Two cross-reacting material–positive (CRM+) factor VII (FVII) mutations, associated with similar reductions in coagulant activity (2.5%) but with mild to asymptomatic (Gly331Ser, c184 [in chymotrypsin numbering]) or severe (Gly283Ser, c140) hemorrhagic phenotypes, were investigated. The affected glycines belong to structurally conserved regions in the c184 through c193 and c140s activation domain loops, respectively. The natural mutants 331Ser-FVII and 283Ser-FVII were expressed, and in addition 331Ala-FVII and 283Ala-FVII were expressed because 3 functional serine-proteases bear alanine at these positions. The 331Ser-FVII, present in several asymptomatic subjects, showed detectable factor Xa generation activity in patient plasma (0.7% ± 0.2%) and in reconstituted system with the recombinant molecules (2.7% ± 1.1%). The reduced activity of recombinant 283Ala-FVII (7.2% ± 2.2%) indicates that the full function of FVII requires glycine at this position, and the undetectable activity of 283Ser-FVII suggests that the oxydrile group of Ser283 participates in causing severe CRM+ deficiency. Furthermore, in a plasma system with limiting thromboplastin concentration, 283Ser-FVII inhibited wild-type FVIIa activity in a dose-dependent manner.
Introduction
Factor VII (FVII) deficiency is associated with a wide spectrum of coagulation and hemorrhagic phenotypes.1,2 Although a large number of FVII mutations have been described,3 the relationship of molecular and clotting defects to the clinical picture has been poorly defined,3-6 particularly for cross-reacting material–positive (CRM+) deficiencies.7-10
Functional studies were conducted in plasma and with recombinant variants to provide molecular elements useful to define this relationship in 2 homozygous CRM+ FVII deficiencies associated with markedly different hemorrhagic phenotypes.
Study design
Patients
Patients with normal FVII antigen and low FVII coagulant activity (FVIIc) (2.5%), not previously characterized, were studied.
Patient FVII33 (male, age 83, FVIIc 2%); patient FVII34 (male, age 26, FVIIc 0.7%); and patient FVII35 (female, age 30, FVIIc 0.7%) were clinically asymptomatic, while patient FVII36 (male, age 39, FVIIc 0.6%) experienced epistaxis and gum hemorrhage. Patient FVII37 (female, age 71, FVIIc 1.1%) was referred for anemia following chronic bleeding (rectorrhagia, hemorrhoids).
Patient FVII38 (female, age 30, FVIIc 2.5%) experienced recurring hemorrhages (menometrorrhagia, melena) and had hemarthroses. Two episodes of hemoperitoneum occurred following rupture of ovarian cysts. Fresh plasma, FVII concentrates, or FVII (Provertin-UM, Immuno, Pisa, Italy) were administered on several occasions.
Genetic analysis
Mutation search and detection of FVII polymorphisms were performed as previously described.11,12 Mutagenesis and transfection expression vectors were created by site-directed mutagenesis of plasmid pCDNA3-FVII.13Oligonucleotides for mutagenesis spanned the following complementary DNA nucleotides: (1) 1193 through 1220 containing the 1206G > A transition (Gly331Ser) or the 1207G > C transversion (Gly331Ala); (2) 1040 through 1073 containing the 1062G > A transition (Gly283Ser) or the 1063 G > C transversion (Gly283Ala).
Factor Xa generation assay
Plasma.
The reaction was initiated by adding an excess (30 μL) of thromboplastin (Thromborel S) (Behring, Marburg, Germany) to 50 μL diluted plasma (1:20, 1:40, 1:80, and 1:140 in 20 mM HEPES, 150 mM NaCl, 0.1% PEG 8000, 5 mM CaCl2, pH 7.4.
Recombinant FVII.
FVII in 2 nM conditioned medium was incubated 5 minutes at 37°C with 1 nM human factor Xa (hFXa) (Sigma, St Louis, MO); 300 μM 75% phosphatidylcholine/25% phosphatidylserine14; and 5 mM CaCl2 in 50 μL final volume. After addition of 30 μL thromboplastin, FXa generation was started with 40 nM hFX (Sigma).
Quenching reactions.
Reactions were quenched with 30 μL 20 mM HEPES, 150 mM NaCl, 0.1% PECT 8000, 50 mM EDTA, pH 7.4 after 5 minutes' incubation at ambient temperature. FXa fluorogenic substrate (MeSO2-D-CHA-Gly-Arg-AMCAcOH) (American Diagnostica, Greenwich, CT) was added (200 μM), and fluorescence (360 nm excitation, 465 nm emission) was measured on SpectraFluorPlus microplate reader (TECAN, Salzburg, Austria).
Standardizing assays.
Assays were standardized with dilutions of pooled normal plasma (PNP) or of recombinant wild-type (rWt) FVII. Molecular modeling of FVII was performed by means of Swiss Model (GLAXO, Geneva, Switzerland) through the ProMod program,15 and CHARMs software. The crystal coordinates of the tissue factor–FVIIa complex (1DAN), FVIIa (1QFK, 1CVW, 1DVA) and FVII (1JBU),16-20 were from the protein data bank.
Results and discussion
FVII gene sequencing indicated that 5 patients (patient FVII33 through FVII37), all from southern Italy, were homozygous for the 10908G > A transition, resulting in the Gly331Ser substitution. In contrast, patient FVII38 was homozygous for the 10764G > A transition, resulting in the Gly283Ser substitution. In spite of a similar reduction in FVIIc levels (Table1), the Gly331Ser homozygotes showed asymptomatic to mild phenotypes, whereas the Gly283Ser homozygote was characterized by severe bleeding tendency.
Several genetic and methodological factors could contribute to loosening the relationship among causative mutations, FVII activity, and clinical severity of these CRM+ FVII deficiencies. Among the genetic factors, we have investigated the influence of FVII polymorphisms, previously found to be associated with FVII levels in plasma.12 All patients were homozygous for a common genotype characterized by the most frequent allelic forms A1, b, and M1,12 thus excluding their differential contribution to FVII levels.
Since the low sensitivity of the routine laboratory assays hampers the comparative evaluation of FVIIc values,5 21 the residual FVII activity in patient plasma was further evaluated by measuring the FXa generation with the use of an FXa fluorogenic substrate (Table1). Activity in plasma from a Gly331Ser homozygote (0.7% ± 0.2%) was significantly higher (P < .001, analysis of variance) than in plasma from the Gly283Ser homozygote, which, investigated at different dilutions, was indistinguishable (P = .97) from FVII-depleted plasma. This indicates very low, if any, activity of the stable Gly283Ser-FVII. FXa generation curves from 2 Gly331Ser homozygotes overlapped (not shown).
To validate the causative role of mutations, the recombinant FVII molecules (r331Ser-FVII, r283Ser-FVII) were transiently expressed in BHK cells. Their antigen levels (Table 1) did not differ significantly (P = .93) from those of rWt-FVII. Functional assays in a reconstituted system showed a clearly detectable FXa generation activity (Table1) for the r331Ser-FVII. In contrast, the activity of the r283Ser-FVII was indistinguishable from that of the mock medium, and of medium containing the active site (rSer344Ala-FVII) or the cleavage site (rArg152Gln-FVII) mutants,9 22 used as 2 inactive reference CRM+ FVII variants.
Findings in plasma and reconstituted system help to interpret the markedly different hemorrhagic phenotypes associated to the mutations. The 331Ser-FVII residual activity would trigger coagulation, thus preventing severe bleeding symptoms in the several Gly331Ser homozygotes. On the other hand, the undetectable activity of the 283Ser-FVII is in accordance with the severe phenotype observed in PFVII38.
Additional insights into the causative role of these mutations were sought by inspection of FVII crystallographic structures,16-20 molecular modeling,15 and comparative analysis,23 coupled with further mutagenesis, expression, and functional studies.
Gly331 (c184 in chymotrypsin numbering) belongs to the activation loop c184 through c193, makes a hydrogen bond with Pro303 of the shifting β-strand-B220 (c153 through c162) and is in close contact with Asp338 at the bottom of the specificity pocket of FVIIa.
Gly283 (c140) is at the hinge of the c140s loop19 and, both in the zymogen and activated form, is in close contact with Asp343, which forms the salt bridge with Ile153.
In FVII models, the Gly283Ser and Gly331Ser substitutions are tolerated without major structural changes, which could explain the presence of normal amounts of circulating proteins resulting in CRM+deficiencies.
Gly283 and Gly331 belong to structurally conserved regions in serine-proteases23 (Table 2). Among functional members, only urokinase and complement factors B and 2 present alanine at the topologically equivalent positions (Table 2, italics), which led us to investigate the r331Ala-FVII and r283Ala-FVII (Table 1). Although clearly lower than that of rWt-FVII, FXa generation by r331Ala-FVII and r283Ala-FVII was remarkably higher than that of the corresponding natural variants. These findings suggest that the full activity of FVII is not compatible with the presence of side chains at positions 331 and 283. The 331Ala-FVII, lacking the serine oxydrile group, showed a 7-fold higher activity than the 331Ser-FVII. The appreciable activity of 283Ala-FVII, compared with the impaired function of the 283Ser-FVII, suggests that the oxydrile group of Ser283, potentially affecting proper salt bridge formation and c140s loop conformational changes, participates in producing a clinically severe form of CRM+FVII deficiency.
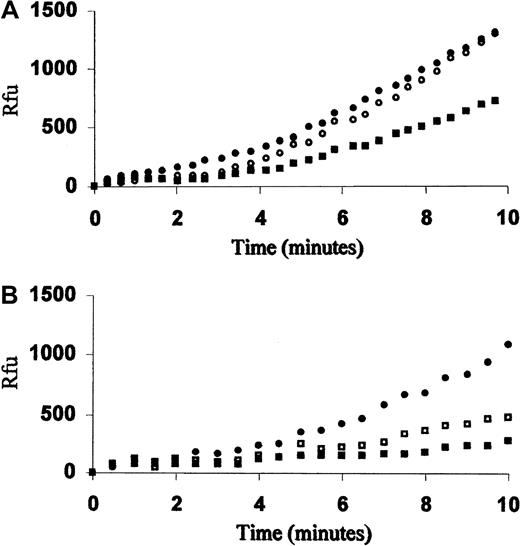
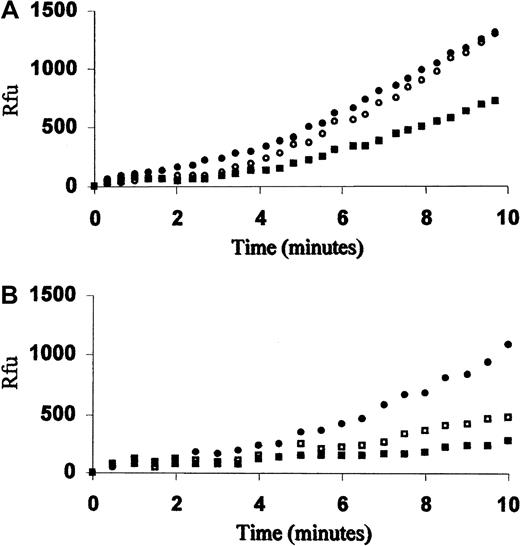
Previous studies of FVIIa inhibition at tissue factor–limiting concentrations24 prompted us to investigate the activity of the recombinant FVII (rFVIIa) in Gly283Ser homozygote plasma (Figure1A). In 3 independent experiments, a reduction of 54.6% ± 8.2% in rFVIIa activity was observed at a 1:100 ratio between rFVIIa and plasma 283Ser-FVII. The rFVIIa was not inhibited in plasma from a severe CRM-FVII–deficient patient (FVIIc, FVIIag less than 1%) homozygous for the double mutation Ala294Val/404delC.25 Furthermore, FXa generation rate by rFVIIa in Ala294Val/404delC homozygote plasma was inhibited in a dose-dependent manner by the addition of medium containing r283Ser-FVII (Figure 1B). A 500-fold excess of r283Ser-FVII (1 nM) reduced rFVIIa (2 pM) activity of 68.8% ± 14.7%. A dose-dependent inhibition was confirmed in plasma-free reconstituted system (not shown).
Inhibition of the rFVIIa activity in plasma by 283Ser-FVII.
(A) FXa generation curves produced by 2 pM rFVIIa (NovoNordisk, Bagsvaed, Denmark) in diluted (1:20) patient FVII38 plasma (FVIIa/283Ser-FVII molar ratio of 1:100) (▪), Ala294ValdelC homozygote plasma (●), or FVII-depleted plasma (○). (B) FXa generation curves produced by 2 pM rFVIIa in Ala294ValdelC homozygote diluted plasma (1:20) with the addition of conditioned medium containing 0.2 nM (■), 1 nM (▪) r283Ser-FVII, or mock medium (●). Reactions were initiated by adding limiting amount of thromboplastin, 20 nM hFX, and 100 μM FXa fluorogenic substrate, and fluorescence was measured over time. The limiting amount was determined before each assay by evaluating the FXa generation obtained with 2 pM rFVIIa concentration and decreasing the amounts of thromboplastin. The decrease in activity was estimated by comparison of FXa generation rates (relative fluorescence units per minute) between 4 and 8 minutes. Rfu indicates relative fluorescence units.
Inhibition of the rFVIIa activity in plasma by 283Ser-FVII.
(A) FXa generation curves produced by 2 pM rFVIIa (NovoNordisk, Bagsvaed, Denmark) in diluted (1:20) patient FVII38 plasma (FVIIa/283Ser-FVII molar ratio of 1:100) (▪), Ala294ValdelC homozygote plasma (●), or FVII-depleted plasma (○). (B) FXa generation curves produced by 2 pM rFVIIa in Ala294ValdelC homozygote diluted plasma (1:20) with the addition of conditioned medium containing 0.2 nM (■), 1 nM (▪) r283Ser-FVII, or mock medium (●). Reactions were initiated by adding limiting amount of thromboplastin, 20 nM hFX, and 100 μM FXa fluorogenic substrate, and fluorescence was measured over time. The limiting amount was determined before each assay by evaluating the FXa generation obtained with 2 pM rFVIIa concentration and decreasing the amounts of thromboplastin. The decrease in activity was estimated by comparison of FXa generation rates (relative fluorescence units per minute) between 4 and 8 minutes. Rfu indicates relative fluorescence units.
Because of the decrease over time of infused FVII and the constant amount of endogenous dysfunctional variants, further studies are needed to establish whether the inhibition observed in vitro might have implications for substitution therapy of severe CRM+deficiency.
We thank Dr F. H. Herrmann, University of Greifswald, Germany, for providing us with plasma from the A294V/404delC homozygote.
Support by the Consiglio Nazionale Della Ricerche Biotech Target Project and Ministero Dell 'Universitá, Della Ricerca Scientifica E Tecnologica.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Francesco Bernardi, Dipartimento di Biochimica e Biologia Molecolare, Università di Ferrara, Via L Borsari 46, 44100 Ferrara, Italy; e-mail: ber@unife.it.