a direct measure of the capacity of Tc1 cells to function in situ.17,38 48 Therefore, the fact that we did not see any delay in this parameter in CCR5−/− mice further supports the assumption that Tc1 cells can be recruited to sites of infection and are able to position themselves appropriately in the absence of this receptor.
Also in the case of subdermal inflammation did we find that CCR5 expression was redundant, and similar results were obtained in LCMV-immune mice, demonstrating that the redundancy of CCR5 could not be explained simply by the high numbers of effector cells nor by their activation state (effector Vs memory cells).64
Regarding the memory phase, we found that the frequency of virus-specific CD8+ T cells in LCMV-immune mice (postinfection day 60) was somewhat higher in CCR5−/−mice. This could indicate that CCR5 expression is important for the long-term control of LCMV infection. A typical indication of impaired long-term virus control is an increased turnover of virus-specific T cells due to an ongoing T-cell response.51 52 However, analysis of T-cell turnover in CCR5−/− mice did not reveal an increase in the frequency of cycling LCMV-specific CD8+ T cells. Nor did we see any impairment of long-term virus control in LCMV-immune CCR5−/− mice. The higher frequency of virus-specific CD8+ T cells observed in LCMV-immune CCR5−/− mice could potentially lead to an accelerated footpad swelling reaction in LCMV-immune CCR5−/− mice. However, no differences in neither the kinetic nor the magnitude of the footpad response was observed between CCR5−/− mice and wild-type mice. Together these results demonstrate a redundant role of CCR5 in the memory phase of LCMV infection.
Our study does, however, point toward a role for CCR5 as a down-modulator of the clonal expansion of CD8+ and CD4+ T cells during the acute phase of LCMV infection, which for CD8+ T cells at least might be maintained into the memory phase. Absence of signaling through CCR5 has previously been reported to result in an enhanced CD4+ T cell-mediated DTH reaction and in improved production of IFN-γ by T cells.47 It has therefore been speculated that CCR5 deficiency in humans might in fact improve their immune response toward T cell-dependent infections. Although this might be true for CD4+ T cell-mediated immune responses, our results strongly suggest that this does not apply to Tc1-mediated virus control.
In conclusion, this study fails to reveal a significant role for CCR5 in virus-induced Tc1-mediated inflammatory reactions. Whether this finding may be extrapolated to other types of Tc1-mediated reactions is not known for certain. However, the observation that CCR5 is also redundant for a DTH response following local peptide challenge suggests that redundancy could in fact be a general phenomenon. Although data from knockout mice should always be interpreted with care, this finding is encouraging if looked on in the light of the possibility of developing CCR5-based therapies for treatment of HIV infection. Thus, if anything, the antiviral T-cell response tends to be augmented in CCR5−/− mice. Generally this would constitute no problem and might even be beneficial, although in rare situations augmented immunopathology may be observed. On the downside, our results also suggest that it may be futile to target CCR5 in an attempt to inhibit other Tc1-mediated reactions, for example, allograft rejection or graft-versus-host reaction.
The authors would like to thank Grethe Thørner Andersen and Lone Malte for their skillful technical assistance.
Supported by the Danish Medical Research Council, the Beckett Foundation, and the Novo Nordisk Foundation.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Allan Randrup Thomsen, Institute of Medical Microbiology and Immunology, Panum Institute, 3C Blegdamsvej, DK-2200 N, Copenhagen, Denmark; e-mail: a.r.thomsen@immi.ku.dk.