Abstract
Oncogenic anaplastic lymphoma kinase (ALK) fusion proteins (NPM/ALK and associated variants) are expressed in about 60% of anaplastic large cell lymphomas (ALCLs) but are absent in normal tissues. In this study, we investigated whether ALK, which is expressed at high levels in lymphoma cells, could be a target for antigen-specific cell-mediated immunotherapy. A panel of ALK-derived peptides was tested for their binding affinity to HLA-A*0201 molecules. Binding peptides were assessed for their capacity to elicit a specific immune response mediated by cytotoxic T lymphocytes (CTLs) both in vivo, in HLA-A*0201 transgenic mice, and in vitro in the peripheral blood lymphocytes (PBLs) from healthy donors. Two HLA-A*0201–restricted CTL epitopes, p280-89 (SLAMLDLLHV) and p375-86 (GVLLWEIFSL), both located in the ALK kinase domain were identified. The p280-89– and p375-86–induced peptide-specific CTL lines were able to specifically release interferon-γ (IFN-γ) on stimulation with ALK peptide-pulsed autologous Epstein-Barr virus–transformed B cells (LCLs) or T2 cells. Anti-ALK CTLs lysed HLA-matched ALCL and neuroblastoma cell lines endogenously expressing ALK proteins. CTL activity was inhibited by anti-HLA-A2 monoclonal antibody CR11.351, consistent with a class I–restricted mechanism of cytotoxicity. These results show the existence of functional anti-ALK CTL precursors within the peripheral T-cell repertoire of healthy donors, clearly indicating ALK as a tumor antigen and ALK-derived peptides, p280-89 and p375-86, as suitable epitopes for the development of vaccination strategies.
Introduction
The discovery of tumor-associated antigens (TAAs) demonstrated that tumor cells can be specifically recognized by the immune system, raising the hypothesis that most if not all tumors express antigens that cytotoxic T lymphocytes (CTLs) can potentially attack.1 The identification of immunogenic epitopes led to their use as targets to mediate the specific clearance of neoplastic cells by TAA targeting strategies such as vaccination.2-4
A major issue in the development of efficient vaccination protocols is the identification of the appropriate and effective TAAs, able to induce an immune response that will affect tumor growth. The ideal target for immune destruction is a tumor-specific protein that is not expressed in normal cells and is essential for the malignant phenotype.5
In an effort to identify new and specific tumor antigens, we examined whether anaplastic lymphoma kinase (ALK) could induce a CTL-mediated antitumor immune response, and therefore be considered as a TAA.
More than half the patients with anaplastic large cell lymphoma (ALCL) possess a t(2;5) chromosomal translocation that leads to the expression of the NPM/ALK fusion protein. This fusion protein is composed of the N-terminal portion of the nuclear phosphoprotein nucleophosmin (NPM) linked to the entire intracellular portion of ALK, which contains its kinase catalytic domain.6,7 It has been extensively demonstrated that constitutive activated NPM/ALK is a potent oncogene having transforming and tumorigenic properties.8-11
Although NPM is a “housekeeping” gene that is ubiquitously expressed in normal cells, ALK expression is restricted in embryonic and adult mouse to the central nervous system (CNS).12,13 In humans, ALK was detected at low levels in some pericytes and scattered glial cells in specific areas of the CNS. ALK is not normally expressed in lymphohematopoietic tissues.14 However, as a result of the t(2;5) chromosomal translocation, ALK is ectopically expressed at high levels in lymphoid cells and its constitutive tyrosine kinase activity leads to deregulated lymphocyte growth.9 15
It has been demonstrated that partners other than NPM can promote the oncogenic expression of the ALK intracytoplasmic domain. ALK variant fusion proteins so far identified in patients with ALCL are TPM3/ALK,16,17 TFG/ALK,18,19ATIC/ALK,20,21 CLTCL/ALK,22 and MSN/ALK.23
The high level of expression of NPM/ALK and its variants in lymphoma cells and their direct role in lymphomagenesis, combined with the fact that normal ALK is expressed at low levels in the immune-privileged CNS, suggests that ALK could potentially be an ideal target for immunotherapy. Indeed, circulating antibodies recognizing NPM/ALK were recently detected in ALCL patient sera.24
Here we studied a panel of ALK-derived peptides with binding motif for the HLA-A*0201 molecules. We evaluated their ability to provoke an in vivo and in vitro peptide-specific CTL response in HLA-A*0201 transgenic mice and in peripheral blood lymphocytes (PBLs) of HLA-matched healthy donors. We demonstrated that the anti-ALK CTLs generated from PBLs of healthy donors elicited an antigen-specific, HLA-A2.1–restricted response, able to effectively kill tumor targets endogenously expressing ALK.
Materials and methods
Animals
The HHD transgenic mice have been previously described.25 They are β2m−/−, Db−/− double knockout and express an HLA-A*0201 monochain composed of a chimeric heavy chain (α1 and α2 domains of HLA-A*0201, α3 and intracellular domains of Db) linked by its N-terminus to the C-terminus of the human β2-microglobulin (β2m) light chain by a 15-amino acid peptide. Mice were housed in a temperature-controlled, light-cycled room. All in vivo experiments were performed in accordance with local ethical guidelines.
Cell lines
Murine RMA-S/HHD cells were obtained by transfection of murine RMA TAP-deficient variant RMA-S cells with the HHD monochain construct as previously described.25
Human cell lines used in the experiments were grown in RPMI 1640 supplemented with 10% heat-inactivated fetal calf serum (FCS), 2 mmol/L l-glutamine, and antibiotics. The following HLA-A*0201 tumor cell lines were used: SU-DHL1 and SUP-M2 (NPM/ALK+ lymphomas), FM3 (melanoma), HCT 116 (colon carcinoma), MCF-7 (breast carcinoma), IMR-32 (ALK+neuroblastoma), NB5 (ALK− neuroblastoma), and T2 cells (TAP1 and TAP2 deficient). SK-N-SH is an HLA-A*0201−, ALK+ neuroblastoma cell line. Before their use as targets in the 51Cr release assay, all neuroblastoma cells were treated with 2000 IU/mL recombinant human interferon (IFN)–γ (Peprotech, Rocky Hill, NJ) for 72 hours.
Peptides
The NPM/ALK sequence was reviewed for peptides that could potentially bind to HLA-A*0201, the most common HLA class I allele among whites, using a peptide-motif scoring system (http://bismas.dcrt.nih.gov/molbio/hla_bind/index.html). Twenty-two among the highest scored predicted peptides present in the ALK sequence were considered. Peptides were also chosen to cover the entire ALK sequence: 11 peptides were within the kinase domain (p197-205: GMPNDPSPL; p217-25: SEQDELDFL; p246-54: SLQSLPRFI; p246-55: SLQSLPRFIL; p249-58: SLPRFILLEL; p280-89: SLAMLDLLHV; p281-89: LAMLDLLHV; p282-90: AMLDLLHVA; p376-85: GVLLWEIFSL; p377-85: VLLWEIFSL; p394-402: SNQEVLEFV), 2 were upstream (p139-47: KLSKLRTST; p148-57: IMTDYNPNYC), and 2 were downstream (p438-46: IILERIEYC; p456-65: ALPIEYGPLV) of the kinase domain and 7 in the C-terminal portion of the protein (p592-600: NLGLEGSCTV; p593-601: LGLEGSCTV; p609-17: RLPGASLLL; p614-22: SLLLEPSSL; p615-23: LLLEPSSLT; p615-24: LLLEPSSLTA; p621-29: SLTANMKEV). FLU matrix58-66 (GILGFVFTL) peptide was used as an irrelevant peptide in human CTL assays.
Peptides purified by high-performance liquid chromatography were synthesized and purchased from American Peptide Company (APC) (Sunnyvale, CA) at a minimum purity of 90%.
HLA-A*0201 binding assays
To determine the binding ability of predicted peptides to HLA-A*0201 molecules, an in vitro cellular binding assay was performed using the TAP-deficient cell line T2. T2 cells were incubated with 100 μM peptide in serum-free RPMI 1640 supplemented with 5 μg/mL human β2m (Fluka, Buchs, Germany) for 18 hours at 37°C. HLA-A*0201 expression was then measured by flow cytometry using the anti-HLA-A2.1 monoclonal antibody (mAb) BB7.2 followed by incubation with fluorescein isothiocyanate (FITC)–conjugated F(ab′)2goat antimouse Ig (Biosource, Camarillo, CA). The results are expressed as fluorescence index (FI) defined as a ratio (median channel of fluorescence) between the sample and a control containing an irrelevant peptide (PML/RARα: VASGAGEAA). An increase over the control of at least 65% (FI > 2) was arbitrarily chosen as the cutoff point.
Measurement of HLA-A*0201/peptide complex stability
To assess the HLA-A*0201/peptide complex stability, T2 cells (106/mL) were incubated overnight with 100 μM of each peptide in serum-free RPMI 1640 supplemented with 100 ng/mL human β2m at 37°C. Cells were then washed 4 times to remove free peptides, incubated for 1 hour with 10 μg/mL Brefeldin A (Sigma-Aldrich, Lyon, France) to block cell surface expression of newly synthesized HLA-A*0201 molecules, washed, and incubated at 37°C for 0, 2, 4, 6, or 8 hours. Subsequently, cells were stained with anti-HLA-A2.1 mAb BB7.2. For each time point, peptide-induced HLA-A*0201 expression was calculated as mean fluorescence value of peptide incubated T2 cells/mean fluorescence value T2 cells in the absence of the peptide. Dissociation complex50(DC50) was defined as the time required for the loss of 50% of the HLA-A*0201/peptide complexes stabilized at t = 0.
Generation of CTLs in HHD mice
HHD mice were injected subcutaneously at the base of the tail with 100 μg peptide emulsified in incomplete Freund adjuvant (IFA) in the presence of 140 μg IAb-restricted hepatitis B virus (HBV) core antigen-derived T-helper epitope (p128-40: TPPAYRPPNAPIL). After 11 days, spleen cells from primed mice (5 × 107 cells in 10 mL) were restimulated in vitro with 10 μM peptide. On day 6 of culture, cells were tested for specific cytotoxicity in a 4-hour 51Cr release assay using as targets RMA-S/HHD cells pulsed with 1 μM peptide or alone.
Generation of CTLs in healthy donors
Blood was collected from HLA-A*0201 healthy volunteer donors. Peripheral blood mononuclear cells (PBMCs) were isolated from whole blood by Ficoll/Hypaque (Pharmacia, Uppsala, Sweden) density gradient centrifugation. Dendritic cells (DCs) were generated according to the protocol of Sallusto and Lanzavecchia.26 Adherent monocyte-enriched PBMCs were cultured in RPMI 1640 medium supplemented with 10% FCS, 20 ng/mL recombinant human interleukin-4 (IL-4) (R &D Systems, Minneapolis, MN) and 800 U/mL recombinant human granulocyte-macrophage colony-stimulating factor (GM-CSF; Sandoz, Basel, Switzerland). On day 5 of culture, 10 ng/mL recombinant human tumor necrosis factor (TNF)–α (Knoll, Ludwigshafen, Germany) was added to the medium. After 40 hours, the surface phenotype of monocyte-derived DCs was determined by single-color flow cytometry using the following mAbs: fluorescein-conjugated anti-CD1a (Valter Occhiena, Torino, Italy), anti-CD86 (Caltag, Burlingame, CA), anti-CD83 (Caltag), anti-CD14 (Becton Dickinson, Franklin Lakes, NJ), and rhodamine-conjugated anti-CD80 (Becton Dickinson). DCs were used to prime autologous PBLs as follows. DCs were pulsed with 10 μM peptide for 2 hours at 37°C, irradiated at 30 Gy, and added to autologous PBLs at a ratio variable from 3:1 to 6:1 (PBL/DC). PBLs (36 × 106 total) were cultured at 1.5 × 106 cells/2 mL culture medium (CM: 50% RPMI 1640/50% X-VIVO) supplemented with 10% heat-inactivated autologous plasma and 10 ng/mL IL-7 (R & D Systems) in the presence of autologous peptide-loaded DCs. At day 10 to 12 of culture, lymphocytes were restimulated with irradiated (30 Gy) autologous peptide-loaded monocytes in CM supplemented with 10% heat-inactivated autologous plasma. After 24 to 48 hours 10 IU/mL IL-2 (Chiron, Emeryville, CA) and 10 ng/mL IL-7 were added to the CM. Lymphocytes were then restimulated weekly in the same way. Starting from the forth round of stimulation, the specificity of the resulting T-cell lines was evaluated weekly in a cytolytic assay. When any specific activity was detected, CD8+ T cells were isolated from the bulk culture by CD4+ cells negative depletion using mAb-coated beads (Dynal Dynabeads, Oxoid, Oslo, Norway) and the CTL lines were maintained in culture in RPMI 1640 containing 10% human AB serum supplemented with 50 U/mL IL-2 in the presence of irradiated (70 Gy) PBMCs as feeder.
CTL assays
Cytolytic activity was measured in a conventional 4-hour51Cr release assay. A 10-fold excess of unlabeled K562 cells was added to offset natural killer (NK) activity. Target cells and effector-to-target (E/T) ratio are indicated in each figure. Specific lysis was determined according to the following formula: % specific lysis = cpm (sample−spontaneous)/cpm (total−spontaneous) × 100.
For cytokine release assay, 105 target cells (Epstein-Barr virus [EBV]-transformed B cells and TAP−/− T2 cells) and 105 lymphocytes were incubated overnight in round-bottomed microtiter plates in 250 μL/well total volume of RPMI 1640 supplemented with 10% FCS. Plates were then centrifuged and a 200-μL aliquot of supernatant was removed from each well. The IFN-γ level was determined by enzyme-linked immunosorbent assay (ELISA; MABTECH, Nacka, Sweden).
HLA-A*0201 blocking of T-cell activity (lysis or cytokine release or both) was performed by preincubating target cells with the anti-HLA-A2 mAb CR11.351.
Results
Identification of ALK-derived peptides binding to HLA-A*0201 molecules
Using a computer-assisted analysis, the ALK amino acid sequence present in the NPM/ALK fusion protein was screened for the presence of HLA-A*0201 binding motifs. We selected 14 nonamer and 8 decamer candidate peptides with the highest predicted half-time of dissociation from HLA-A*0201 molecules.
The ability of the 22 selected ALK-derived peptides to bind to HLA-A*0201 molecules and to form stable major histocompatibility complex (MHC)/peptide complexes was assessed by measuring the binding affinity, expressed as FI, and stability, expressed as DC50.
Nine of the 22 predicted peptides were shown to be high-affinity peptides, with strong binging to HLA-A*0201 (FI > 2.3) and efficient stabilizers (DC50 ranging from 2 to 6 hours; Table 1). Such HLA binding and stabilizing capacities correlate well with potential immunogenicity.
In vivo immunogenicity of ALK-derived peptides in HHD mice
To evaluate the in vivo immunogenic potential of ALK-derived high-affinity binding peptides, we immunized H-2Db−/−, β2m−/−, HLA-A*0201 monochain transgenic (HHD) mice. In these mice, the peripheral CD8+ T-cell repertoire is educated, both at the thymic and peripheral levels, with transgenic HLA class I human molecules. Therefore, the HHD mouse is a useful animal model to assess the ability of individual peptides to induce HLA-A*0201-restricted CTL responses in vivo.27 28
Seven of the 9 HLA-binding peptides were able to induce peptide-specific CTL responses.
Taking into account the binding affinity, the number of responding mice and the level of specific lysis, ALK-derived peptides can be classified as strong (p280-89, p282-90), intermediate (p376-85, p377-85, p621-29), weak (p281-89, p456-465), or inefficient (p609-17, p615-24) CTL inducers (Table 1).
The ALK-derived peptide CTL induction was comparable to that associated with well-known dominant tumor epitopes, such as gp100p154 and HER-2/neup369,27 29 in HHD mice.
As previously described for different HLA-A2.1–associated peptides,29 ALK-derived peptides that failed to generate significant peptide-specific CTLs are also those demonstrating weaker stabilization capacity (DC50, 2 hours), irrespective of their binding affinity. This observation is in accord with the need of MHC affinity and MHC/peptide stability in determining peptide immunogenicity.
Stimulation of ALK-specific CTLs derived from healthy donor PBLs
To determine the capacity of ALK peptides to mobilize a human repertoire, anti-ALK–specific CTLs were generated by in vitro sensitization of HLA-A*0201 healthy donor PBLs with ALK peptides previously screened in HHD mice. For each peptide PBL samples from 3 donors were tested.
To prime ALK-specific lymphocytes, peptide-pulsed autologous DCs derived from PBMCs were used after their in vitro maturation confirmed by flow cytometric analysis of DC surface markers: CD1a, CD83, CD80, CD86, CD14 (data not shown). After 3 cycles of weekly restimulation, the specificity of T lymphocytes bulk cultures for ALK peptides was evaluated as antigen-restricted lysis of peptide-loaded T2 cells and NPM/ALK+ ALCL cell lines. At least 1 of 3 tested donors were responders to stimulation with peptides p280-89, p376-85, p377-85, and p456-65 (donors [d]: 01, 02, 03, 04, 05, Figure1). No specific reactivity was detected in the cell lines generated from PBLs stimulated with peptides p281-89, p282-90, and p621-29 (data not shown).
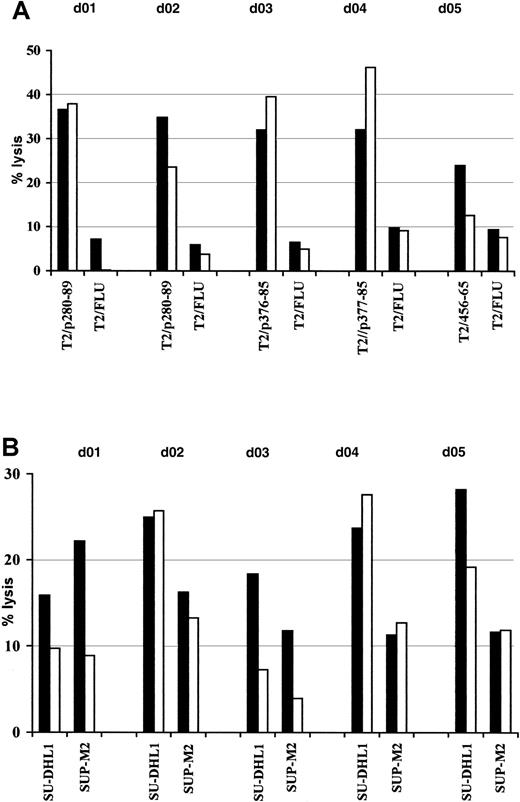
Induction of anti-ALK–specific effectors in healthy donor PBLs.
PBLs were activated with autologous peptide-pulsed DCs and restimulated weekly with peptide-loaded autologous monocytes. The resulting T-cell bulk cultures were tested for antigen specificity starting at the third round of stimulation. Cytotoxic activity was determined in a standard51Cr release assay at an E/T ratio of 50:1 (▪) and 25:1 (■) using as targets T2 cells pulsed with 5 μM cognate peptide or irrelevant influenza matrix-derived peptide (FLU) (A) and NPM/ALK+ lymphoma cell lines SU-DHL1 and SUP-M2 (B). Results of 1 representative experiment of 2 performed are shown.
Induction of anti-ALK–specific effectors in healthy donor PBLs.
PBLs were activated with autologous peptide-pulsed DCs and restimulated weekly with peptide-loaded autologous monocytes. The resulting T-cell bulk cultures were tested for antigen specificity starting at the third round of stimulation. Cytotoxic activity was determined in a standard51Cr release assay at an E/T ratio of 50:1 (▪) and 25:1 (■) using as targets T2 cells pulsed with 5 μM cognate peptide or irrelevant influenza matrix-derived peptide (FLU) (A) and NPM/ALK+ lymphoma cell lines SU-DHL1 and SUP-M2 (B). Results of 1 representative experiment of 2 performed are shown.
Attempts at generating anti-ALK CTLs from responding donors led to the establishment of 2 cell lines specific for peptide p280-89 (d01 and d02) and 1 line specific for peptide p376-85 (d03). Flow cytometric analysis demonstrated that more than 99% of the cells were CD3+, CD8+, CD4− (data not shown).
Antigen specificity of anti-ALK–specific CTLs was investigated. Autologous EBV-transformed B cells (LCLs) or T2 cells were prepulsed with the indicated peptide and used as targets under different conditions (Figure 2).
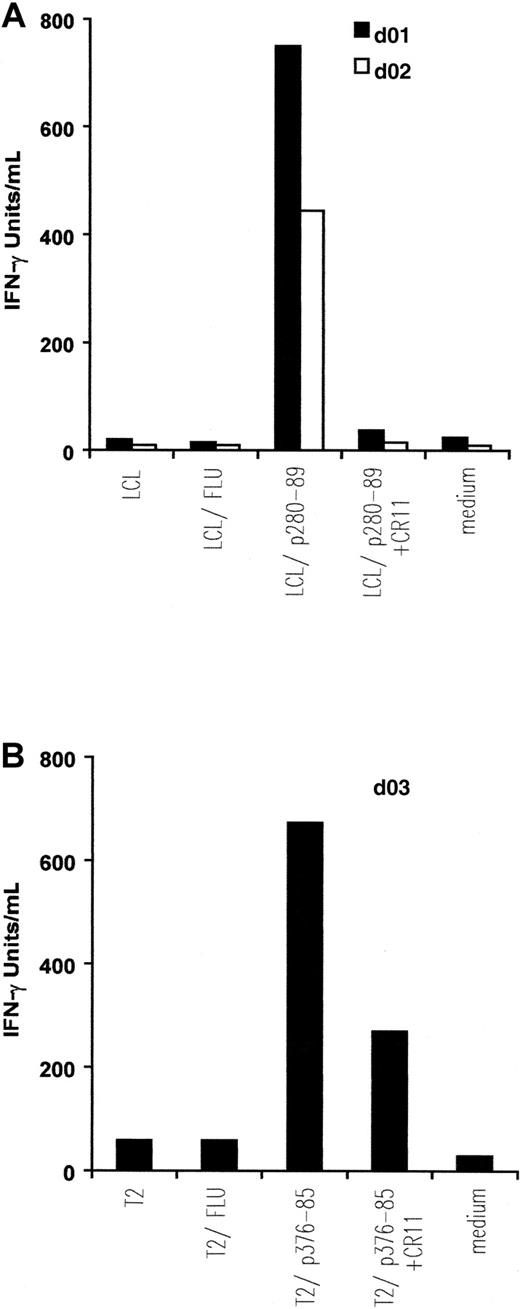
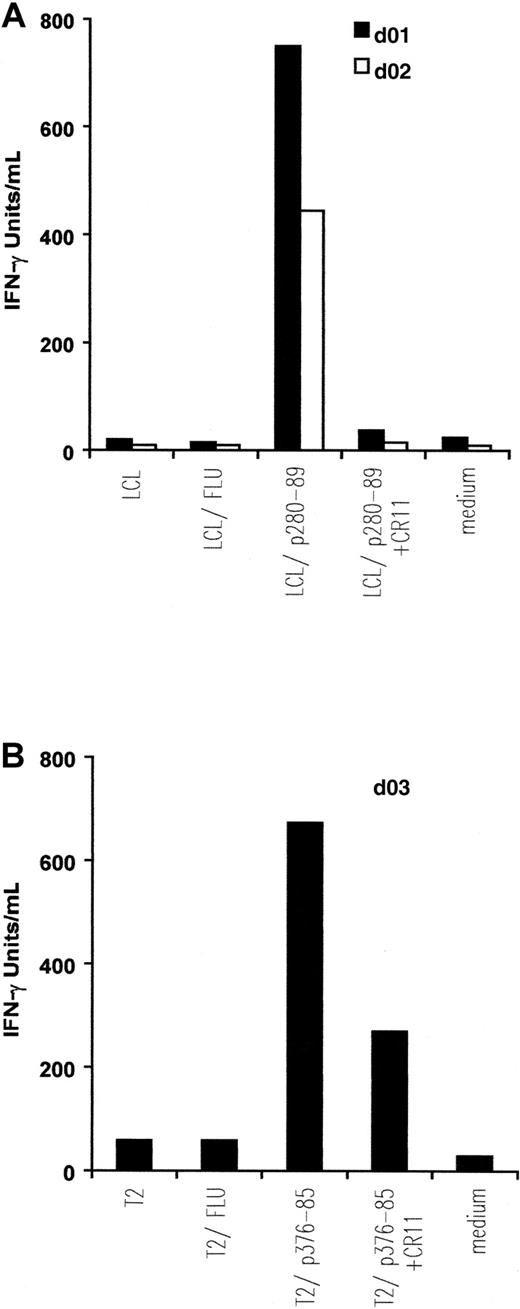
IFN-γ release by in vitro–induced anti-ALK CTL lines.
Autologous EBV-transformed B cells (LCL) (A) and T2 cells (B) were pulsed with 5 μM, respectively, p280-89 and p376-85 peptides as well as influenza matrix peptide (FLU: irrelevant peptide) and were then used as stimulator in an IFN-γ release assay. Preincubation with 5 μg/mL anti-HLA-A2 mAb CR11.351 inhibits IFN-γ secretion. Results of 1 representative experiment of at least 3 performed are shown.
IFN-γ release by in vitro–induced anti-ALK CTL lines.
Autologous EBV-transformed B cells (LCL) (A) and T2 cells (B) were pulsed with 5 μM, respectively, p280-89 and p376-85 peptides as well as influenza matrix peptide (FLU: irrelevant peptide) and were then used as stimulator in an IFN-γ release assay. Preincubation with 5 μg/mL anti-HLA-A2 mAb CR11.351 inhibits IFN-γ secretion. Results of 1 representative experiment of at least 3 performed are shown.
CTLs from the 2 donors responding to ALK peptide p280-89 released IFN-γ (d01: 750 IU/mL; d02: 445 IU/mL) when incubated with p280-89–loaded LCLs. The cytokine secretion was completely blocked in the presence of anti-HLA-A2 mAb CR11.351. No IFN-γ production was observed when CTLs were incubated with autologous LCLs alone or loaded with irrelevant peptide (influenza matrix: FLU; Figure 2A).
Similar quantities of IFN-γ (674 IU/mL) were released by CTLs generated from d03 and incubated with p376-85–pulsed T2 cells. No, or a limited quantity of, IFN-γ was detected after incubating CTLs in the presence of anti-HLA-A2 mAb CR11.351, alone or with FLU-pulsed T2 cells (Figure 2B).
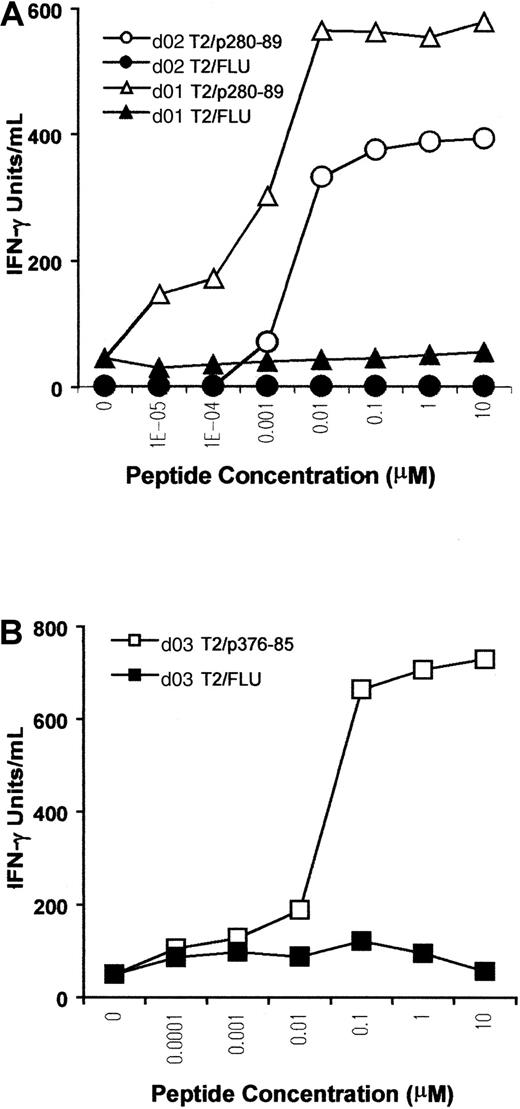
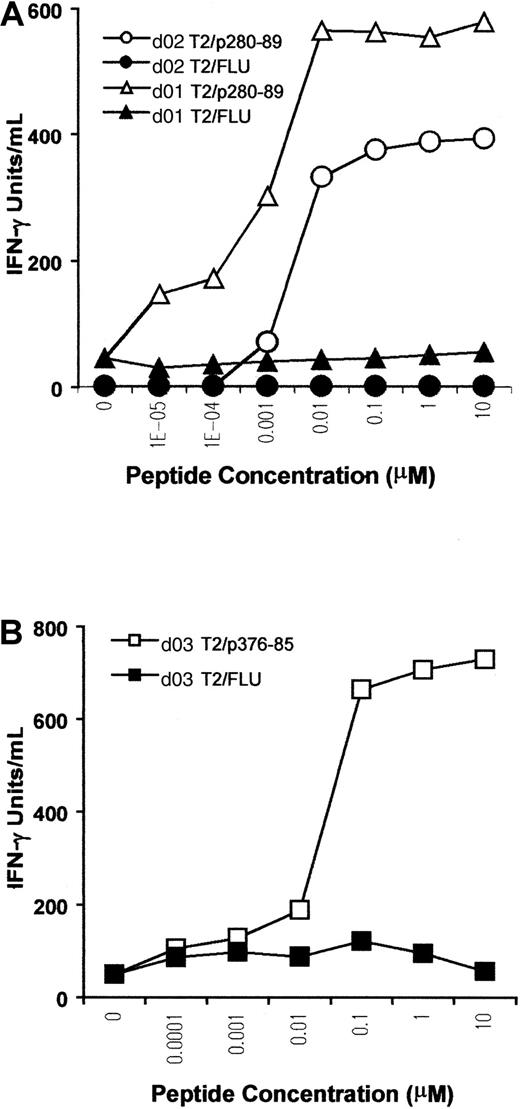
A titration assay was performed to further assess the sensitivity and competence of CTLs in recognizing the antigen. T2 cells were pulsed with p280-89, p376-85, or FLU peptides at different concentrations, and then used as stimulators in a cytokine release assay. For p280-89–specific CTLs, maximal production of IFN-γ was reached with only 10 nM peptide, indicating high affinity of recognition and antigen concentration-dependent IFN-γ production (Figure3A). The CTL line from d03, specific for ALK peptide p376-85, exhibited a lower level of IFN-γ release (maximal release at 100 nM), indicating a lower efficiency for T-cell stimulation (Figure 3B).
Peptide sensitivity of in vitro–induced ALK-specific CTLs.
T2 cells were incubated with titrated amounts of p280-89 (A) and p376-85 (B) peptides as well as influenza matrix peptide (FLU) and were then used as stimulator in an IFN-γ release assay. Results of 1 representative experiment of 2 performed are shown.
Peptide sensitivity of in vitro–induced ALK-specific CTLs.
T2 cells were incubated with titrated amounts of p280-89 (A) and p376-85 (B) peptides as well as influenza matrix peptide (FLU) and were then used as stimulator in an IFN-γ release assay. Results of 1 representative experiment of 2 performed are shown.
Taken together these results indicate that ALK peptides, p280-89 and p376-85, previously tested for their ability to be immunogenic in HHD mice, were also able to mobilize a specific CTL repertoire in PBLs from healthy donors. Moreover, dose-response data indicated a high and a moderate affinity of recognition for p280-89– and p376-85–generated CTLs, respectively, providing further evidence that ALK kinase is immunogenic and not subject to tolerance mechanisms.
Lysis of tumor cell lines
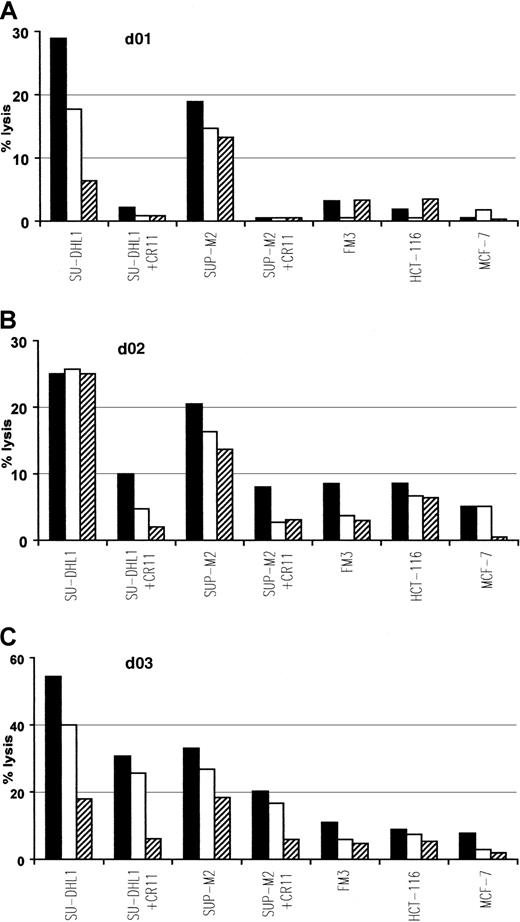
To determine if ALK-derived peptides, demonstrated to be immunogenic in vivo and in vitro, were also naturally processed on human tumor cells, we examined the ability of p280-89– and p376-85–specific CTLs to lyse HLA-A2.1+ ALCL lymphoma cell lines, SU-DHL1 and SUP-M2, expressing the chimeric protein NPM/ALK. ALK-specific CTLs killed these tumor lines and their lytic activity was inhibited by the presence of anti-HLA-A2 mAb CR11.351. No lysis above background level was observed in HLA-A2.1+/ALK− FM3 (melanoma), HCT-116 (colon carcinoma), and MCF-7 (breast carcinoma) human tumor lines expressing irrelevant antigens (Figure4).
Specific lysis of ALCL cell lines endogenously expressing NPM/ALK.
The p280-89–specific CTL lines generated from donors 01 and 02 (A,B) and p376-85–specific CTL line generated from donor 03 (C) efficiently recognize and lyse HLA-matched ALCL lymphoma cell lines (SU-DHL1 and SUP-M2) endogenously expressing NPM/ALK. Lysis was inhibited in the presence of anti-HLA-A2 mAb CR11.351 and no activity was detected against HLA-A2.1+ cell lines FM3 (melanoma), HCT-116 (colon carcinoma), and MCF-7 (breast carcinoma). Cytotoxic activity was determined in a standard 51Cr release assay at an E/T ratio of 50:1 (▪), 25:1 (■), 12:1(▤). Results of 1 representative experiment of at least 3 performed are shown.
Specific lysis of ALCL cell lines endogenously expressing NPM/ALK.
The p280-89–specific CTL lines generated from donors 01 and 02 (A,B) and p376-85–specific CTL line generated from donor 03 (C) efficiently recognize and lyse HLA-matched ALCL lymphoma cell lines (SU-DHL1 and SUP-M2) endogenously expressing NPM/ALK. Lysis was inhibited in the presence of anti-HLA-A2 mAb CR11.351 and no activity was detected against HLA-A2.1+ cell lines FM3 (melanoma), HCT-116 (colon carcinoma), and MCF-7 (breast carcinoma). Cytotoxic activity was determined in a standard 51Cr release assay at an E/T ratio of 50:1 (▪), 25:1 (■), 12:1(▤). Results of 1 representative experiment of at least 3 performed are shown.
Thus, it can be concluded that ALK peptide–induced CTLs efficiently lyse lymphoma targets, in a HLA-A2–restricted fashion, by relevant antigen-specific recognition.
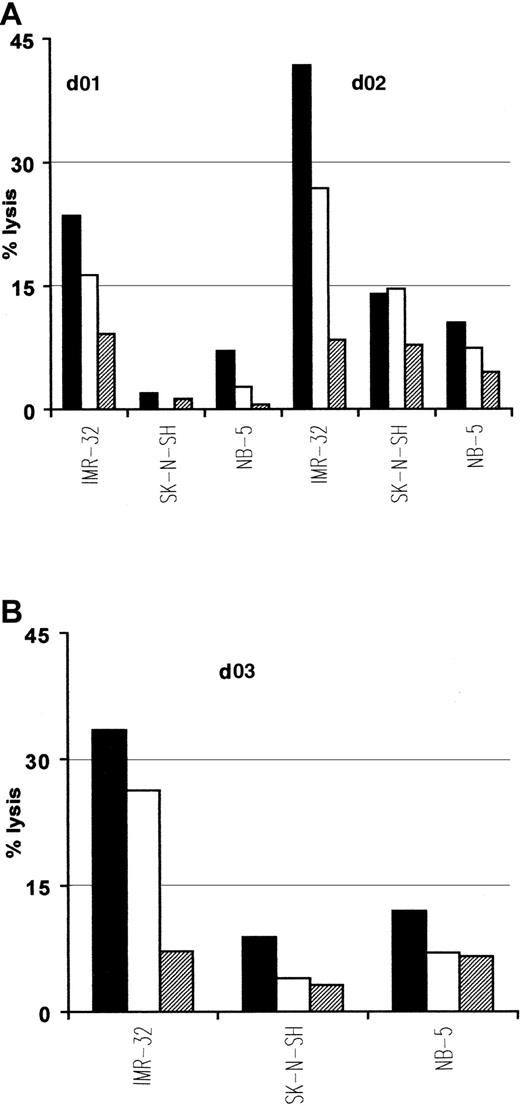
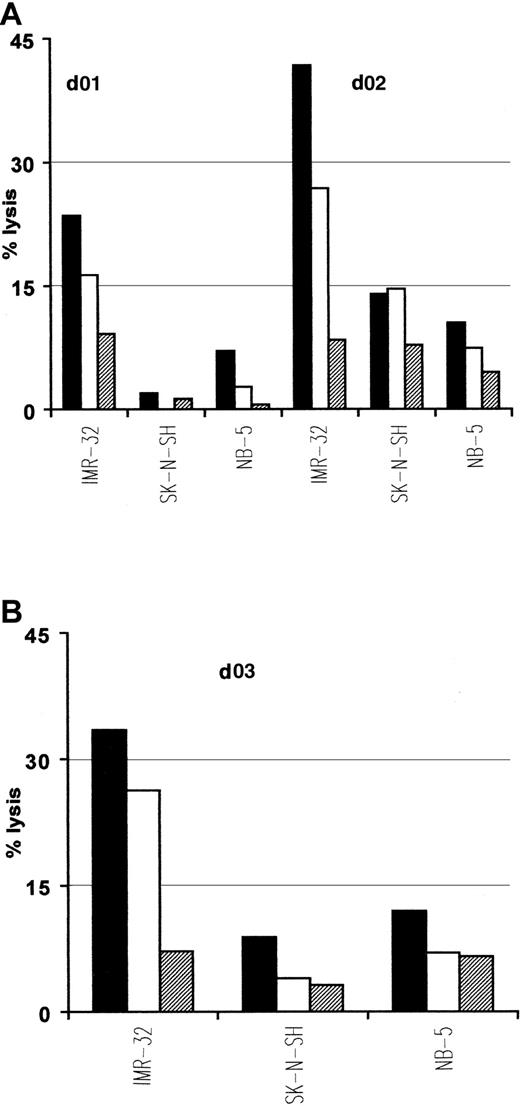
Interestingly, native ALK tyrosine kinase expression has also been demonstrated in cell lines and tumors of neural origins such as neuroblastoma.30 Because p280-89 and p376-85 peptides lie within the kinase domain, which is present in both native and translocated ALK, we used neuroblastoma cell lines as targets in cytotoxic experiments to further verify the natural processing of these 2 epitopes. Due to the limited expression of class I MHC molecules characterizing neuroblastomas that could interfere with the antigen recognition by CTLs, we treated tumor cells with INF-γ before their use as targets. p280-89– and p376-85–specific CTLs efficiently lysed HLA-A2.1+/ALK+ neuroblastoma cell line IMR-32. No lysis was detected either for HLA-A2.1−/ALK+ neuroblastoma cell lines SK-N-SH or for HLA-A2.1+/ALK− NB-5 cell line (Figure 5).
Lysis of neuroblastoma cells expressing native ALK.
Anti-p280-89 (A) and p375-86 (B) CTL lines recognize and kill in an HLA-restricted way neuroblastoma cells. IMR-32 (HLA-A2.1+/ALK+), SK-N-SH (HLA-A2.1−/ALK+), and NB-5 (HLA-A2.1+/ALK−) cell lines were used as targets in a standard 51Cr release assay. E/T ratio: 50:1 (▪), 25:1 (■), 12:1(▤). Results of 1 representative experiment of at least 3 performed are shown.
Lysis of neuroblastoma cells expressing native ALK.
Anti-p280-89 (A) and p375-86 (B) CTL lines recognize and kill in an HLA-restricted way neuroblastoma cells. IMR-32 (HLA-A2.1+/ALK+), SK-N-SH (HLA-A2.1−/ALK+), and NB-5 (HLA-A2.1+/ALK−) cell lines were used as targets in a standard 51Cr release assay. E/T ratio: 50:1 (▪), 25:1 (■), 12:1(▤). Results of 1 representative experiment of at least 3 performed are shown.
These results indicate that both p280-89 and p376-85 epitopes are efficiently processed and presented for CTL recognition at the cell surface of tumor cells and may therefore provide targets for antigen-specific immunotherapy.
Discussion
In this study, using a reverse immunology approach, we identified 2 new tumor-associated CTL epitopes (p280-89 and p376-85) derived from the ALK tyrosine kinase domain, which (1) bind to HLA-A2.1 molecules, (2) are immunogenic in vivo in the HLA-A*0201 transgenic mice, (3) stimulate in vitro–specific CTL responses using PBLs from healthy donors, and (4) are spontaneously processed and presented at the surface of tumor cells.
ALK is a receptor tyrosine kinase identified for the first time by Morris et al6 as part of the fusion protein NPM/ALK in ALCL-derived cell lines. NPM/ALK contains the NPM oligomerization domain and the intracytoplasmic region of ALK. NPM/ALK homodimerization determines the constitutive activation of the ALK catalytic domain in the transformed cell. It has been demonstrated that constitutive activation of ALK plays a key role in lymphomagenesis, by aberrant phosphorylation of intracellular substrates that likely contributes to the molecular pathogenesis of ALCL.8,9,15 31
We postulate that ALK could represent an ideal tumor antigen mostly because it is not expressed by normal cells, except at low levels in some CNS cells.14 As a consequence, we expected that anti–ALK-specific CTL precursors were not physiologically eliminated by clonal deletion in the thymus and were not restricted by self-tolerance in the periphery. Following this hypothesis, ALK-derived peptides that experimentally showed strong HLA-A2.1 binding features and immunogenicity in HHD mice were tested for in vitro anti-ALK repertoire mobilization capacity from human circulating blood. Our results confirm the existence of an efficient anti-ALK precursor population present within the T-cell repertoire of healthy donors. Moreover, considering the relatively low starting number of PBLs (36 × 106) used to start each single sensitization experiment, such precursors may be present at high frequency. Given the known difficulty in generating immune responses in vitro, it is remarkable that the screening of only 3 healthy donors for each peptide induced a specific immune response in at least one donor, suggesting the strength of ALK in being immunogenic.
The anti-ALK–specific CTL repertoire is unlikely to react against normal CNS cells, because the CNS is an immunologically privileged site where, under physiologic conditions, antigens are not exposed to the immune system. More importantly, the scarce level of expression in normal CNS is most likely too low to ensure CTL recognition as shown with human MAGE-specific32 and murine p53-specific33 CTLs. In contrast, the high level of expression of NPM/ALK, driven by the strong NPM promoter, should result in a substantial amount of NPM/ALK processing by the proteosome, thereby leading to a significant expression of ALK-derived epitopes at the cell surface. This should make the immune response against ALK selective for malignant cells.
The down-regulation of tumor antigen expression represents a problem for the development of immunotherapies because of the possible immunoselection of nonexpressing tumor clones. Because ALK is required for neoplastic transformation of lymphoid cells, it should be unlikely to observe tumor escape phenomena by ALK antigen loss variants.
Translocated ALK is expressed in approximately 83% and 31% of children and adults with ALCL, respectively,34 indicating that ALK is a stable lymphoma-specific hallmark. Recently, ALK+ lymphoma or “Alkoma” has been recognized as a distinct tumor entity.35 ALK broad expression not restricted to one or few patients, as in the case of point-mutated oncogenes, should make its hypothetical use in vaccination protocols more easily and widely applicable.
Finally, we demonstrated that ALK could be targeted for killing of ALK+ neuroblastoma tumor lines. Despite a lower level of ALK expression compared with ALCL and an unclear involvement in the transformed phenotype,30 our results demonstrate that neuroblastoma cell lines can still be efficiently lysed by ALK-specific CTLs. The killing of neuroblastoma cells by a CTL response specific for ALK epitopes reveals the potential of ALK as a shared tumor antigen for multiple malignancies.
In conclusion, ALK kinase is a novel tumor-specific antigen able to induce an antigen-specific CTL response in humans. Two novel ALK-derived epitopes (p280-89 and p376-85) were identified. We propose that these CTL epitopes could serve as good candidates for the design of future immunotherapy strategies of ALK-expressing tumors such as ALCL and neuroblastoma. ALK immunogenicity could be linked to the more favorable prognosis, with a better 5-year survival rate observed in ALK+ ALCL patients compared with ALK− ALCL cases.36-38 Circulating antibodies recognizing NPM/ALK were detected in ALCL patient sera.24 However, how and whether the immune system contributes to the relatively good prognostic outlook of ALK+ ALCL patients will require further investigations.
The contributions of Drs G. Parmiani, R. H. Gunby, P. Dalerba (Istituto Nazionale Tumori, Milan, Italy) and Dr S. Morris (St. Jude Children's Hospital, Memphis, TN) in critically reading the manuscript are gratefully acknowledged. We thank Edoardo Marchesi (Istituto Nazionale Tumori, Milan, Italy) for the excellent technical contribution, S. Ragsdale (St. Jude Children's Hospital, Memphis, TN) for providing the NB neuroblastoma cell lines, Dr F. Arienti (Istituto Nazionale Tumori, Milan, Italy) for supplying us with several buffy coats, and Dr C. Russo (Merck Research Laboratories, West Point, PA) for the anti-HLA-A2 mAb CR11.351.
Supported by grants from Italian Association for Cancer Research (AIRC, Milan), European Community (EUCAPS program), INSERM (PROGRES program), and “Fondation pour la Recherche Medicale.”
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Lorena Passoni, Oncogenic Fusion Genes and Proteins Unit, Istituto Nazionale Tumori, Via Venezian 1, 20133 Milan, Italy; e-mail: passoni@istitutotumori.mi.it.