Antithrombin (AT) deficiency is an autosomal disorder associated with venous thromboembolism. However, a diagnosis of homozygous AT deficiency is seldom made. Most patients are heterozygous and have approximately 50% AT activities, and they are at higher risk for the development of thromboembolism. Through gene targeting we generated AT-deficient mice and previously reported that completely AT-deficient mice could not survive the prenatal period because of extensive thrombosis in the myocardium and liver sinusoids. In contrast, heterozygous AT-deficient mice with 50% AT activities have not shown spontaneous thromboembolic episodes. To demonstrate a thrombotic tendency in heterozygous AT deficiency, we challenged heterozygous AT-deficient mice (AT+/− mice) with the administration of lipopolysaccharide (LPS) or with restraint stress by immobilization. LPS injection markedly induced fibrin deposition in the kidney glomeruli, myocardium, and liver sinusoids in AT+/− mice compared with wild-type mice (AT+/+ mice). Restraint stress tests were performed by placing mice in 50-mL conical centrifuge tubes for 20 hours. Fibrin deposition was observed in the kidney ofAT+/+ and AT+/− mice, but AT+/−mice exhibited more extensive fibrin deposition thanAT+/+ mice. After prophylactic administration of human AT concentrates to increase plasma AT activities of AT+/−mice, LPS-induced fibrin deposition was effectively prevented. These results suggest that heterozygous AT deficiency is significantly associated with a tendency toward thrombosis formation in the kidney. The AT+/− mouse thus is a useful model for studying the effect of environmental or genetic risk factors on thrombogenesis.
Introduction
Antithrombin (AT) is a plasma glycoprotein with a molecular weight of 58 000. It is one of the most important inhibitors of blood coagulation, and it inactivates thrombin and several serine proteases, including factors IXa, Xa, Xia, and XIIa, by forming a 1:1 molar complex between the active site of the serine protease and its reactive site. Heparin has an accelerating effect on the formation of AT–protease complexes. In the presence of heparin, the active site of the protease is brought into close contact with the reactive site of AT, and the rate of inhibition is enhanced up to several thousand times.1-4
Congenital AT deficiency is an autosomal disorder associated with venous thromboembolism. The mean prevalence of venous thromboembolism among heterozygous subjects was 51% compared with controls without the deficiency (1.5%).5 The incidence of the disorder in the general population is estimated at 1 in 2000 to 5000.6,7 AT deficiency is classified into 2 types. Type 1 is a quantitative deficiency, and AT antigens and activities are lowered. Type 2 is a qualitative defect without reduction of AT antigens.2,8 Patients with undetectable AT activities or antigens appear to have homozygous AT deficiency, but homozygous AT deficiency is extremely rare. Such patients have been reported to have severe thrombotic diseases of early onset.9 Most patients with AT deficiency are heterozygous, and AT activity is approximately half the normal level. Previous population studies had indicated that heterozygous patients were expected to live as long as the general population in spite of the greater risk for thromboembolic episodes.10 11
We generated congenitally AT-deficient mice through gene targeting and reported that completely AT-deficient mice could not survive the prenatal period because of extensive thrombosis in the myocardium and liver sinusoids along with massive bleeding.12 In contrast, heterozygous AT-deficient mice (AT+/− mice) were born normally, and their external appearance was similar to that of wild-type mice (AT+/+ mice).12 To further investigate the role of AT and the impact of its deficiency on the tendency toward thrombosis, we induced a hypercoagulable state by lipopolysaccharide (LPS) challenge and by restraint stress with immobilization in AT+/− mice. Purified human AT was tested for the prevention of thrombosis in LPS-challenged AT+/−mice, and the requirement of AT for the treatment of the kidney thrombosis was examined.
Materials and methods
Mice
The generation of AT-deficient mice was described previously.12 In brief, the region including exon 2 of theAT gene was replaced by homologous recombination with plasmid MC1neo containing DTA (diphtheria toxin fragment A gene) and the AT gene fragments cloned from the genomic DNA library of 129SV mice. Male chimeric mice were mated with wild-type C57BL/6J female mice (CLEA, Tokyo, Japan). Littermates of 6-month-old mice were used for thrombogenic challenges. For each genotype, sex and weight were matched for all the experiments in this study. Experimental designs and protocols, including plasmid construction, generation of gene-targeting mice, and thrombogenic challenges, were reviewed by the Nagoya University Animal Research Committee.
Assays for antithrombin
Blood samples were collected into 3.8% sodium citrate at a ratio of 9:1 and were centrifuged at 2000g for 10 minutes to obtain plasma. The plasma was stored at −80°C until assay. Plasma AT antigen levels were determined using N-assay TIA AT-III (Nittobo, Tokyo, Japan), a turbidimetric immunoassay for the antigen–antibody complex. AT activities were measured using N-test ATIII-S (Nittobo), which determines anticoagulant activity using a chromogenic substrate, according to the manufacturer's instructions.
Thrombogenic challenge
Mice were injected intraperitoneally with 5 mg/kg LPS (Escherichia coli serotype 0111: B4; Sigma, St Louis, MO). Four hours later, mice were killed and the lungs, livers, hearts, and kidneys were removed and fixed overnight in Carnoy solution (methanol:chloroform:acetic acid, 6:3:1). For the rescue experiment of thrombotic disease, AT+/− mice were injected through the tail vein with AT concentrates purified from human plasma (Welfide, Osaka, Japan) at doses of 50 U/kg or with physiological saline 30 minutes before LPS challenge.
In another experiment, mice were exposed to restraint stress by placement in 50-mL conical centrifuge tubes in which they could hardly move (Yamamoto et al, manuscript submitted). Air and water were supplied through small punctures in the tube walls. After 20 hours, the mice were killed, and their organs were removed and used for immunohistochemical analysis.
Immunohistochemical analysis
Fixed tissues were dehydrated, embedded in paraffin, and sectioned (6 μm thick). Tissue sections were deparaffinized in xylene, transferred to 100% ethanol, and incubated for 30 minutes in 0.3% hydrogen peroxide–methanol. After rinsing with phosphate-buffered saline (PBS), the slides were incubated successively with 5% normal goat serum–PBS (20 minutes, room temperature), rabbit anti-human fibrin–fibrinogen antibody (DAKO, Glostrup, Denmark) (1:100 dilution, 1 hour, room temperature), anti–rabbit IgG antibody conjugated with biotin (1:500 dilution, 1 hour, room temperature), and avidin–biotin complex conjugated with horseradish peroxidase (Vector Laboratories, Burlingame, CA) (30 minutes, room temperature). Staining was visualized with diaminobenzidine tetrahydrochloride–Ni3+, Co2+ (Amersham Pharmacia Biotech, Piscataway, NJ). The percentage of the glomeruli with fibrin deposition (%GFD) was calculated in all areas of each histologic specimen of the kidney. Partially stained glomeruli were categorized as positive (Figure 2A).
Statistical analysis
Stat-View 4.5 (SAS Institute, Cary, NC) was used for statistical analysis. P values were calculated using the Studentt test, and P = .05 was considered statistically significant. Data represented means ± SD.
Genotype determination of AT+/− pups
Genomic DNA was extracted from tails of mice as previously described12 and was used for polymerase chain reaction (PCR) analysis. PCR was performed using the external primers of the replaced gene fragment. The wild-type allele and the mutant allele gave different band sizes. Primer sequences and PCR conditions have been described.12
Results
Genotypes and plasma AT levels of AT+/− mice
One hundred forty-one pups were born from 20 pairings ofAT+/− and AT+/+ mice, and the genotypes were determined by PCR analysis. All 141 pups grew normally; 66 pups wereAT+/−, and 75 were AT+/+. There was no deviation between the 2 genotypes, indicating that the AT+/− genotype does not cause embryonic lethality (Figure1A). The external appearance ofAT+/− mice could not be distinguished from that ofAT+/+ mice. No spontaneous thromboembolic episodes were observed during the longest follow-up period of 14 months. In humans, pregnant women are at risk for thrombotic disease, but no AT+/−female mice developed thrombosis during gestation.
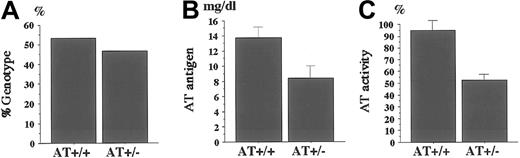
Genotype of newborn pups and plasma levels of AT antigens and activities.
(A) Genotype of newborn mice from the pairing of AT+/− andAT+/+ mice. One hundred forty-one pups were born from 20 pairings, and the genotypes were determined by PCR analysis. Plasma levels of AT antigens (B) and activities (C) are measured as described in “Materials and methods.” AT antigens and activities were significantly reduced in AT+/− mice compared withAT+/+ mice (n = 8; P < .01). Values are means ± SD.
Genotype of newborn pups and plasma levels of AT antigens and activities.
(A) Genotype of newborn mice from the pairing of AT+/− andAT+/+ mice. One hundred forty-one pups were born from 20 pairings, and the genotypes were determined by PCR analysis. Plasma levels of AT antigens (B) and activities (C) are measured as described in “Materials and methods.” AT antigens and activities were significantly reduced in AT+/− mice compared withAT+/+ mice (n = 8; P < .01). Values are means ± SD.
Plasma levels of AT antigens and activities were determined for 8AT+/− mice and 8 AT+/+ mice. Mean values of antigens and activities for AT+/− mice were 8.4 mg/dL and 52.6%, respectively, whereas those for AT+/+ mice were 13.8 mg/dL and 95.1% (Figure 1B,C). Compared to AT+/+ mice, mean relative values for AT antigens and activities were 60.9% and 55.3% of AT+/+ mice, respectively; both values were significantly decreased in AT+/− mice (P < .01; Figure 1B,C). Hence, the AT+/− mice have a heterozygous AT deficiency that would be a risk factor for thrombosis in humans.
Lipopolysaccharide challenge of AT+/− mice
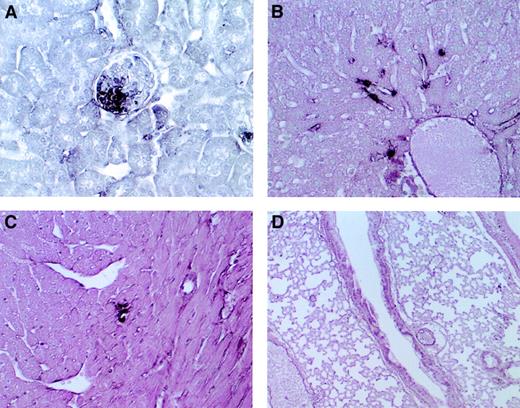
Through immunohistochemical analysis, fibrin deposition was detected in the glomeruli and in the peritubular capillaries of the kidney of AT+/− mice that were intraperitoneally injected with 5 mg/kg LPS (Figure 2A). Fibrin deposition was also detected in the liver sinusoids (Figure 2B) and small vessels of the myocardium (Figure 2C). We previously found that the AT−/− fetus developed degeneration of the myocardium and liver from extensive fibrin deposition,12 but it did not occur in AT+/− mice. In the lung, no fibrin deposition was observed (Figure 2D). Fibrin deposition in the kidney, liver, and myocardium was also observed in AT+/+ mice after LPS challenge, but their levels were lower than those of AT+/−mice (data not shown). All mice survived after LPS administration.
Microscopic findings in AT+/− mice after LPS challenge.
Immunohistochemical staining with anti-fibrin–fibrinogen antibody of the kidney (A), liver (B), heart (C), and lung (D) of AT+/−mice. Fibrin deposition was detected in the kidney glomeruli (A), liver sinusoids (B), and small vessels of the myocardium (C). Almost no deposition was observed in the lung (D). Magnifications, ×400 (A-C), ×100 (D).
Microscopic findings in AT+/− mice after LPS challenge.
Immunohistochemical staining with anti-fibrin–fibrinogen antibody of the kidney (A), liver (B), heart (C), and lung (D) of AT+/−mice. Fibrin deposition was detected in the kidney glomeruli (A), liver sinusoids (B), and small vessels of the myocardium (C). Almost no deposition was observed in the lung (D). Magnifications, ×400 (A-C), ×100 (D).
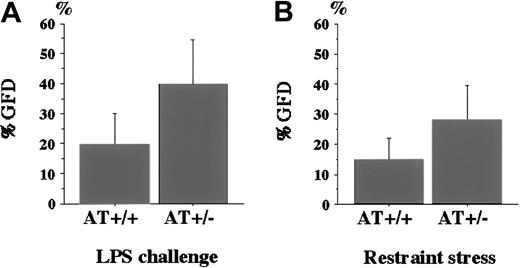
To compare the degree of fibrin deposition in the kidney, we determined the %GFD and compared it between AT+/− andAT+/+ mice (Figure 3). The %GFD was higher in AT+/− mice than in AT+/+mice (39.8% ± 14.6% vs 19.7% ± 10.3%). Student ttest revealed that the difference was statistically significant (P < .01, Figure 3A). As a control, we also examined the kidneys of AT+/− mice not challenged with LPS, but no fibrin deposition was observed (data not shown).
Percentage of glomeruli with fibrin deposition (%GFD) in AT+/+ and AT+/− mice.
(A) The %GFD of AT+/+ mice and AT+/− mice after LPS challenge. LPS was intraperitoneally injected into mice as described in “Materials and methods.” After 4 hours, kidney specimens were subjected to immunohistochemical analysis as described in the legend to Figure 2. The %GFD was significantly higher inAT+/− mice than in AT+/+ mice (n = 12,P < .01). (B) The %GFD of AT+/+ mice andAT+/− mice after exposure to restraint stress. Mice were placed in 50-mL tubes for 20 hours with appropriate water and air supply. The %GFD was significantly higher in AT+/− mice than in AT+/+ mice (n = 8; P < .05). Values are means ± SD.
Percentage of glomeruli with fibrin deposition (%GFD) in AT+/+ and AT+/− mice.
(A) The %GFD of AT+/+ mice and AT+/− mice after LPS challenge. LPS was intraperitoneally injected into mice as described in “Materials and methods.” After 4 hours, kidney specimens were subjected to immunohistochemical analysis as described in the legend to Figure 2. The %GFD was significantly higher inAT+/− mice than in AT+/+ mice (n = 12,P < .01). (B) The %GFD of AT+/+ mice andAT+/− mice after exposure to restraint stress. Mice were placed in 50-mL tubes for 20 hours with appropriate water and air supply. The %GFD was significantly higher in AT+/− mice than in AT+/+ mice (n = 8; P < .05). Values are means ± SD.
Restraint stress
It has been reported that mental or physical stress may affect coagulation level or fibrinolytic factors.13 14 Our preliminary observations suggest that restraint stress is a risk factor for thrombosis (Yamamoto et al, manuscript submitted), and we restrained AT+/+ and AT+/− mice by placing them in narrow centrifuge tubes for 20 hours. Figure 3B indicates that the %GFD was significantly higher in AT+/− mice than inAT+/+ mice (28.1% ± 11.4% vs 14.9% ± 7.1%, n = 8, P < .05).
AT supplementation before lipopolysaccharide challenge
Next we studied whether AT supplementation before LPS challenge could rescue the kidney thrombus formation in AT+/− mice. To determine the appropriate dosage to which to increase plasma AT activities, human AT concentrates were intravenously administered toAT+/− mice at doses of 0, 25, 50, 100, and 250 U/kg. Thirty minutes later, plasma levels of AT activities were measured, and the mean levels were 46.7%, 73.9%, 103.6%, 152.4%, and 261.5%, respectively (n = 3). Thus we chose 50 U/kg AT concentrates to normalize plasma AT levels of AT+/− mice.
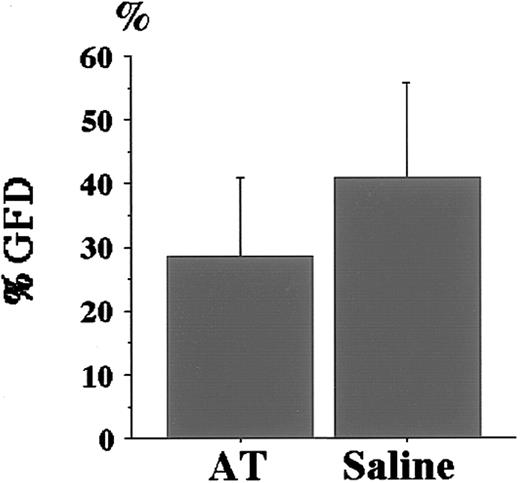
The same volume of human AT concentrates or physiological saline was injected into the tail veins of AT+/− mice 30 minutes before LPS challenge. Prophylactic AT treatment significantly reduced the %GFD (28.5% ± 12.4% for AT supplementation vs 40.8% ± 14.8% for saline; P < .05, Figure4). The improved %GFD (28.5%) was, however, slightly higher than that of the LPS-challengedAT+/+ mice without AT supplementation (19.7%, Figure 3A), though the difference was not significant (P = .07), suggesting the possibility that supplemented AT was not enough to suppress LPS-induced thrombus formation completely.
Effect of AT supplementation before LPS challenge toAT+/− mice.
Prophylactic AT treatment was performed 4 hours before LPS challenge using 50 U/kg human AT concentrates (AT) as described in “Materials and methods.” The same volume of control physiological saline was injected. AT supplementation significantly reduced %GFD of AT+/− mice (n = 12;P < .05). Values are means ± SD.
Effect of AT supplementation before LPS challenge toAT+/− mice.
Prophylactic AT treatment was performed 4 hours before LPS challenge using 50 U/kg human AT concentrates (AT) as described in “Materials and methods.” The same volume of control physiological saline was injected. AT supplementation significantly reduced %GFD of AT+/− mice (n = 12;P < .05). Values are means ± SD.
Discussion
During our 14-month observation period, no heterozygous AT-deficient mice developed spontaneous thrombotic disease. In patients with heterozygous AT deficiency, decreased plasma AT levels may not be the absolute risk factor for thrombosis but may play an additional and important role with other risk factors.3,15 In most instances, thromboembolic episodes of such patients are associated with acquired conditions, including acute infection, surgery, pregnancy, delivery, major trauma, and the use of oral contraceptives.15 Other genetic risk factors such as factor V Leiden also predispose to the development of thrombosis in heterozygous AT-deficient subjects.16
LPS or endotoxin is the specific cell membrane component of microorganisms, and it triggers the activation of the coagulation cascade.17 We induced a hypercoagulable state inAT+/− mice by LPS challenge and demonstrated that AT deficiency was strongly associated with fibrin deposition in glomeruli of the kidney (Figure 3A). Normalizing AT levels by the purified human AT concentrates before LPS challenge significantly reduced fibrin deposition (Figure 4). These facts indicate that fibrin deposition in the kidney of AT+/− mice is attributed to decreased AT levels and that heterozygous AT deficiency is a risk factor for renal thrombosis. Although patients with heterozygous AT deficiency have deep vein thrombosis, no AT+/− mice exhibited the thrombotic disease in hands or feet. Mice harboring the factor V Leiden mutation have thrombosis in the lower limbs, but this phenotype is reported to be rare.18 Such differences in disease phenotype between humans and mice may not be interpreted by this study, and further observations may be required to study the blood flow in arms and legs of mice.
Systemic infectious diseases occasionally lead to the release of endotoxin, a major thrombogenic agent. Indeed, thromboembolic episodes of patients with heterozygous AT deficiency are reported to be associated occasionally with acute infection,15 suggesting that patients with heterozygous AT deficiency are susceptible to endotoxin-induced hypercoagulation. Our experiment clearly indicated that prophylactic AT supplementation successfully rescued the thrombotic kidney disease of AT+/− mice (Figure 4), suggesting the therapeutic roles of AT for thrombotic human diseases. A recent randomized control study by Warren et al19 used high-dose intravenous AT for patients with severe sepsis. Mean baseline AT level before treatment was approximately 60%, and it was increased to 180% 24 hours after AT administration. However, AT administration did not significantly improve the mortality rate of patients compared with placebo controls. Taken together, the observed effects on %GFD inAT+/− mice might be restricted to diseases caused by AT deficiency.
The improved %GFD achieved by AT supplementation (Figure 4) was still higher than %GFD of AT+/+ mice (Figure 3A), which were also challenged by LPS without pretreatment (28.5% ± 12.4% vs 19.7% ± 10.3%; P = .07). It has been reported that plasma AT levels begin to decrease early in sepsis because of excessive consumption.20 21 Indeed, AT activities of AT+/−mice 4 hours after LPS challenge were decreased to 63.0%, whereas 30 minutes after AT injection they measured 103.6%. InAT+/+ mice, the activity was also decreased to 71.8% 4 hours after LPS challenge, and the decrease was statistically significant. Therefore, AT appeared to be consumed during LPS-induced thrombin generation. Dose escalation of administered AT may be required to rescue the fibrin deposition of AT+/− mice with the comparable level of AT+/+ mice without pretreatment.
Mental or physical stress appears to affect plasma coagulation and fibrinolysis. In humans, it is one of the triggers of unstable angina, myocardial infarction, and sudden death.22-24 The levels of several coagulation or fibrinolytic factors—including von Willebrand factor, factor VIII, factor VII,13 or plasminogen activator inhibitor-1 (PAI-1)14—appear to increase in response to mental stress, suggesting the possibility that prothrombic potential may be increased, thus promoting thrombotic complications under stressful conditions. In this study, as an alternative thrombogenic challenge, we exposed AT+/+ andAT+/− mice to 20 hours of restraint stress. AT+/−mice developed renal thrombosis to a greater degree than didAT+/+ mice (Figure 3B), indicating that heterozygous AT-deficient mice are susceptible to stress-induced renal thrombosis. In our preliminary observation, restraint stress increased PAI-1 expression in the kidney glomerulus (Yamamoto et al, manuscript submitted). Presumably, the thrombogenic risk for AT-deficient mice might be increased by the lower fibrinolytic potential.
In this study, we confirmed that congenital heterozygous AT deficiency is associated with a tendency to thrombosis as well as complete AT deficiency, but these disorders are not identical in pathophysiology.AT+/− mice are at risk for thrombosis, but additional thrombogenic stimulations are required. The addition of other genetic risk factors into the AT+/− genotype will provide further understanding of the role of AT in thrombogenesis.
We thank Takayuki Nakayama and Kyosuke Takeshita for their helpful advice and discussions. We greatly thank Kazuo Kagami, Kayo Sakakura, and Eriko Yamafuji for their technical assistance.
Supported in part by Grants-in-Aid for Scientific Research (11470209 and 13470203) from Ministry of Education, Science, Sports and Culture, Center of Excellence Research (COE), and Welfide Medicinal Research Foundation (H.S.).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Hidehiko Saito, First Department of Internal Medicine, Nagoya University School of Medicine, 65 Tsurumai-cho, Showa-ku, Nagoya, Aichi 466-8550, Japan; e-mail:hsaito@med.nagoya-u.ac.jp.