Abstract
Neurotrophins, such as nerve growth factor (NGF) and neurotrophin-3 (NT-3), are essential for development, function, and survival of peripheral sympathetic and sensory neurons. Most eosinophilic leukocytes in the human body are localized in mucosal tissues; however, the roles of eosinophils in human diseases are not fully understood. We found that human eosinophils constitutively express messenger RNA for NGF and NT-3, synthesize and store these proteins intracellularly, and continuously replenish them. Incubation of eosinophils with a transcription inhibitor, actinomycin D, for 8 hours completely depletes intracellular NGF and NT-3. New synthesis of NGF is enhanced by Fc-receptor–mediated stimuli, such as immunoglobulin (Ig)A and IgG immune complexes; in contrast, production of NT-3 is not affected by these stimuli. Notably, supernatants of eosinophils stimulated with IgA immune complex and interleukin 5 promote neurite extension of the PC-12 pheochromocytoma cell line; this effect is abolished by pretreatment of the supernatants with anti-NGF–neutralizing antibody. By enzyme-linked immunosorbent assay, substantial amounts of NGF protein are also detected in the supernatants of stimulated eosinophils. Furthermore, in patients with seasonal allergic rhinitis, the concentrations of NGF in nasal secretions correlate with the magnitudes of eosinophilic inflammation in the airway, suggesting a potential clinical implication of eosinophil NGF. Our observations propose a new pathologic mechanism by which eosinophils may contribute to enhanced neurologic responses in patients with allergic diseases and other eosinophilic disorders. Alternatively, eosinophils may play important roles in maintenance and restoration of homeostatic functions of mucosal tissues through the pleitropic activities of NGF.
Introduction
Eosinophils are leukocytes associated with helminth infection and allergic diseases (reviewed in Kita et al1). Although the eosinophil is a formed element of the peripheral circulation, it primarily resides in the tissues. More than 99% of eosinophils in the human body are distributed in the mucosal tissues in which the epithelial surfaces are exposed to the external environment.1 However, the physiologic or pathologic roles of tissue-resident eosinophils in host defense and human diseases are not fully understood. Eosinophils release a number of proinflammatory mediators, such as cytotoxic cationic proteins and lipid mediators, which could be important in the pathophysiology of asthma and allergic disease.1 Several lines of evidence, many from correlative studies, have also implicated eosinophils in the mechanisms of heightened neurologic responses in diseases, especially in bronchial asthma2-4 and atopic dermatitis.5 Indeed, airway eosinophilic inflammation and increased bronchial responsiveness to bronchoconstrictive stimuli are hallmarks of human bronchial asthma.2-4 Eosinophils are also potential sources of cytokines and growth factors.6 These factors include autocrine cytokines, such as granulocyte-macrophage colony-stimulating factor7 and interleukin-5 (IL-5);8immunomodulatory cytokines, such as IL-49,10 and IL-10;10 chemokines and chemotactic cytokines, such as regulated on activation, normal T cell–expressed and –secreted11 and IL-16;12 and factors involved in fibrosis and tissue repair, such as tumor growth factor (TGF)–α,13 TGF-β,14 and vascular endothelial cell growth factor/vascular permeability factor.15 Although the importance of eosinophils as a source of cytokines and growth factors remains to be determined, eosinophils could influence inflammatory reactions and other biologic responses through a broader spectrum of mechanisms than previously appreciated.
In this study, we investigated whether eosinophils secrete the neurotrophic cytokines, nerve growth factor (NGF) and neurotrophin-3 (NT-3). NGF is essential for survival, development, and function of peripheral sympathetic and sensory neurons (reviewed in Levi-Montalcini16). Indeed, overexpression of NGF in the lungs of mice promotes innervation of tachykinin-containing sensory neurons, hyperreactivity to capsaicin, and thickening of the basement membrane in the airways.17 Increased expression and release of NGF were also found in patients with allergic rhinitis18 and are implicated in the development of neural hyperresponsiveness in patients.19 In addition to its neurotrophic activity, NGF exerts broad biologic activities on nonneuroral cells involved in innate and adaptive immune systems, such as lymphocytes,20 mast cells,21basophils,22 and eosinophils themselves.23Furthermore, NGF also promotes hemopoietic colony growth24and may participate in wound healing and the tissue repair process.25,26 NGF was shown to be expressed by various cells.23,27 28 However, little is currently known about any cell type that could produce NGF or NT-3 on immunologically specific activation. Furthermore, our knowledge about the regulatory mechanisms that produce these essential NGFs is limited. In this study, we found that human eosinophils constitutively synthesize NGF and NT-3. Moreover, when the cells are activated with inflammatory stimuli, they secrete substantial amounts of NGF and promote the development of neuronal cells by NGF activity.
Materials and methods
Isolation of eosinophils, neutrophils, and mononuclear cells
Eosinophils were purified from peripheral blood obtained from healthy volunteers by negative selection using anti-CD16–bound immunomagnetic beads and a magnetic cell sorting system.29The preparations were more than 98% eosinophils, and the contaminating cells were neutrophils. All the isolation procedures were performed at 4°C or on ice to avoid eosinophil activation during isolation. Neutrophils and peripheral blood mononuclear cells (PBMCs) were isolated from peripheral blood of the same eosinophil donors and were used as reference cells for eosinophils. For that purpose, neutrophils were isolated by using a gradient material, 1-Step Polymorphs (Accurate Chemical & Scientific Corp, Westbury, NY), following the procedure recommended by the manufacturer. PBMCs were isolated by density centrifugation using Histopaque (Sigma Chemical, St Louis, MO; 1.077 g/mL). The purities of the neutrophil and PBMC preparations were more than 96%. All isolated cells were used immediately. The human mast cell line, HMC-1,30 was used as a positive control for reverse transcriptase–polymerase chain reaction (RT-PCR) analyses of NGF and NT-3 messenger RNA (mRNA). The HMC-1 cell line was maintained in Iscoves modified Dulbecco medium supplemented with 10% fetal calf serum, 2 mM l-glutamine, 100 IU/mL penicillin, and 50 μg/mL streptomycin (Sigma) with passage every 3 to 4 days.
Detection of NGF and NT-3 mRNA by RT-PCR
Total RNA was prepared from 2 × 106 cells of freshly isolated eosinophils and HMC-1 cells using TRIzol Reagent (GIBCO BRL/Life Technologies, Gaithersburg, MD). The isopropanol-precipitated RNA was washed with 75% ethanol, dried, resuspended in 12 μL oligo-dT (20 μg/mL; Mayo Molecular Biology Core Facility, Rochester, MN), and incubated at 65°C for 6 minutes. The RT reactions were performed by using 25 U avian myeloblastosis virus RT in 20 μL reaction mixture. Complementary DNA was amplified from a 20-μL reaction mixture in a thin-wall tube containing 1 UTaq DNA polymerase (Boehringer Mannheim, Indianapolis, IN). Primers for NGF, NT-3, and β2 microglobulin (β2-MG) were made by Mayo Clinic Core facility according to the published primer sequences31 32: NGF, 5′-CACTCAGGATCTGGACTTCGAGG-3′ (5′ primer) and 5′-CGGCAGGTCAGGCTCTTCTCAA-3′ (3′ primer), amplified product 437 base pair (bp); NT-3, 5′-ATCTTACAGGTGAACAAGGT-3′ (5′ primer) and 5′-TCGGTGACTCTTATGCTCCG-3′ (3′ primer), amplified product 459 bp; β2-MG, 5′CTCGCGCTACTCTCTCTTTCTGG3′ (5′ primer) and 5′GCTTACATGTCTCGATCCCACTTAA3′ (3′ primer), amplified product 320 bp.
The specificities of the amplified products were confirmed by dot blot analyses using internal oligonucleotide probes (data not shown). PCR was performed in a thermal cycler for 35 cycles (94°C for 30 seconds, 60°C for 30 seconds, and 72°C for 90 seconds), followed by a 7-minute extension at 72°C. Five microliters of the PCR products with 1 μL 6× DNA loading buffer was subject to electrophoresis on 1.5% agarose gels and stained with 0.5 μg/mL ethidium bromide.
Activation of eosinophils
Eosinophils were suspended in RPMI 1640 medium supplemented with 25 mM HEPES and l-glutamine (Celox Laboratories, Hopkins, MN) containing 10% (vol/vol) 56°C heat-inactivated defined calf serum (RPMI-DCS; HyClone Laboratories, Logan, UT). Soluble immune complex in the presence or absence of interleukin-5 (IL-5) was used to stimulate eosinophils as previously described.8,9 33Briefly, eosinophils suspended in RPMI-DCS at 1 × 106cells/mL were incubated in 48-well tissue culture plates with or without human secretory immunoglobulin A (sIgA; ICN Pharmaceutical, Aurora, OH), human serum IgA (ICN Pharmaceutical), or human serum IgG (ICN Pharmaceutical) at a final concentration of 20 μg/mL. After a 1-hour incubation at 4°C and without washing, eosinophils were stimulated with corresponding antihuman sIgA monoclonal antibody (diluted 1:1000; Sigma), goat antihuman IgA (20 μg/mL; ICN Pharmaceutical), or goat antihuman IgG (20 μg/mL; ICN Pharmaceutical) and cultured in the presence or absence of 25 ng/mL IL-5 at 37°C and 5% CO2 for 24 hours. After incubation, cell-free supernatants were collected and stored at −20°C until used for bioassay of NGF activity and for NGF protein assay (see below). To quantitate intracellular contents of NGF, NT-3, and an eosinophil granule protein, eosinophil-derived neurotoxin (EDN), eosinophil cell pellets were lysed with 1 mL 1% Triton-X in phosphate-buffered saline (PBS) and 3 freeze-thaw cycles. To quantitate intracellular NGF, NT-3, and EDN, eosinophils were cultured without stimuli and either no inhibitor or with the transcription inhibitor, actinomycin D (1 μM; Sigma), for up to 8 hours and lysed. By trypan blue dye exclusion, eosinophil viability after 8 hours of treatment with actinomycin D was more than 96%.
Measurement of NGF, NT-3, and EDN proteins
To measure NGF and NT-3 proteins in cell lysates and supernatants, we used commercial enzyme-linked immunosorbent assay (ELISA) kits (Promega Corporation, Madison, WI) according to the manufacturer's recommended procedure with the standard curve slightly modified. The specificities of the assay were confirmed by the manufacturer, and the low end of the standard curve was 3.9 pg/mL. Eosinophil granule protein, EDN, in cell lysates was quantitated by radioimmunoassay (RIA). The RIA is a double antibody competition assay performed by using radioiodinated EDN, rabbit anti-EDN antibody, and burro antirabbit IgG.34 The minimum detection limit of this EDN assay was 2 ng/mL. All assays were performed in duplicate.
Biologic assay of NGF activity
The bioassay using the PC-12 pheochromocytoma cell line for NGF activity is commonly used to detect and to measure biologically active NGF, which stimulates neurite outgrowth of PC-12 cells.35This cell line does not respond to NT-3.35 The PC-12 cell line (ATCC, Manassas, VA) was expanded and maintained in complete medium consisting of 85% F-12 medium, 10% heat-inactivated horse serum, and 5% fetal calf serum as previously described.36To detect and quantitate the biologic activity of NGF in eosinophil supernatants, PC-12 cells were replated onto collagen-coated 24-well tissue culture plates at 2 × 104 cells/well, and eosinophil culture supernatants or serial dilutions of NGF as controls were added to the wells. After 24 hours, the numbers of PC-12 cells with neurite outgrowth were counted by using the dark field inverted microscope (Nikon). Cells with at least 2 neurites and each neurite more than about 50 μm long were judged as neurite outgrowth–positive cells. All the samples were tested in duplicate, and the enumeration was performed in a blinded manner. The specificity of the assay was confirmed by adding neutralizing goat antihuman NGF antibody (100 ng/mL; R&D Systems) or goat IgG (Sigma) to the replicate samples.
Immunocytochemistry
Immunocytochemistry for NGF and NT-3 in eosinophil sections was performed with goat antibodies for these proteins and fluorescein isothiocyanate (FITC)–conjugated rabbit antigoat IgG. Briefly, freshly isolated eosinophils were collected and mixed with 1% agar in PBS and then fixed in 4% freshly prepared paraformaldehyde, processed, and embedded in paraffin as previously described.37Five-micrometer sections were mounted on microscope slides, deparaffinized with xylene, and rehydrated. After partial digestion with 0.1% trypsin (Sigma), the slides were blocked with 10% normal rabbit serum overnight at 4°C. Serial sections were incubated with goat antihuman NGF antibody (5 μg/mL; R&D Systems), goat antihuman NT-3 antibody (5 μg/mL; R&D Systems), or normal goat IgG (5 μg/mL) at 37°C for 30 minutes. The slides were washed and blocked with 1% chromotrope 2R (EM Science, Cherry Hill, NJ). Sections were then incubated with affinity-purified FITC-conjugated rabbit antigoat IgG (Southern Biotechnology Associates, Birmingham, AL) at 37°C for 30 minutes. Sections were washed and mounted with 10% PBS:90% glycerol solution containing p-phenylenediamine, coverslipped, and sealed. The fluorescence images were captured with a confocal laser-scanning microscope (LSM 310; Carl Zeiss, Oberkochen, Germany) at a pinhole setting of 50. The excitation wavelength from an argon/krypton laser was set to 488 nm, and the emission wavelength was set to 530 ± 15 nm. Transmission images were also captured from the same field to visualize the morphology of the cells.
NGF and NT-3 levels in nasal lavage fluids from patients with ragweed hay fever during allergy season
Fifteen patients, aged 18 to 60 years, with moderate to severe ragweed allergic rhinitis were enrolled in the study. Patients had histories of ragweed hay fever within the previous 2 years, positive skin prick tests to ragweed extract (403 000 AU/mL) with wheal more than 8 mm, and elevated levels of ragweed-specific IgE antibody. Patients were not taking any form of glucocorticoid treatment within 1 month before the study. Nasal lavages were obtained from patients at the beginning of September when the ragweed hay fever season reaches a peak in Rochester, MN. Lavage was performed with the head tilted back by instilling 5 mL normal saline into one nostril, followed by bringing the head forward to collect the fluid in a 150-mL beaker. This procedure was performed twice in each nostril using a total of 20 mL normal saline, and the recovery was regularly more than 80%. The nasal lavage fluid was passed through a 42-μm nylon mesh filter (Nitex; Tetko, Briarcliff Manor, NY), and the filtrate was centrifuged at 420g for 12 minutes at 4°C. The supernatant fluids were recovered and stored at 4°C for analysis of NGF, NT-3, and EDN by using ELISA or RIA as described above. The Institutional Review Board at the Mayo Clinic reviewed and approved this study.
Statistical analyses
All results are presented as mean ± SEM from the number of experiments indicated. Statistical significance of the differences was assessed with Student paired or unpaired t test. Correlations were assessed by using the Pearson or Spearman correlation of the InStat statistics package (GraphPad Software, San Diego, CA).
Results
Human eosinophils constitutively express NGF and NT-3 mRNA and synthesize these proteins
We used RT-PCR to investigate whether eosinophils express mRNA for interstitial cell growth factors. Eosinophils from all 6 donors constitutively and reproducibly expressed mRNA for TGF-β1,10 TGF-β2 (data not shown), NGF, and NT-3 (this report). In Figure 1A, human mast cell line, HMC-1 cells, expressed NT-3 and NGF mRNA, as shown previously.27 Furthermore, consistent with previous findings,23 freshly isolated human eosinophils constitutively expressed mRNA for NT-3 and NGF. The control, PCR of eosinophil RNA preparations without RT reaction, showed no detectable signals (data not shown). As shown in Figure 1B, these mRNAs were expressed in other eosinophil donors.
RT-PCR analysis of eosinophil neurotrophin gene expression.
(A) Total mRNA was extracted from 2 × 106 freshly isolated eosinophils or cultured HMC-1 cells. The RNA was reverse transcribed to complementary DNA and amplified with specific primers for NGF, NT-3, or β2-MG. A 35-cycle PCR was performed. The products were electrophoresed on a 3% agarose gel followed by staining with ethidium bromide. (B) The experiments were repeated 3 times using eosinophil preparations from 3 different donors. Amplified complementary DNA bands are compatible with the size of estimated PCR products for NGF (437 bp), NT-3 (459 bp), and β2-MG (positive control, 320 bp).
RT-PCR analysis of eosinophil neurotrophin gene expression.
(A) Total mRNA was extracted from 2 × 106 freshly isolated eosinophils or cultured HMC-1 cells. The RNA was reverse transcribed to complementary DNA and amplified with specific primers for NGF, NT-3, or β2-MG. A 35-cycle PCR was performed. The products were electrophoresed on a 3% agarose gel followed by staining with ethidium bromide. (B) The experiments were repeated 3 times using eosinophil preparations from 3 different donors. Amplified complementary DNA bands are compatible with the size of estimated PCR products for NGF (437 bp), NT-3 (459 bp), and β2-MG (positive control, 320 bp).
Eosinophils characteristically store many eosinophil-derived cytokines and growth factors, providing a preformed pool of cytokines available for release (reviewed in Lacy and Moqbel38). Therefore, we examined eosinophil storage of NGF and NT-3 proteins and compared these stored amounts with those in neutrophils and PBMCs. Freshly isolated eosinophils contained 127.3 ± 40 pg/106 cells and 112 ± 21.6 pg/106 cells of NGF and NT-3, respectively, within or associated with the cells (mean ± SEM, n = 4). In contrast, both NGF and NT-3 were undetectable in neutrophils. PBMCs contained a small amount (5.8 ± 3.2 pg/106 cells) of NGF, but NT-3 was undetectable, suggesting that NGF and NT-3 are uniquely stored in eosinophils.
Immunocytochemistry and confocal microscopy were used to localize stored NGF in eosinophils. As shown in Figure2, eosinophil sections stained with anti-NGF antibody revealed a diffusely labeled cytoplasm, vesicular staining within the cells, and intense staining on or beneath the cell membrane. Sections stained with control immunoglobulin (goat IgG) yielded no or barely visible staining (data not shown).
Immunocytochemistry for NGF in eosinophil sections.
Freshly isolated eosinophils were collected and mixed with 1% agar and embedded in paraffin. Five-micrometer sections were blocked with normal rabbit serum and incubated with goat antihuman NGF antibody, followed by additional blocking with chromotrope 2R. Bound antibody was then visualized by affinity-purified FITC-conjugated rabbit antigoat IgG. The fluorescence images (upper panel) were captured with a confocal laser-scanning microscope. Transmission images (lower panel) were also captured from the same field to visualize the morphology of the cells. Note that the fluorescence image is captured from an approximately 1-μm optical section and that the transmission image is from a whole 5-μm section. Original magnification × 600.
Immunocytochemistry for NGF in eosinophil sections.
Freshly isolated eosinophils were collected and mixed with 1% agar and embedded in paraffin. Five-micrometer sections were blocked with normal rabbit serum and incubated with goat antihuman NGF antibody, followed by additional blocking with chromotrope 2R. Bound antibody was then visualized by affinity-purified FITC-conjugated rabbit antigoat IgG. The fluorescence images (upper panel) were captured with a confocal laser-scanning microscope. Transmission images (lower panel) were also captured from the same field to visualize the morphology of the cells. Note that the fluorescence image is captured from an approximately 1-μm optical section and that the transmission image is from a whole 5-μm section. Original magnification × 600.
To examine whether stored NGF and NT-3 proteins have been phagocytized or newly synthesized by blood eosinophils, isolated eosinophils were cultured in vitro in the presence of the transcription inhibitor, actinomycin D,39 for up to 8 hours. A 4-hour treatment with actinomycin D reduced NGF and NT-3 by 86% and 80%, respectively, compared with cells without actinomycin D. Furthermore, an 8-hour treatment with actinomycin D almost depleted intracellular NGF and NT-3 (95% reduction of both NGF and NT-3). The estimated half-lives of NGF and NT-3 in the presence of actinomycin D were short, 1.5 hours and 1.8 hours, respectively. Thus, these experiments suggest that mature peripheral blood eosinophils continuously transcribe mRNA for and synthesize and store NGF and NT-3 proteins.
Synthesis of NGF protein is enhanced by immunologic stimuli
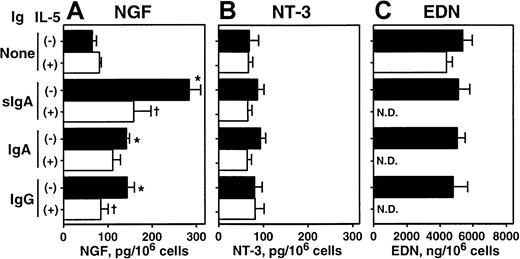
We further investigated whether this synthesis and storage of NGF and NT-3 proteins are regulated by immunologic stimuli. IL-5 has multiple effects on eosinophils, including maturation, survival, and activation, and it is considered an important cytokine in eosinophilic inflammation and allergic disorders (reviewed in Costa et al40 and Cuss41). Eosinophils express receptors for IgA and IgG, namely FcαR42 and FcγRII43; the sIgA immune complex is a potent agonist for eosinophil cytokine synthesis.8,9 33 As shown in Figure3A, eosinophils incubated for 24 hours with sIgA–anti-sIgA immune complexes, but without IL-5, showed a 3-fold increase in intracellular NGF. IgA–anti-IgA and IgG–anti-IgG immune complexes also doubled intracellular NGF, suggesting that immune complexes stimulate production and accumulation of NGF. IL-5 by itself slightly increased intracellular NGF. Interestingly, combinations of IL-5 and immune complexes significantly reduced the intracellular NGF compared with immune complexes alone (P < .05 in sIgA and IgG), suggesting that these combinations either induce extracellular secretion of NGF protein or inhibit the production of NGF. This question was addressed subsequently with a PC-12 cell bioassay and protein ELISA (see below). In contrast to NGF, the amount of NT-3 was neither increased nor decreased by immune complexes, IL-5, or their combinations (Figure 3B). Furthermore, the intracellular content of preformed granule protein, EDN, was not affected by immune complexes (Figure 3C).
Effects of cellular stimulation with immune complexes and/or IL-5 on intracellular NGF and NT-3.
Eosinophils were suspended in RPMI 1640 medium supplemented with 10% DCS and incubated with medium alone or 20 μg/mL sIgA, serum IgA, or serum IgG for 1 hour at 4°C. Cells were then stimulated with the corresponding antihuman sIgA monoclonal antibody, goat antihuman IgA, or goat antihuman IgG and cultured in the absence (closed columns) or presence (open columns) of 25 ng/mL IL-5 at 37°C for 24 hours. After culture, cells were collected and lysed with 1% Triton-X and 3 freeze-thaw cycles. The amounts of NGF (A), NT-3 (B), and EDN (C) in cell lysates were measured by ELISA or RIA. The data show mean ± SEM from 3 experiments. *denotes significant difference from the values cultured with medium alone (None) (P < .05); †denotes significant difference from the values cultured with the same stimuli but without IL-5 (P < .05). ND, not determined.
Effects of cellular stimulation with immune complexes and/or IL-5 on intracellular NGF and NT-3.
Eosinophils were suspended in RPMI 1640 medium supplemented with 10% DCS and incubated with medium alone or 20 μg/mL sIgA, serum IgA, or serum IgG for 1 hour at 4°C. Cells were then stimulated with the corresponding antihuman sIgA monoclonal antibody, goat antihuman IgA, or goat antihuman IgG and cultured in the absence (closed columns) or presence (open columns) of 25 ng/mL IL-5 at 37°C for 24 hours. After culture, cells were collected and lysed with 1% Triton-X and 3 freeze-thaw cycles. The amounts of NGF (A), NT-3 (B), and EDN (C) in cell lysates were measured by ELISA or RIA. The data show mean ± SEM from 3 experiments. *denotes significant difference from the values cultured with medium alone (None) (P < .05); †denotes significant difference from the values cultured with the same stimuli but without IL-5 (P < .05). ND, not determined.
Eosinophils secrete biologically active NGF on immunologic stimuli
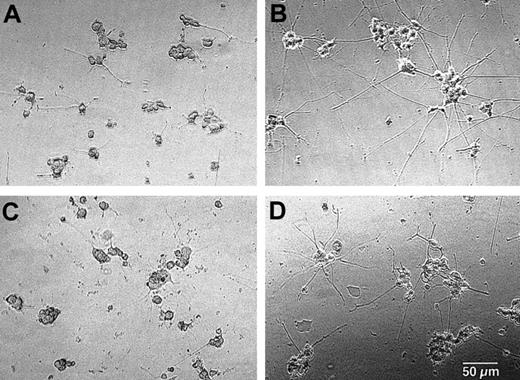
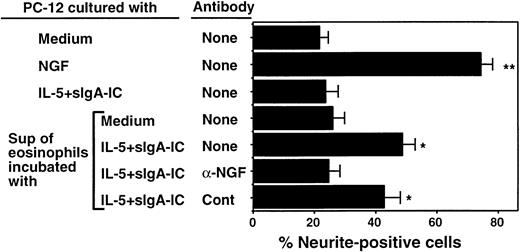
To address whether NGF is secreted by activated eosinophils and whether NGF release by eosinophils is biologically relevant, we cultured PC-12 cells with supernatants of eosinophils stimulated with IL-5 and sIgA immune complexes. As shown in Figures4A and 5, 22% of PC-12 cells cultured with medium alone expressed at least 2 neurites ≥ 50 μm. NGF, at 2 ng/mL, strikingly promoted neurite outgrowth of PC-12 cells, resulting in 76% of cells expressing neurites (Figures 4B, 5). When supernatants of eosinophils incubated with IL-5 and sIgA immune complex for 24 hours were added to PC-12 cells instead of NGF, they significantly increased the number of neurite-positive cells (49% ± 4%, n = 5, P < .05; Figures 4D, 5). In contrast, supernatants from eosinophils incubated with medium alone (Figures 4C, 5) or sIgA immune complex plus IL-5 without eosinophils (Figure 5) did not promote neurite outgrowth. Furthermore, the effects of supernatants from eosinophils incubated with IL-5 and sIgA were abolished by anti-NGF antibody but not by control antibody (Figure 5). Thus, supernatants from eosinophils incubated with IL-5 plus sIgA immune complex likely contain biologically active NGF that can stimulate neurite elongation of PC-12 cells.
Supernatants from activated eosinophils promote elongation of neurites in PC-12 cells.
Eosinophils were suspended in RPMI 1640 medium supplemented with 10% DCS and incubated with medium alone or sIgA (20 μg/mL) and antihuman sIgA monoclonal antibody (1:10 000) plus 25 ng/mL IL-5 for 24 hours at 37°C. After culture, cell-free supernatants were collected. PC-12 cells were incubated with medium alone (A), 2 ng/mL NGF (B), supernatant of eosinophils cultured with medium alone (C), or supernatant of eosinophils cultured with sIgA immune complex and IL-5 (D) for 24 hours. The cell morphology was examined under the inverted microscope. Bar = 50 μm. Original magnification × 100.
Supernatants from activated eosinophils promote elongation of neurites in PC-12 cells.
Eosinophils were suspended in RPMI 1640 medium supplemented with 10% DCS and incubated with medium alone or sIgA (20 μg/mL) and antihuman sIgA monoclonal antibody (1:10 000) plus 25 ng/mL IL-5 for 24 hours at 37°C. After culture, cell-free supernatants were collected. PC-12 cells were incubated with medium alone (A), 2 ng/mL NGF (B), supernatant of eosinophils cultured with medium alone (C), or supernatant of eosinophils cultured with sIgA immune complex and IL-5 (D) for 24 hours. The cell morphology was examined under the inverted microscope. Bar = 50 μm. Original magnification × 100.
Effect of eosinophil supernatants to promote neurite elongation is neutralized by anti-NGF antibody.
Eosinophils were suspended in RPMI 1640 medium supplemented with 10% DCS and incubated with medium alone or sIgA (20 μg/mL) + antihuman sIgA monoclonal antibody (1:10 000) + 25 ng/mL IL-5 (IL-5 + sIgA-IC) for 24 hours at 37°C. The replicate culture contained sIgA, antihuman sIgA monoclonal antibody, and IL-5 but no eosinophils. After culture, cell-free supernatants were collected. PC-12 cells were incubated with medium alone, 2 ng/mL NGF, supernatant of sIgA + anti-sIgA + IL-5 without eosinophils, supernatant of eosinophils cultured with medium alone, or supernatant of eosinophils cultured with sIgA + anti-sIgA + IL-5. Replicate eosinophil supernatants were treated with 100 ng/mL anti-NGF antibody or control goat IgG (cont). After 24 hours, the numbers of PC-12 cells with at least 2 neurites approximately 50 μm or longer were counted by using the dark field inverted microscope. Data show mean ± SEM from 5 experiments. * and ** denote significant difference from PC-12 cells incubated with medium alone (P < .05 andP < .01, respectively).
Effect of eosinophil supernatants to promote neurite elongation is neutralized by anti-NGF antibody.
Eosinophils were suspended in RPMI 1640 medium supplemented with 10% DCS and incubated with medium alone or sIgA (20 μg/mL) + antihuman sIgA monoclonal antibody (1:10 000) + 25 ng/mL IL-5 (IL-5 + sIgA-IC) for 24 hours at 37°C. The replicate culture contained sIgA, antihuman sIgA monoclonal antibody, and IL-5 but no eosinophils. After culture, cell-free supernatants were collected. PC-12 cells were incubated with medium alone, 2 ng/mL NGF, supernatant of sIgA + anti-sIgA + IL-5 without eosinophils, supernatant of eosinophils cultured with medium alone, or supernatant of eosinophils cultured with sIgA + anti-sIgA + IL-5. Replicate eosinophil supernatants were treated with 100 ng/mL anti-NGF antibody or control goat IgG (cont). After 24 hours, the numbers of PC-12 cells with at least 2 neurites approximately 50 μm or longer were counted by using the dark field inverted microscope. Data show mean ± SEM from 5 experiments. * and ** denote significant difference from PC-12 cells incubated with medium alone (P < .05 andP < .01, respectively).
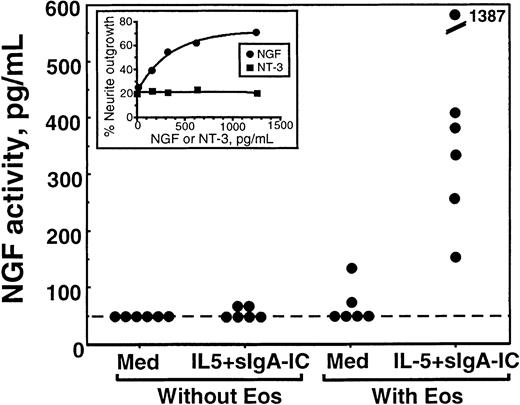
The NGF activity in eosinophil supernatants was semiquantitated by using a standard curve made by serial dilutions of recombinant NGF included in each experiment. As shown in Figure6, there was no detectable NGF activity in medium or medium containing IL-5 plus sIgA immune complex. When eosinophils were incubated without stimuli, only 1 of 6 eosinophil supernatants contained detectable NGF activity. In contrast, all 6 supernatants from eosinophils incubated with IL-5 plus sIgA immune complex contained strong NGF activity, varying from 150 to 1400 pg/mL (490 ± 200 pg/mL, mean ± SEM, n = 6). We were unable to do similar experiments for NT-3 because cell lines responding to NT-3 were not easily available.
Quantitation of NGF activity in supernatants from stimulated eosinophils.
Eosinophils were incubated with medium alone (med) or stimulated with sIgA + antihuman sIgA monoclonal antibody + IL-5 (IL-5 + sIgA-IC) for 24 hours as described above; the replicate culture contained sIgA + antihuman sIgA monoclonal antibody + IL-5, but no eosinophils. Supernatants were collected and used for the PC-12 cell biologic assay as described in “Materials and methods.” The NGF activities of the supernatants were semiquantitated by using a standard curve generated by PC-12 cells incubated with serial dilutions of recombinant NGF. Each dot represents different experiments using eosinophils from 6 different donors. Inset graph shows an example of a standard curve. PC-12 cells were incubated with serial dilutions of NGF (closed circle) or NT-3 (closed square) for 24 hours, and the numbers of cells with neurite elongation were determined. Note that NT-3 does not promote neurite elongation of PC-12 cells.
Quantitation of NGF activity in supernatants from stimulated eosinophils.
Eosinophils were incubated with medium alone (med) or stimulated with sIgA + antihuman sIgA monoclonal antibody + IL-5 (IL-5 + sIgA-IC) for 24 hours as described above; the replicate culture contained sIgA + antihuman sIgA monoclonal antibody + IL-5, but no eosinophils. Supernatants were collected and used for the PC-12 cell biologic assay as described in “Materials and methods.” The NGF activities of the supernatants were semiquantitated by using a standard curve generated by PC-12 cells incubated with serial dilutions of recombinant NGF. Each dot represents different experiments using eosinophils from 6 different donors. Inset graph shows an example of a standard curve. PC-12 cells were incubated with serial dilutions of NGF (closed circle) or NT-3 (closed square) for 24 hours, and the numbers of cells with neurite elongation were determined. Note that NT-3 does not promote neurite elongation of PC-12 cells.
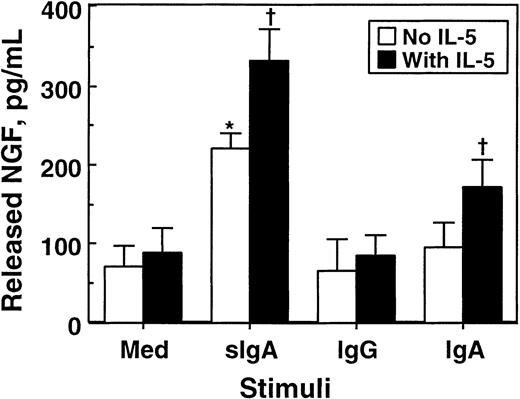
To confirm the results of the bioassay, we used ELISA and quantitated NGF proteins in eosinophil supernatants. As shown in Figure7, approximately 70 pg/mL NGF was detected in the supernatants of eosinophils cultured with medium alone, suggesting that some NGF protein is spontaneously secreted without apparent stimuli. Incubation of eosinophils with sIgA immune complex significantly increased the amounts of NGF in the supernatants (P < .05). In contrast, IgG or IgA immune complexes by themselves did not show significant effects. IL-5 by itself did not induce NGF secretion; however, it increased the secretion of NGF by eosinophils stimulated with sIgA or IgA immune complexes. A combination of sIgA immune complex and IL-5 produced 335 ± 42 pg/mL NGF (mean ± SEM, n = 4), which is roughly comparable to the value obtained from the PC-12 bioassay. Thus, eosinophils stimulated with sIgA immune complex secrete substantial amounts of NGF protein and bioactivity.
Effects of cellular stimulation with immune complexes and/or IL-5 on secretion of NGF by eosinophils.
Eosinophils (1 × 106 cells) were suspended in 1 mL RPMI 1640 medium supplemented with 10% DCS and incubated with medium alone or 20 μg/mL sIgA, serum IgA, or serum IgG for 1 hour at 4°C. Cells were then stimulated with the corresponding antihuman sIgA monoclonal antibody, goat antihuman IgA, or goat antihuman IgG and cultured in the absence (open columns) or presence (closed columns) of 25 ng/mL IL-5 at 37°C for 24 hours. After culture, cell-free supernatants were collected, and the amounts of NGF were measured by ELISA. The data show mean ± SEM from 5 experiments. *denotes significant difference from the values cultured with medium alone (Med) (P < .05). †denotes significant difference from the values cultured with the same stimuli but without IL-5 (P < .05).
Effects of cellular stimulation with immune complexes and/or IL-5 on secretion of NGF by eosinophils.
Eosinophils (1 × 106 cells) were suspended in 1 mL RPMI 1640 medium supplemented with 10% DCS and incubated with medium alone or 20 μg/mL sIgA, serum IgA, or serum IgG for 1 hour at 4°C. Cells were then stimulated with the corresponding antihuman sIgA monoclonal antibody, goat antihuman IgA, or goat antihuman IgG and cultured in the absence (open columns) or presence (closed columns) of 25 ng/mL IL-5 at 37°C for 24 hours. After culture, cell-free supernatants were collected, and the amounts of NGF were measured by ELISA. The data show mean ± SEM from 5 experiments. *denotes significant difference from the values cultured with medium alone (Med) (P < .05). †denotes significant difference from the values cultured with the same stimuli but without IL-5 (P < .05).
Eosinophils, NGF, and NT-3 in vivo
The experiments described above show that eosinophils constitutively make and spontaneously secrete some NGF and that they produce and secrete increased amounts of NGF when exposed to immunologic stimuli in vitro. Can these in vitro phenomena be reproduced in vivo in disease? To address this question, we collected nasal lavage fluids from patients with allergic rhinitis during the peak of the ragweed hay fever season and measured the NGF concentrations by ELISA. To monitor the magnitude of eosinophil infiltration and activation, we also determined the EDN concentrations in the same lavage fluids.44 As shown in Figure8, NGF was clearly detected in nasal secretions of patients with allergic rhinitis, and there was a significant correlation between EDN and NGF concentrations in these patients' lavage samples (P < .001). In contrast, there was no correlation between EDN and NT-3 concentrations. Thus, there is a strong association between eosinophilic inflammation and NGF levels, but not NT-3 levels, in the airways of patients with allergic disease.
Concentrations of NGF, but not NT-3, in nasal secretions from patients with allergic rhinitis correlate with EDN concentrations.
Nasal lavage fluids were collected from patients with ragweed hay fever during the peak of the allergy season, as described in detail in “Materials and methods.” Concentrations of NGF, NT-3, and EDN in the cell-free supernatants of lavage fluids were measured by ELISA and RIA. (A) This panel shows the correlation between the EDN and NGF concentrations in the lavage fluids. (B) This panel shows the correlation between the EDN and NT-3 concentrations in the lavage fluids. Each dot represents the data from each patient. Pearson coefficient of correlation and significance of correlation are shown in the lower right corner.
Concentrations of NGF, but not NT-3, in nasal secretions from patients with allergic rhinitis correlate with EDN concentrations.
Nasal lavage fluids were collected from patients with ragweed hay fever during the peak of the allergy season, as described in detail in “Materials and methods.” Concentrations of NGF, NT-3, and EDN in the cell-free supernatants of lavage fluids were measured by ELISA and RIA. (A) This panel shows the correlation between the EDN and NGF concentrations in the lavage fluids. (B) This panel shows the correlation between the EDN and NT-3 concentrations in the lavage fluids. Each dot represents the data from each patient. Pearson coefficient of correlation and significance of correlation are shown in the lower right corner.
Discussion
One of the novel findings in this study is that eosinophils stimulated with immune complexes have an enhanced ability to produce and secrete NGF, suggesting that NGF can be expressed in an immunologically specific, FcR-dependent manner. Previous studies suggested that mast cells,45 T cells,28 and eosinophils23 could express NGF mRNA and protein. Although little is known about the regulatory mechanism of NGF secretion, one study suggests that perturbation of high-affinity IgE receptors on mast cells triggers NGF release.46 Our observations add to this knowledge and suggest that eosinophils interacting with sIgA and IL-5 secrete substantial and biologically significant amounts of NGF. Therefore, immunologic or inflammatory responses may affect the functions of the peripheral nervous system through increased production of NGF by immune cells. It is well known that neurogenic mediators, such as substance P and other neuropeptides, affect the functions of immune cells.47 Therefore, 2-way communication likely exists between the immune and the nervous systems. Furthermore, NGF may be a particularly important eosinophil-derived mediator at mucosal sites of chronic allergic inflammation where eosinophils may encounter large quantities of allergen-specific immunoglobulins and allergens.48 This concept was buttressed by the strong correlations between NGF levels and the magnitude of eosinophilic inflammation in the airways of patients with allergic rhinitis during hay fever season (Figure 8).
Another original finding is the constitutive nature of NGF and NT-3 production and storage by human eosinophils. Human eosinophils, unlike lymphocytes, tend to store synthesized cytokines; so far, at least 9 eosinophil-derived cytokines are reported to be stored intracellularly as preformed mediators (reviewed in Lacy and Moqbel38). Immunochemical and subcellular fractionation studies suggest that these proteins are likely stored in crystalloid granules and small secretory vesicles.11,49 Our study shows that NGF and NT-3 may also be cytokines falling into this category. Although the exact locations of the NGF and NT-3 storage were not the major focus of this study, Figure 3 clearly indicates that the production and storage of NGF by eosinophils are regulated differently from those of the crystalloid granule proteins, such as EDN. Furthermore, synthesis and storage of NGF and NT-3 were constitutive and were quite sensitive to actinomycin D–induced depletion. In addition, a small but detectable amount of NGF was secreted into supernatants without exogenous stimuli. Previously, we showed that eosinophils constitutively produce and store IL-4 and IL-10 and that the process is inhibited by actinomycin D treatment.10 In contrast, in the same experiments, eosinophils stored minimal amounts of IL-8, but, once stimulated with immunoglobulins, they produced and secreted a large quantity of IL-8.10 Therefore, at least 2 classes of cytokines and growth factors are likely produced by eosinophils; one is constitutively produced and stored, and the other is produced mainly after cellular activation. In the future, it will be important to know which eosinophil mediators belong to which classes and to investigate the biologic relevance of these mediators in the context of the physiologic and pathologic functions of eosinophils. Perhaps some factors are associated with the homeostatic roles of eosinophils and some factors are associated with the proinflammatory roles of eosinophils.
NGF increases nerve conductance and sensitivity in vitro.50 Increased expression of NGF in vivo in the airways of transgenic mice correlates positively with increased peribronchial nerve density.17 NGF transgenic mice show increased airway levels of tachykinins, such as substance P, heightened mucous production, and airway hyperreactivity to capsaicin17; these findings are distinctly reminiscent of findings in patients with asthma and allergic diseases.51Furthermore, intravenous and intranasal administration of NGF induced airway hyperreactivity in guinea pigs and mice, respectively.52,53 Increased levels of NGF were observed in the airways of symptomatic patients with allergic rhinitis18 or patients with asthma 18 hours after segmental allergen challenge.54 Interestingly, histologic examination of airways from patients with asthma or from allergen-challenged guinea pigs revealed eosinophils physically associated with the nerve bundles.55,56 Therefore, given the NGF effects in vitro and in vivo, eosinophils, by production of NGF, could be involved in the increased sensitivity or amplitude or both of the sensory terminal neuron's responses to exogenous stimuli. In fact, we found significant correlations between the nasal levels of NGF and eosinophilic inflammation in patients with allergic rhinitis (P < .001; Figure 8). We recognize that this study does not exclude other potential sources of NGF, such as mast cells45,46 and T cells.28 However, our findings in vivo, as well as eosinophils' ability to produce NGF on immunologic stimuli, strongly suggest that eosinophils produce NGF in various human diseases. Thus, the degree of eosinophil activation may contribute to the neuronal hyperresponsiveness or pathologic pain states frequently accompanying chronic eosinophilic inflammation, such as asthma, allergic diseases, and several eosinophilic disorders (eg, eosinophilia myalgia syndrome).57
Our observations may also have important implications for the physiologic roles of eosinophils in mucosal immunity and inflammation. Perhaps soluble immune complexes formed in vivo in mucosal tissues of patients with allergic diseases48,58 induce cytokine and growth factor production in eosinophils, making eosinophils a “regulator” of inflammation and tissue homeostasis. This may be especially important because eosinophils can produce factors inhibiting inflammation and promoting wound healing, such as TGF-α37and TGF-β,14 and factors affecting development and maintenance of peripheral sympathetic and sensory neurons, such as NGF (this report). Furthermore, NGF also promotes hemopoietic colony growth24 and may participate in wound healing and tissue repair processes.25,26 Thus, eosinophil production of NGF may represent a well-integrated mechanism for maintaining and/or restoring homeostatic function of mucosal tissues during or after inflammation. In addition, eosinophils express functional receptors for neurotrophins,23 59 suggesting that the synthesis and release of NGF and NT-3 by eosinophils could be an autocrine mechanism of this leukocyte. This suggestion raises a key question: Is eosinophil-derived NGF always detrimental to the host by causing neuronal hyperreactivity or is it necessary to maintain integrity of the nervous, immune, and hematologic systems in mucosal tissues? Further studies to address this question will elucidate the, as yet unexplored, roles of eosinophils in communication between cells of the immune and the nervous systems and the roles of NGF in the complex inflammatory network and repair processes in human diseases.
We thank Ms Kay Bachman, Ms Judith Blomgren, Ms Sandra Dunnette, and Ms Kathleen Bartemes for their help in studies of patients with hay fever. We also thank Ms Cheryl Adolphson for editorial assistance, Ms Linda Arneson for secretarial assistance, Ms Gail Kephart for technical assistance, and Dr Douglas Plager for critical reading of the manuscript.
Supported by grants AI 34486 and AI 34577 from the National Institutes of Health and by the Mayo Foundation.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Hirohito Kita, Department of Medicine, Guggenheim Bldg Rm 406, 200 First St SW, Mayo Clinic, Rochester, MN 55905; e-mail: kita.hirohito@mayo.edu.