We read with interest the article by Hsu et al1analyzing the activation status of AKT in plasma cells from patients with multiple myeloma (MM) or monoclonal gammopathy of undetermined significance (MGUS). Recent studies based on MM cell lines indicate that the phosphatidylinositol 3–kinase (PI3K) signaling pathway plays a positive role in the survival of myeloma cells.2 Although various cellular intermediary proteins are activated by PI3K, recent studies suggest that AKT/PKB activity alone is sufficient to block apoptosis. Possible activators of the AKT/PKB signaling pathways in MM include several growth factors such as insulinlike growth factor–1, epidermal growth factor, basic fibroblast growth factor, interleukin-3 (IL-3), IL-6, and macrophage colony-stimulating factor.3 Alternatively, loss of the tumor suppressor gene, PTEN, may also promote AKT signal activation. Regardless of the initiating stimulus, full activation of AKT requires phosphorylation at Thr 308 and Ser 473 by the protein kinases, PDK1 and PDK2.
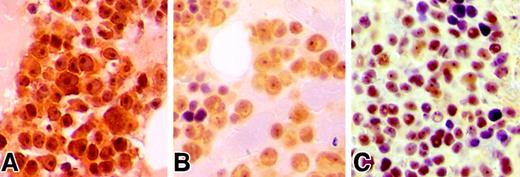
Hsu et al analyzed the phosphorylation status of AKT by using an antibody that recognizes the phosphorylation site of AKT at Ser473 (pSer473-AKT) and demonstrated primarily a cytoplasmic membrane–specific staining pattern in MM cells. We have also analyzed the expression pattern of pSer473-AKT in 18 MM patients. By immunohistochemical staining with an anti–pSer473-AKT antibody (Cell Signaling Technology, Beverly, MA), we found expression of phosphorylated-AKT in 16 of 18 patients, indicative of constitutively phosphorylated-AKT in primary MM cells. However, unlike the findings of Hsu et al, the majority of our samples showed marked nuclear expression and weaker cytoplasmic reactivity in the plasma cells (Figure1). Interestingly, plasma cells from 8 patients demonstrated a marked nucleolar staining pattern. Overall, our findings were consistent with previous reports demonstrating nuclear localization of phosphorylated-AKT following activation.4,5 In addition to its well recognized role at the plasma membrane, AKT is also known to be important in nuclear transduction.3 Furthermore, AKT has been shown to migrate to the nucleus following receptor activation as seen with the B-cell antigen receptor in B lymphocytes.6 Once in the nucleus, activated AKT is believed to influence the functions of several regulatory proteins, such as AFX/Forkhead transcription factors, primarily through regulation of their subcellular localization.7 8
Immunohistochemical analysis of pS473-AKT in MM patients.
(A-C) Bone marrow biopsies obtained from MM patients demonstrate immunopositivity for pSer473-AKT in plasma cells. Biopsies were fixed in 10% zinc formalin for 2 hours, subsequently decalcified in Decalcifier II (Surgipath Medical Industries, Richmond, IL) for 2 hours and processed by a standard automated processor. Four μM sections mounted on glass slides underwent microwave antigen retrieval process in citrate buffer, subsequently incubated with anti–pSer473-AKT antibody at 1/100 dilution overnight at 4°C similar to described previously.9 Slides were then stained with a streptavidin/peroxidase detection system.9 Note that panels A and C illustrate prominent nuclear and nucleolar staining in the neoplastic plasma cells. Magnification × 500.
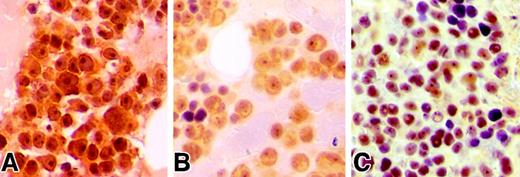
Immunohistochemical analysis of pS473-AKT in MM patients.
(A-C) Bone marrow biopsies obtained from MM patients demonstrate immunopositivity for pSer473-AKT in plasma cells. Biopsies were fixed in 10% zinc formalin for 2 hours, subsequently decalcified in Decalcifier II (Surgipath Medical Industries, Richmond, IL) for 2 hours and processed by a standard automated processor. Four μM sections mounted on glass slides underwent microwave antigen retrieval process in citrate buffer, subsequently incubated with anti–pSer473-AKT antibody at 1/100 dilution overnight at 4°C similar to described previously.9 Slides were then stained with a streptavidin/peroxidase detection system.9 Note that panels A and C illustrate prominent nuclear and nucleolar staining in the neoplastic plasma cells. Magnification × 500.
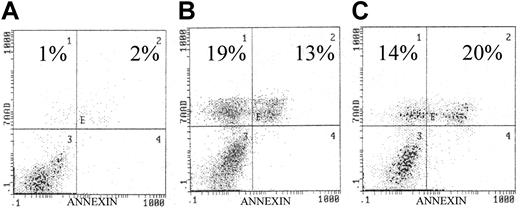
Because IL-6 is known to be an important cytokine for myeloma cell survival and is known to mediate phosphorylation of AKT, we analyzed the effect of AKT inhibitors (wortmannin and LY294 002) on the IL-6–dependent human myeloma cell line U266. Similar to the analysis of other MM cell lines, both AKT inhibitors caused marked apoptosis as detected by annexin/7–AAD staining (Figure 2).2 These results indicate that IL-6–mediated activation of AKT/PKB signaling is important for cellular survival of plasma cells in MM.
Effects of AKT inhibitors on U266 cell line.
(A) Control. Exposure to the AKT inhibitors (panel B, wortmannin, 10 μM/L; panel C, LY294002, 10 μM/L) resulted in significantly increased apoptosis as indicated in each histogram. U266 cells were incubated with/without the presence of each inhibitor for 72 hours. Similar to our previous study, apoptosis was quantified by annexin/7AAD staining, indicating early and late apoptosis, respectively.9
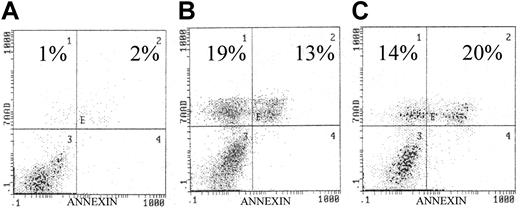
Effects of AKT inhibitors on U266 cell line.
(A) Control. Exposure to the AKT inhibitors (panel B, wortmannin, 10 μM/L; panel C, LY294002, 10 μM/L) resulted in significantly increased apoptosis as indicated in each histogram. U266 cells were incubated with/without the presence of each inhibitor for 72 hours. Similar to our previous study, apoptosis was quantified by annexin/7AAD staining, indicating early and late apoptosis, respectively.9
In summary, our study confirms that AKT plays a significant role in MM cell survival. However, the predominant immunohistochemical localization pattern of phosphorylated-AKT differs from that reported by Hsu et al. This is most likely related to the differences in the techniques used for pSer473-AKT detection, including the source of antibody, fixation (Bouin vs formalin), and utilization of antigen retrieval. Overall, our data and those reported by Hsu et al indicate that overexpression and activation of AKT plays a significant role in MM cell survival. Thus selective inhibitors that specifically target the AKT signaling pathway may have important future therapeutic implications in the treatment of patients with MM.