Angiogenesis is required for tumor growth and metastasis. It has recently been suggested that thrombin is a potent promoter of angiogenesis. We therefore examined the possibility that thrombin could be inducing the expression of angiopoietin-2 (Ang-2), necessary for remodeling. Human umbilical vein endothelial cells were incubated with or without thrombin (1 U/mL) for 1 to 24 hours and then examined for messenger RNA (mRNA) by Northern analysis. Enhanced mRNA expression (about 4-fold over baseline) was noted at 4 hours. Enhanced expression of Ang-2 mRNA was secondary to enhanced transcription (about 4-fold), with no effect on stabilization. Enhanced Ang-2 mRNA transcription was inhibited by H7 and PD98059, indicating the requirement of serine/threonine kinases as well as the mitogen-activated protein kinase pathway. Up-regulation of mRNA was associated with enhanced Ang-2 protein synthesis and secretion as assayed by immunoblot. Thrombin-induced secreted Ang-2 inhibited the binding of recombinant 35S–Ang-1 to its Tie-2–Fc receptor, demonstrating functionality. Hirudin reversed this effect, demonstrating thrombin specificity. Thus, thrombin-induced tumorigenesis and metastasis is associated with enhanced Ang-2 protein synthesis and secretion via enhanced transcription of Ang-2. This could help explain how thrombin promotes angiogenesis.
Introduction
Angiogenesis, the process of new blood vessel growth, is essential for embryonic development as well as the support of normal metabolic demands. It is also essential in pathologic conditions, such as wound healing and tumor growth.1,2Various growth factors, particularly vascular endothelial growth factor (VEGF), angiopoietin-1 (Ang-1), and angiopoietin-2 (Ang-2), have been implicated in the regulation of vessel formation. VEGF promotes angiogenesis in vitro by inducing confluent microvascular endothelial cells to invade collagen gels and form capillarylike structures.3 VEGF consists of a family of 6 ligands (placental growth factor, VEGF, and VEGF B, C, D, and E) that bind to 3 receptor tyrosine kinases on endothelial cells (VEGFR-1/Flt-1, VEGFR-2/Flk-1/KDR, and VEGFR-3/Flt-4).4 5
A second family of proteins exerting an important role in vascular development and stabilization has now been identified and designated angiopoietins. Angiopoietins regulate angiogenesis by activating or blocking the activation of Tie-2/Tek, a surface receptor tyrosine kinase generally restricted to endothelial cells. Ang-1 helps to maintain vascular integrity and is involved in vessel maturation.6-8 Ang-2 acts as an antagonist of the Ang-1/Tie2 interaction.9 Ang-2 is expressed in areas undergoing vascular remodeling and is involved in neovascularization. By competing with Ang-1 for binding to their common receptor Tie2, Ang-2 leads to decreased vessel maturation and either vessel regression (in the absence of VEGF) or enhanced vessel sprouting (in the presence of VEGF).10 Both hypoxia and VEGF up-regulate Ang-2 expression.11
It has recently been suggested that thrombin is a potent promoter of angiogenesis.12-15 The relationship between thrombosis and cancer/metastasis was first recognized by Trousseau in 1872.16 Many studies have described a systemic activation of the blood coagulation cascade in patients with cancer.17-20 Recent studies have shown that thrombin-treated tumor cells have an increased ability to adhere to von Willebrand factor, fibronectin, platelets, and endothelial cells and form pulmonary metastases in syngeneic mice.21-25 However, the data do not define the mechanism by which thrombin promotes tumor progression and metastasis. Thrombin has a multitude of enzymatic and cellular actions on a variety of cell types.26 In this communication, we report the induction of Ang-2 messenger RNA (mRNA) and the synthesis and secretion of protein by thrombin and investigate its mechanism.
Materials and methods
Cell culture and materials
Human diploid primary culture fibroblasts (FS4) were kindly provided by Dr J. Vilcek (New York University Medical Center, New York, NY) and cultured in Dulbecco modified Eagle medium with 10% fetal calf serum and 1% penicillin and streptomycin. Human umbilical vein endothelial (HUVE) cells were derived from fresh umbilical cord. The cord was washed 3 times with phosphate-buffered saline, treated with 0.5% trypsin (Sigma, St Louis, MO) for 3 to 5 minutes, and the HUVE cells isolated by washing and centrifugation in phosphate-buffered saline. HUVE cells were cultured in EBM-2 media (Clonetics) containing 10% fetal calf serum, 1% penicillin/streptomycin, and endothelial growth supplements (Sigma). Cells were starved for 4 hours in Dulbecco modified Eagle medium and then treated with different concentrations of thrombin for various time intervals. Thrombin, wortmannin, H7, and PD998059 were obtained from Sigma.
Northern Blot analysis
Total RNA was extracted, fractionated by electrophoresis on a 1% agarose gel in 6.7% formaldehyde, and transferred onto a Genescreen Plus nylon membrane (NEN Life Science Products, Boston, MA). The membrane was hybridized at 42°C overnight with32P-dCTP–labeled probes specific for Ang-2 (1.5 kb reverse transcriptase–polymerase chain reaction product, which contains the complete coding region). The blots were sequentially washed with varying dilutions of SSC, the last being 0.1× SSC at 65°C for 30 minutes. Autoradiography was carried out at −70°C with an intensifying screen. The autoradiographic signals were quantified by densitometric analysis (Personal Densitometer, Molecular Dynamics/Amersham).
Immunoblotting of secreted Ang-2
Cells were treated with 1 U/mL thrombin for 8 hours. Culture media were collected and concentrated with Centricom (Sigma), and cells were extracted in lysis buffer: 1% Triton X-100, 150 mM NaCl, 10 mM Tris (pH 7.4), 50 μg/mL pepstatin, 20 μg/mL aprotinin, 2 μg/mL leupeptin, 0.2 mM phenylmethylsulfonyl fluoride, 1 mM ethylenediaminetetraacetic acid, 0.2 mM sodium orthovanadate, and 0.5% Nonidet P-40. A total of 100 μg of protein, determined by BioRad protein assay, was run on sodium dodecyl sulfate (SDS)–polyacrylamide gel, transferred to a membrane, incubated with blocking solution (2% powdered milk, 0.2% Tween 20 in phosphate-buffered saline), and reacted with a specific goat anti–Ang-2 polyclonal antibody (Santa Cruz, CA). After washing, peroxidase-conjugated second antibodies were applied and chemiluminescence generated by incubation with ECL reagents (Amersham Life Science).
Nuclear run-on analysis of transcription
HUVE cells were stimulated with or without thrombin (1 U/mL) for 1 hour. The cells were then lysed and nuclei isolated by centrifugation at 12 000g for 15 minutes at 4°C. The nuclear suspension was incubated with 0.5 mM each of CTP, ATP, and GTP and with 250 μCi (9.25 MBq) of 32P-UTP. The samples were extracted with phenol/chloroform, precipitated, and resuspended at equal counts per milliliter in hybridization buffer. Denatured plasmid DNA harboring the Ang-2 fragment as well as GAPDH were blotted onto nitrocellulose filters and then hybridized with the radiolabeled samples. The filters were washed as described above for Northern analysis and the membranes subjected to autoradiography.
Actinomycin D chase studies
Confluent cells were incubated in the presence of thrombin (1 U/mL) for 1 hour at 37°C. The cells were then treated with media containing actinomycin D (5 μg/mL) and incubated for an additional 1 to 7 hours. Total RNA was extracted and analyzed for Ang-2 mRNA levels by Northern blot analysis.
Ang-2 inhibition of binding of Ang-1 to Tie-2–Fc
35S-labeled recombinant Ang-1 was prepared by in vitro translation of the complementary DNA27 using the coupled TNT transcription/translation ribosomal system (Promega, Madison, WI). The recombinant Tie-2–Fc fusion protein was prepared from 293 cells and purified with protein A beads as previously described.6 Cultured HUVE cells were treated with thrombin (1 U/mL) or thrombin plus hirudin (1:1) for 8 hours at 37°C. The media were removed and concentrated 10-fold with Centricon (AMICON, Beverly, MA). A total of 1 mL concentrated media was mixed with 5 μL recombinant 35S–Ang-1, incubated with 0.25 μg Tie-2–Fc for 3 hours at 4°C, and then precipitated with protein A beads and run on 12% SDS–polyacrylamide gel electrophoresis for autoradiography as described.27
Results
Effect of thrombin on the expression of Ang-2 mRNA
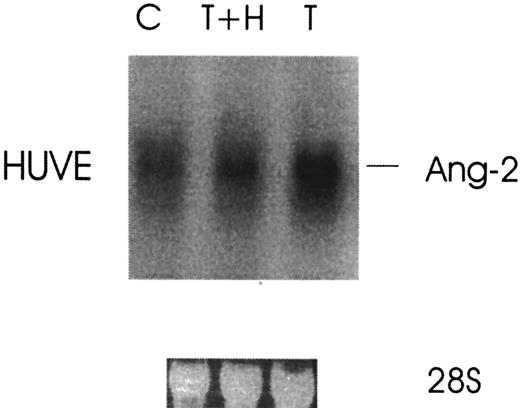
Because thrombin stimulates tumor growth and metastasis and enhanced angiogenesis is required for both, we investigated the effect of thrombin on the induction of Ang-2 mRNA, an angiogenesis growth factor required for blood vessel remodeling. Figure1 demonstrates that the expression of Ang-2 mRNA was upregulated about 4-fold by thrombin in HUVE cells with peak at 4 hours. Thrombin specificity was demonstrated by the more than 3-fold inhibition of Ang-2 mRNA with hirudin in HUVE cells (Figure 2).
Thrombin-induced increased expression of Ang-2 mRNA in HUVE cells.
Cells were starved in media containing 1% fetal calf serum overnight and then treated with or without thrombin for various time intervals. Cells were collected and analyzed for Ang-2 mRNA by Northern blot. Ribosomal 28S RNA is shown as an internal gel-loading standard.
Thrombin-induced increased expression of Ang-2 mRNA in HUVE cells.
Cells were starved in media containing 1% fetal calf serum overnight and then treated with or without thrombin for various time intervals. Cells were collected and analyzed for Ang-2 mRNA by Northern blot. Ribosomal 28S RNA is shown as an internal gel-loading standard.
Thrombin specificity of Ang-2 mRNA up-regulation.
Cells were treated without (C) or with 1 U/mL thrombin (T) or with thrombin plus hirudin (T+H) for 3 hours and then analyzed by Northern blot.
Thrombin specificity of Ang-2 mRNA up-regulation.
Cells were treated without (C) or with 1 U/mL thrombin (T) or with thrombin plus hirudin (T+H) for 3 hours and then analyzed by Northern blot.
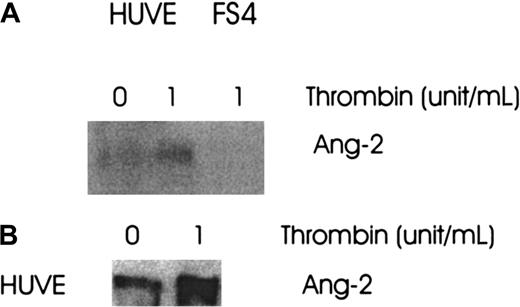
Thrombin increases the production and secretion of Ang-2 protein
To define whether the up-regulation of Ang-2 mRNA was accompanied by an increase in protein synthesis, cells were cultured in media without or with thrombin (1 U/mL) for 8 hours. The cells were extracted with lysis buffer and the media collected and concentrated. Both were analyzed for Ang-2 production by Western blot. The production (Figure 3A) and secretion (Figure 3B) of Ang-2 was increased 2.5- and 3.5-fold, respectively, in HUVE cells.
Effect of thrombin on Ang-2 protein production and secretion.
FS4 and HUVE cells were cultured in media with or without thrombin, 1 U/mL, for 8 hours at 37°C. The media were collected and concentrated and the cells extracted with lysis buffer. Both were then analyzed for Ang-2 production by Western blot. (A) Ang-2 in HUVE cells, not in FS4 cells. (B) Ang-2 in supernatant of HUVE cells.
Effect of thrombin on Ang-2 protein production and secretion.
FS4 and HUVE cells were cultured in media with or without thrombin, 1 U/mL, for 8 hours at 37°C. The media were collected and concentrated and the cells extracted with lysis buffer. Both were then analyzed for Ang-2 production by Western blot. (A) Ang-2 in HUVE cells, not in FS4 cells. (B) Ang-2 in supernatant of HUVE cells.
Thrombin induces increased transcription of Ang-2 mRNA
Thrombin-induced increased expression of Ang-2 mRNA could reflect increased gene transcription, increased mRNA stability, or both. To determine whether the increased Ang-2 mRNA was a result of increased gene transcription, cells were examined by nuclear run-on experiments. As shown in Figure 4, the rate of transcription of Ang-2 was increased by 3-fold in HUVE cells stimulated with thrombin.
Effect of thrombin on nuclear transcription of Ang-2 mRNA.
HUVE cells were treated with and without 1 U/mL thrombin for 3 hours, lysed, and nuclei extracted. Radiolabelled run-on RNAs were synthesized from isolated nuclei and hybridized to immobilized complementary DNA for Ang-2 and GAPDH (internal loading control).
Effect of thrombin on nuclear transcription of Ang-2 mRNA.
HUVE cells were treated with and without 1 U/mL thrombin for 3 hours, lysed, and nuclei extracted. Radiolabelled run-on RNAs were synthesized from isolated nuclei and hybridized to immobilized complementary DNA for Ang-2 and GAPDH (internal loading control).
As shown in Figure 5, the mRNA stability of Ang-2 was not increased with thrombin in HUVE cells, although the level of expression of Ang-2 was clearly enhanced upon thrombin stimulation.
Effect of thrombin on Ang-2 mRNA stability.
Cells were treated with and without 1 U/mL thrombin for 3 hours, followed by addition of 5 μg/mL actinomycin D to inhibit RNA synthesis. Northern analysis was then performed at various time intervals of 1 to 24 hrs.
Effect of thrombin on Ang-2 mRNA stability.
Cells were treated with and without 1 U/mL thrombin for 3 hours, followed by addition of 5 μg/mL actinomycin D to inhibit RNA synthesis. Northern analysis was then performed at various time intervals of 1 to 24 hrs.
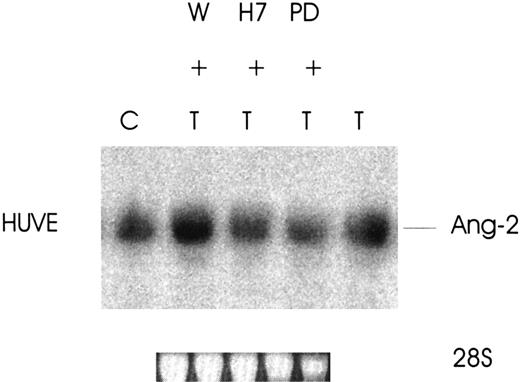
Thrombin promotes the expression of Ang-2 via the serine/threonine kinase and MAPK pathways
To define the signaling pathways responsible for the increased expression of Ang-2 by thrombin, 3 major cellular transduction mechanisms involved in thrombin receptor activation28-30were studied (phosphatidylinositol-3 [PI-3] kinase, serine/threonine kinases, and mitogen-activated protein kinase [MAPK]). As shown in Figure 6, the increased expression of Ang-2 by thrombin in HUVE cells is regulated by 2 of the 3 pathways examined. Ang-2 induction was totally inhibited by H7 (serine/threonine kinase inhibitor) and by PD98059 (MAPK kinase inhibitor) (3-fold less for both) but not inhibited by wortmannin (PI-3 kinase inhibitor). These data indicate that the mechanism of thrombin-induced up-regulation of Ang-2 in HUVE cells is associated with activation of serine/threonine kinases and the MAPK pathway.
Effect of signaling pathway protein kinase inhibitors on thrombin-induced expression of Ang-2 mRNA.
Cells were treated without (C) or with wortmannin (W) 1 μM, H7 (20 μM), or PD98059 (100 μM) for 2.5 hours at 37°C and thrombin, 1 U/mL, added for an additional 3 hours. Northern blot was then performed for Ang-2 mRNA. Note inhibition by H7 and PD98059, which inhibit serine/threonine kinases and the MAPK pathway.
Effect of signaling pathway protein kinase inhibitors on thrombin-induced expression of Ang-2 mRNA.
Cells were treated without (C) or with wortmannin (W) 1 μM, H7 (20 μM), or PD98059 (100 μM) for 2.5 hours at 37°C and thrombin, 1 U/mL, added for an additional 3 hours. Northern blot was then performed for Ang-2 mRNA. Note inhibition by H7 and PD98059, which inhibit serine/threonine kinases and the MAPK pathway.
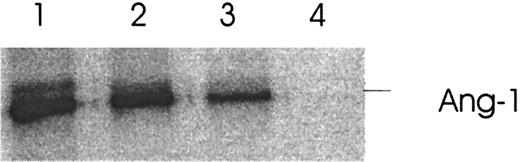
Thrombin-induced Ang-2 inhibits the binding of Ang-1 to its receptor, Tie-2. Functional evidence that the thrombin-induced Ang-2 secretion is biologically active is provided by the following experiment. Recombinant 35S-labeled Ang-1 produced from an in vitro transcription/translation ribosomal system was shown to bind to its recombinant fusion protein receptor Tie-2–Fc (Figure7, lane 1). Ang-2 derived from the supernatants of thrombin-treated HUVE cells was then preincubated with35S–Ang-1 to incubation with Tie-2–Fc by Ang-2. Figure 7, lane 3, demonstrates inhibition of binding of Ang-1 to Tie-2–Fc by Ang-2. Figure 7, lane 2, demonstrates reversibility of the thrombin effect with hirudin.
Effect of thrombin-induced Ang-2 on binding of Ang-1 to its Tie-2 receptor.
HUVE cells were treated with 1 U/mL thrombin for 5 hours and the concentrated supernatant incubated with 35S–Ang-1 prior to binding of Ang-1 to Tie-2–Fc. Binding was analyzed by autoradiography following immunoprecipitation with protein A and 12% SDS–polyacrylamide gel electrophoresis. Lane 1, 35S–Ang-1 plus Tie-2–Fc. Lane 2, 35S–Ang-1 plus supernatant of thrombin-hirudin–treated HUVE cells. Lane 3, 35S–Ang-1 plus supernatant of thrombin-treated HUVE cells. Lane 4, absence of Tie-2–Fc in the system. Representative of 3 experiments.
Effect of thrombin-induced Ang-2 on binding of Ang-1 to its Tie-2 receptor.
HUVE cells were treated with 1 U/mL thrombin for 5 hours and the concentrated supernatant incubated with 35S–Ang-1 prior to binding of Ang-1 to Tie-2–Fc. Binding was analyzed by autoradiography following immunoprecipitation with protein A and 12% SDS–polyacrylamide gel electrophoresis. Lane 1, 35S–Ang-1 plus Tie-2–Fc. Lane 2, 35S–Ang-1 plus supernatant of thrombin-hirudin–treated HUVE cells. Lane 3, 35S–Ang-1 plus supernatant of thrombin-treated HUVE cells. Lane 4, absence of Tie-2–Fc in the system. Representative of 3 experiments.
Discussion
The requirement of angiogenesis for tumor growth and metastasis is well documented.1 The effect of thrombin and activated platelets on the promotion of tumor growth and metastasis is similarly well documented.21-25 The role of thrombin in the induction of angiogenesis has recently been explored by several groups and can now be attributed to its effects on VEGF, Ang-1, and Ang-2.
Platelets contain VEGF31,32 and Ang-1,27,33which are released following platelet activation with thrombin. Tumor specimens are surrounded by platelets and when removed at surgery have VEGF and thrombin localized on their surface.34-37 Tumor growth is inhibited by VEGF antibody.38,39 The recombinant Tie-2 receptor, AdExTek, capable of blocking Tie-2 activation by Ang-1, inhibits the growth and metastasis of murine mammary carcinoma (4T1) and melanoma (B16F10.9) cells.40 Ang-2 has been found in hypervascular human hepatocarcinomas as well as in an animal model in which it was highly expressed only in tumor tissue. Ectopic expression of Ang-2 in nonexpressing human hepatocellular cells promoted hepatomas in nude mice.41 Ang-2 has been found in advanced-stage neuroblastoma compared with low-stage tumors as well as in neuroblastoma cell lines,42 in increased intensity in blood vessels of non–small cell lung carcinoma,43uveal melanoma cell lines,44 thyroid tumor progression,45 and endothelial cells of human gliomas.46
However, these associations do not define the mechanism by which thrombin promotes angiogenesis. Recent publications contribute to our understanding of this mechanism. Maragoudakis and coworkers have reported that thrombin promotes endothelial cell alignment in vitro in matrigel and angiogenesis in vivo,12 that angiogenesis is independent of fibin formation,15 and that thrombin potentiates VEGF by upregulating its recepor, KDR.14Herbert et al have demonstrated up-regulation of endothelial cell growth by thrombin by the autocrine release of basic fibroblast growth factor.13 Our group47,48 and Ollivier et al49 have recently demonstrated up-regulation of VEGF mRNA and protein in prostate DU145 cells47 and fibroblasts.47,49 We have recently demonstrated the presence of Ang-1 in platelets27 and its release by thrombin.33
The role of Ang-2 in tumorigenesis and metastasis has recently been more clearly defined by Yancopoulos and coworkers.50 They have observed that tumor cells do not initially require vascular support but then proceed to co-opt existing host endothelial cells in which Ang-2 is highly induced prior to VEGF induction. The co-opted vessels then regress via disruption of endothelial cell interactions and undergo apoptosis, resulting in central necrosis of the tumor. Angiogenesis is then induced at the tumor margin associated with the induction of VEGF and Ang-2, supporting further growth.
Our study indicates, for the first time, up-regulation of functional Ang-2 by thrombin. Our current report on the induction of Ang-2 protein synthesis and secretion contributes to an understanding of the mechanism of thrombin-induced angiogenesis, tumor growth, and metastasis. Unlike the effect of thrombin on up-regulation of VEGF via enhanced stabilization of VEGF mRNA,47 Ang-2 upregulates via enhanced transcription, with absence of enhanced stabilization of mRNA. It has recently been reported by Oh et al11 that both hypoxia and VEGF up-regulate Ang-2 mRNA in bovine microvascular endothelial cells and that VEGF is capable of upregulating Ang-2. One should therefore consider the possibility that thrombin-induced up-regulation of Ang-2 may be secondary to its up-regulation of VEGF. However, this was shown not to be the case by these authors,24 who demonstrated that neutralizing anti-VEGF antibody had no effect on anoxia-induced up-regulation of Ang-2. Our studies similarly do not support this possibility because up-regulation of VEGF mRNA by thrombin is inhibited by wortmannin (PI-3 kinase inhibitor),47 whereas up-regulation of Ang-2 mRNA is not and HUVE cells have no detectable VEGF mRNA before or after thrombin stimulation.
Up-regulation of Ang-2 mRNA was inhibited by both serine/threonine kinase and a highly specific MAPK kinase inhibitor. Both signaling pathways are involved in thrombin stimulation of cells. Thrombin-induced cell protection of astrocytes is inhibited by the serine/threonine kinase inhibitor, H7.28Thrombin-stimulated platelet activation and aggregation requires the activation of a MAPK kinase and the phosphorylation of a serine/threonine kinase.30 Thus, phosphorylated MAPK kinases and serine/threonine kinases are required for the up-regulation of Ang-2 in HUVE cells, whereas the PI-3 kinase pathway does not appear to be involved.
It is generally accepted that VEGF, Ang-1, and Ang-2 are necessary for efficient blood vessel growth and development. It has been proposed that Ang-2, a natural antagonist of Ang-1, may be an important proangiogenic factor in that it may counteract Ang-1–mediated blood vessel stability, thus maintaining the endothelium in a more plastic state and promoting the response of endothelial cells to angiogenesis growth factors.50 Up-regulation of both VEGF and Ang-2 by thrombin indicates that angiogenesis might be facilitated by thrombosis. Thus, the well-described association of thrombosis with cancer16-20 may be contributing to tumorigenesis by the initiation of thrombin-stimulated angiogenesis, which could explain, at least in part, the enhancement of experimental tumorigenesis by thrombin.21-25
Supported by NIH grant HL-13336-28, the Hildegarde D. Becher Foundation, and grants from the Helen Polonsky Research Fund, Dorothy and Seymour Weinstein Research Fund, and New York Community Trust.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Yao-Qi Huang or Simon Karpatkin, Dept of Medicine and Kaplan Cancer Center, New York University Medical School, 550 First Ave, New York, NY 10016; e-mail: huangy02@med.nyu.edu orsimon.karpatkin@med.nyu.edu.