Interleukin-10 (IL-10)–treated dendritic cells (DCs) induce an alloantigen- or peptide-specific anergy in various CD4+ and CD8+ T-cell populations. In the present study, we analyzed whether these anergic T cells are able to regulate antigen-specific immunity. Coculture experiments revealed that alloantigen-specific anergic CD4+ and CD8+ T cells suppressed proliferation of syngeneic T cells in a dose-dependent manner. The same effect was observed when the hemagglutinin-specific CD4+T-cell clone HA1.7 or tyrosinase-specific CD8+ T cells were cocultured with anergic T cells of the same specificity. Anergic T cells did not induce an antigen-independent bystander inhibition. Suppression was dependent on cell-to-cell contact between anergic and responder T cells, required activation by antigen-loaded DCs, and was not mediated by supernatants of anergic T cells. Furthermore, anergic T cells displayed an increased extracellular and intracellular expression of cytotoxic T-lymphocye antigen (CTLA)–4 molecules, and blocking of the CTLA-4 pathway restored the T-cell proliferation up to 70%, indicating an important role of the CTLA-4 molecule in the suppressor activity of anergic T cells. Taken together, our experiments demonstrate that anergic T cells induced by IL-10–treated DCs are able to suppress activation and function of T cells in an antigen-specific manner. Induction of anergic T cells might be exploited therapeutically for suppression of cellular immune responses in allergic or autoimmune diseases with identified (auto) antigens.
Introduction
The task of eliminating autoreactive T cells is mediated in part by clonal deletion in the thymus. Autoreactive T cells that did not undergo negative selection in the thymus are subject to several peripheral mechanisms controlling unwanted T-cell activation.1 These include immunological ignorance, deletion, immunoregulation, and anergy.2-5 Maintenance of tolerance might be of clinical importance not only for self-tolerance but also for the control of pathogen-induced immune processes.6 7
Anergy is immunologically defined as the inability of antigen-specific T cells to produce interleukin (IL)-2 and to clonally expand on rechallenge with fully competent antigen-presenting cells (APCs).8 Induction of anergy is an active process that occurs when T-cell receptors (TCRs) are ligated by antigen in the absence of costimulation.9 Anergy can also be induced in the presence of costimulation when the TCR is ligated by superantigen or by altered peptide ligands that bear a single amino acid substitution in the sequence of the agonistic peptide.10,11 Although quite distinct, these approaches to induce anergy appear to share common biochemical events characterized by hypophosphorylation of several signal transduction–related proteins.12-15 One important aspect of this type of regulation is that alloreactive T cells, tolerant to a specific antigen (alloantigen/autoantigen), can potentially down-regulate the response of other naive allogeneic or antigen-specific T cells.16 17
Dendritic cells (DCs) are highly specialized APCs of the immune system.18 Fully mature DCs are potent activators of naive T cells and are regarded as important initiators of primary T-cell immune responses. In contrast to modulators inducing the maturation of DCs, the immunosuppressive properties of IL-10 on DCs have been well documented in several studies.19 The inhibitory influence of IL-10 on APC function of DCs may be due to several phenotypic and functional alterations, such as down-regulation of major histocompatibility complex class II and costimulatory molecules and the reduced secretion of a variety of inflammatory cytokines.20-23 Additionally, it was shown that IL-10–treated LCs (Langerhans cells) induce an antigen-specific tolerance in TH1, but not in TH2 cell clones.24 Recently, we showed that IL-10–treated human DCs generated from peripheral blood induce a state of antigen-specific anergy in CD4+ and CD8+ T cells.25,26 These anergic T cells are characterized by an impaired proliferative capacity, reduced production of IL-2 and interferon (IFN)-γ, and no secretion of TH2/TC2 cytokines.25,26Furthermore, functional studies demonstrated that anergic melanoma-associated antigen-specific (tyrosinase-specific) T cells failed to lyse malignant melanoma cells.26
In the present study, we analyzed the effect of anergic T cells induced by IL-10–treated DCs on naive syngeneic T cells or activated antigen-specific CD4+ and CD8+ T-cell lines and clones. We will demonstrate that anergic T cells suppress proliferation of cocultured T cells of the same alloantigen or peptide specificity, but induce no bystander suppression. This suppression is mediated in part by stimulation of the cytotoxic T-lymphocye antigen (CTLA)–4 molecule on anergic T cells, is dependent on cell-to-cell contact between the cocultured T cells, and is independent of soluble mediators produced.
Materials and methods
Culture medium
X-VIVO-15 (BioWhittaker, Walkersville, MD), supplemented with 1% autologous plasma, was used for generation of DCs. T cells were cultured and stimulated in X-VIVO-20 (BioWhittaker).
Cytokines
All cytokines used in this study were recombinant human proteins and were used at plateau concentrations to induce optimal growth of DCs. Final concentrations were as follows: 800 U/mL granulocyte-macrophage colony-stimulating factor (GM-CSF) (Leukomax) (Sandoz, Basel, Switzerland); 1000 U/mL IL-4 and IL-6 (Strathmann Biotech, Hannover, Germany); 10 ng/mL IL-1β and tumor necrosis factor (TNF)–α (Strathmann); and 1 μg/mL prostaglandin E2 (PGE2) (Minprostin) (Pharmacia-Upjohn, Heppenheim, Germany). For culture and expansion of T cells, 50 U/mL IL-2 (Proleukin) (Chiron, Emeryville, CA) was used.
Antibodies
For immunostaining, mouse immunoglobulin (Ig)-G, the following were used: CD2, CD3, CD4, CD8, CD11a, CD25, CD28, CD40L, CD58, CD62L, CD69, CD122 (Pharmingen, Hamburg, Germany), CTLA-4 (Alexis Biochemicals, San Diego, CA), mouse subclass-specific isotypes (Coulter/Immunotech, Fullerton, CA). Conjugated secondary reagents were as follows: fluorescein isothiocyanate (FITC)–conjugated goat-antimouse IgG (Jackson Immunoresearch, West Grove, PA). Staining of magnetic activated cell sorting (MACS)–sorted T cells: FITC- or phycoerythrin (PE)-conjugated CD4 or CD8; FITC- and PE-conjugated mouse IgG (Coulter/Immunotech).
Flow cytometric analysis
Immunofluorescence staining was performed after washing the cells twice with phosphate-buffered saline (PBS) plus 0.5% human serum albumin (HSA). Cells were incubated for 20 minutes at 4°C with each monoclonal antibody (mAb) diluted to the optimal concentration for immunostaining. After washing with cold PBS/HSA, the cells were incubated with FITC- and PE-conjugated second-step mAb for 20 minutes at 4°C, washed 3 times, and analyzed by flow cytometry (FACScalibur, Cellquest software) (Becton Dickinson, Mountain View, CA).
For intracellullar analysis of CTLA-4 expression, 48 hours after restimulation T cells were collected, washed, fixed/saponin-permeabilized (perm/fix-solution, Pharmingen), and stained with 0.5 μg CTLA-4–specific or IgG control antibodies per test.
Generation of DCs
DCs were generated from buffy coats or leukapheresis products as described before.27 Briefly, peripheral blood mononuclear cells were isolated by Ficoll density gradient centrifugation and used immediately or stored frozen in aliquots. For each DC preparation, monocytes were isolated by plastic adherence and cultured in X-VIVO-15 plus 1% heat-inactivated autologous plasma including 800 U/mL GM-CSF and 1000 U/mL IL-4. At day 7, nonadherent cells were rinsed off, washed once in PBS, and transferred to 6-well plates at 7 × 105cells in 3 mL per well. For differentiation into mature DCs, immature DCs were additionally stimulated on day 7 with 10 ng/mL IL-1β, 10 ng/mL TNF-α 1000 U/mL IL-6, and 1 μg/mL PGE2. For the generation of anergy-inducing DCs, IL-10 (40 ng/mL) was added to the DCs at day 7 after stimulation with the cytokines. Nonadherent DCs at day 9 were used for T-cell stimulation.
Induction of alloreactive T cells and proliferation assays
Naive CD4+/CD8+ T cells were purified with MACS-Beads as indicated by the manufacturer (Miltenyi, Bergisch-Gladbach, Germany) (purity: greater than 98% CD4+/CD8+, greater than 90% CD45RA+). Subsequently, 106 T cells were cultured with 105 mature DCs or IL-10–treated DCs in 1 mL X-VIVO-20 in 24-well plates to generate anergic or optimally stimulated control T cells. These T cells were used in anergy assays or coculture experiments and are referred to here as anergic T cells or activated control T cells (CTs). Parts of the cultures were used for proliferation assays carried out in X-VIVO-20 with 1 × 105 T cells per well and different numbers of allogeneic DCs in 96-well plates. T-cell proliferation was measured after 3 days of incubation, and an additional 16-hour pulse with3H-Tdr (37 kBq per well) by means of a liquid scintillation counter.
Tyrosinase-specific and melanoma antigen recognized by T cells–specific CD8+ T-cell lines and tetanus-toxoid–specific CD4+ T-cell lines
CD8+ T cells (2 × 105) from HLA-A2+ donors (purity greater than 95% CD8+ T cells, generated as described above) were cultured in X-VIVO 20 and stimulated with mature, HLA-A2+, autologous DCs (2 × 104) pulsed with the specific tyrosinase peptide (Tyr-Met-Asp-Gly-Thr-Met-Ser-Gln-Val) or melanoma antigen recognized by T cells (MART-1) peptide (Glu-Ala-Ala-Gly-Ile-Gly-Leu-Thr-Val) (20 μg/mL). For the generation of tetanus-toxoid (TT)–specific T-cell lines, T cells (2 × 105) from donors (HLA-DR1+) vaccinated against TT were cultured in X-VIVO-20 and stimulated with mature autologous DCs (2 × 104) pulsed with the specific antigen (TT, 10 μg/mL).
After several restimulations (3 to 4 times) every 7 days and expansion of the cell number by addition of IL-2 (10 U/mL), the peptide-specific proliferation was tested by means of specific and unspecific peptides. Prior to use in experiments, T cells were rested 7 to 8 days following the last restimulation. Three tyrosinase-specific CD8+ T-cell lines from 2 unrelated HLA-A2+donors were generated and used for the experiments. MART-1–specific T-cell lines served as controls. Two TT-specific CD4+T-cell lines from 2 unrelated HLA-DR1+ donors were generated and used for the experiments.
Culture of human CD4+-antigen–specific T-cell clone HA1.7
The isolation of cloned T cells reactive with the influenza hemagglutinin antigen (HA peptide 307-319) has been reported in detail previously.28 The T-cell clone HA1.7 (kindly provided by Dr J. R. Lamb, St Mary′s Hospital Medical School, London, United Kingdom) was cultured in RPMI 1640 (Gibco, Grand Islands, NY) supplemented with 100 U/mL penicillin (Gibco), 100 μg/mL streptomycin (Gibco), 2 mM glutamine (Gibco), and 10% screened human AB+ serum. Growing HA-reactive T cells were expanded with specific antigen (HA) in a proliferation-inducing dose (1 μg/mL) and HLA-DR1+ peripheral blood mononuclear cells every 7 days. After stimulation, the T-cell clone was fed with IL-2 (50 U/mL) every 3 to 4 days. Prior to their use in experiments, the T cells were rested 7 to 8 days following the last restimulation.
Anergy assay and coculture experiments
We prepared and cultured anergic and activated allogeneic CD4+ and CD8+ T cells, tyrosinase-specific CD8+ T cells, or the CD4+ T-cell clone HA1.7 as described above. T cells were cocultured during the first incubation at a density of 2 × 105 (allogeneic naive CD4+and CD8+) or 1 × 105 (tyrosinase-specific CD8+ T cells, HA-specific CD4+ T-cell clone) with 1 × 104 DCs, pretreated with IL-10 (40 ng/mL), or left untreated to generate anergic or optimally stimulated activated T cells. At 36 hours later, T cells were separated by CD4+/CD8+ microbeads (Miltenyi) and rested for 2 days in culture medium containing 2 U/mL IL-2. Subsequently, T cells were restimulated with DCs generated from the same donor as was used for the first culture in experiments with allogeneic T cells and tyrosinase-specific T cells or with DCs generated from an HLA-DR1+ (CD4+ T-cell clone HA1.7) donor.
To assess the suppressor activity of anergic T cells in the alloantigen-specific system, anergic CD4+ and CD8+ T cells (1 × 105) and syngeneic activated T cells (1 × 105) restimulated with 2 × 104 DCs (generated from the same donor as used in the primary culture) were used. In experiments with peptide-specific T cells (5 × 104), anergic CD4+ T cells of the clone HA1.7 or tyrosinase-specific CD8+ T cells were cocultured with (5 × 104) syngeneic activated T cells of the same specificity and restimulated with syngeneic mature DCs (5 × 103).
In experiments analyzing the stimulatory effect of APCs on the suppressor activity of the anergic T cells, cocultured T cells were rescued after the first restimulation. Subsequently, these T cells were cultured without additional stimulation (APCs or IL-2) to remove anergic T cells from the culture as anergic T cells undergo rapid apoptosis under these conditions. Simultaneously, control experiments using anergic or activated T cells (stimulated with DCs or unstimulated) were performed to analyze the viability of the T cells. Viability was detected by trypan blue staining or mixed leukocyte reaction (MLR), indicating the complete death of anergic T cells after 2 to 3 days of culture, in contrast to activated control T cells. Afterward, the second restimulation experiments were performed.
Proliferation was measured 48 to 72 hours later by thymidine incorporation. Tests were carried out in triplicate, and results were expressed as mean cpm ± SD.
For blocking experiments, the following were used: antibodies to CTLA-4 (10 μg/mL); B7-1/B7-2 (Pharmingen) (10 μg/mL); IL-10 (R&D, Hamburg, Germany) (10 μg/mL); and transforming growth factor (TGF)–β (R&D) (15 μg/mL). Blocking properties of the anti–CTLA-4 antibody were characterized in privious studies.29 30 To overcome the suppressor activity, IL-2 (100 U/mL) was added in the coculture experiments. In some experiments, supernatants of anergic T cells were harvested and added to coculture of activated control T cells of the same specificity.
Transwell experiments
Transwell experiments were done in 24-well plates. Anergic alloantigen-specific 106 T cells or anergic peptide-specific (tyrosinase-specific) 2 × 105 T cells were stimulated with (105 or 2 × 104) mature DCs. Additionally, 106 control allogeneic or 2 × 105 activated peptide-specific T cells were either added directly to the cultures of anergic T cells or were placed in transwell chambers (0.4 μm) (Millicell) (Millipore, Bedford, MA) in the same well with (105 or 2 × 104) mature DCs (primed with the specific antigen). After 3 days of culture, activated T cells (2 × 105/well) were transferred to 96-well plates in triplicates. Proliferation was measured after an additional 16-hour pulse with 3H-Tdr by means of a liquid scintillation counter.
Cytokine analysis
For assessment of cytokine production, supernatants were collected 48 hours after restimulation of T cells with mature DCs and stored at −70°C. Amounts of IL-2, IL-4, IL-10, and IFN-γ were measured by enzyme-linked immunosorbent assay (ELISA) with the use of commercially available antibodies and standards according to the manufacturer‘s protocols (Pharmingen).
Results
Anergic T cells induced by IL-10–treated DCs suppress the activation of syngeneic alloantigen-specific T cells
In previous studies, we demonstrated that IL-10–treated DCs induce an alloantigen-specific or peptide-specific anergy in cocultured CD4+ and CD8+ T cells. To assess suppressive effects of anergic T cells, we first analyzed the effect of alloantigen-specific anergic T cells on the proliferation of syngeneic activated T cells previously stimulated with mature DCs. First, 1 × 105 anergic CD4+ (Figure1A) or CD8+ (Figure 1B) T cells were cocultured with the an equal number of syngeneic activated control T cells (1 × 105) of the same donor and restimulated with mature DCs (2 × 104) from the same DC donor that was used during the induction of anergy. Cocultures of anergic (1 × 105) or activated control T cells (1 × 105/2 × 105) with allogeneic mature DCs served as control. After 2 days, proliferation of activated control T cells cocultured with anergic T cells was markedly inhibited (Figure1) compared with T lymphocytes stimulated only with mature DCs in the absence of syngeneic anergic T cells. As previously shown, anergic T cells stimulated with allogeneic DCs showed an impaired proliferation in contrast to T cells activated by allogeneic DCs in an MLR. To test whether these effects of anergic T cells in our system are alloantigen-specific, activated control T cells were generated from a second donor and used in the restimulation experiments. In contrast to the results described above, an unrestricted T-cell response was observed in these experiments independent of coculture with anergic or activated control T cells (Figure 1).
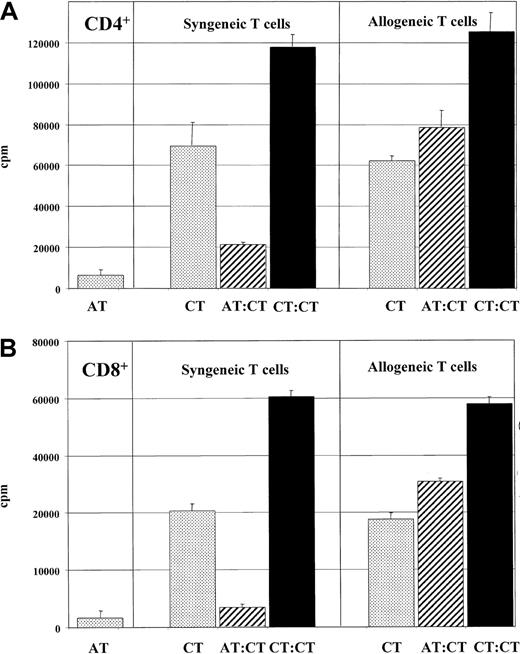
Alloantigen-specific suppressor activity of anergic T cells.
Anergic T cells were found to display alloantigen-specific suppressor activity. First, 1 × 105 anergic CD4+ (panel A) or CD8+ (panel B) T cells (ATs) (generated by stimulation with IL-10–treated DCs) were cocultured with an equivalent number of activated control T cells (CTs) (stimulated with mature DCs) and restimulated with mature DCs (2 × 104) from the same DC donor used during induction of anergy (hatched bars) as described in “Materials and methods.” A coculture of the same number of T cells (2 × 105) (CTs) with mature DCs served as controls (black bars). To test the alloantigen specificity, anergic T cells were cocultured with T cells from a second unrelated donor in a second experiment. Anergic T cells (1 × 105) (ATs) stimulated with allogeneic DCs (1 × 104) and allogeneic and syngeneic activated control T cells (1 × 105) (syn CTs, allo CTs) activated by allogeneic DCs (1 × 104) from the same donor used in the primary culture served as controls (dotted bars). T-cell proliferation was measured after 3 days of incubation and an additional 16-hour pulse with 3H-Tdr (37 kBq per well) by means of a liquid scintillation counter. Similar results were observed in 5 independent experiments.
Alloantigen-specific suppressor activity of anergic T cells.
Anergic T cells were found to display alloantigen-specific suppressor activity. First, 1 × 105 anergic CD4+ (panel A) or CD8+ (panel B) T cells (ATs) (generated by stimulation with IL-10–treated DCs) were cocultured with an equivalent number of activated control T cells (CTs) (stimulated with mature DCs) and restimulated with mature DCs (2 × 104) from the same DC donor used during induction of anergy (hatched bars) as described in “Materials and methods.” A coculture of the same number of T cells (2 × 105) (CTs) with mature DCs served as controls (black bars). To test the alloantigen specificity, anergic T cells were cocultured with T cells from a second unrelated donor in a second experiment. Anergic T cells (1 × 105) (ATs) stimulated with allogeneic DCs (1 × 104) and allogeneic and syngeneic activated control T cells (1 × 105) (syn CTs, allo CTs) activated by allogeneic DCs (1 × 104) from the same donor used in the primary culture served as controls (dotted bars). T-cell proliferation was measured after 3 days of incubation and an additional 16-hour pulse with 3H-Tdr (37 kBq per well) by means of a liquid scintillation counter. Similar results were observed in 5 independent experiments.
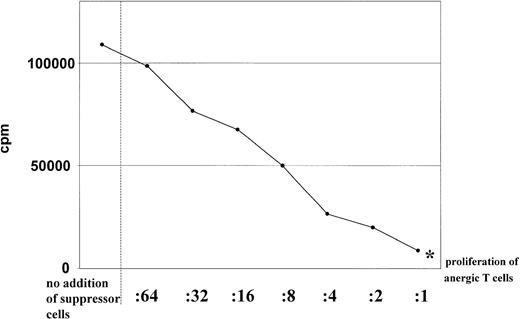
As shown in Figure 2, addition of various numbers of anergic CD4+ T cells to cultures of activated CD4+ T cells revealed that anergic T cells suppressed the proliferation of syngeneic T cells in response to allogeneic mature DCs in a dose-dependent manner. At a 1:1 ratio of anergic to activated T cells, the proliferation of T cells is nearly reduced to the level of proliferation of the anergic T lymphocytes alone (Figure 2). Similiar results were obtained when naive syngeneic CD4+ T cells were cocultured with anergic CD4+ T cells (data not shown).
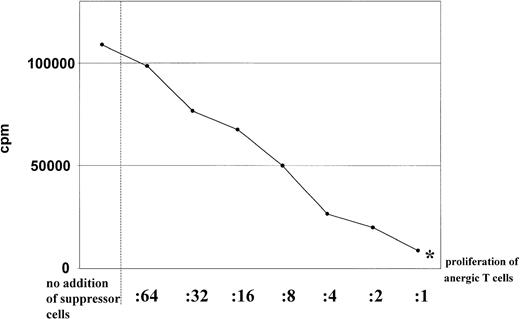
Dose dependence of the suppressor activity.
Suppressor activity of anergic T cells was found to be dose dependent. Different numbers of anergic CD4+ (in the ratios indicated) were restimulated with allogeneic DCs (1 × 104) (generated from the same donor used for the primary culture) in the presence of syngeneic activated CD4+ T cells (1 × 105) stimulated with mature DCs. Proliferation of anergic T cells alone were indicated by an asterisk. Proliferation of T cells was determined by addition of 3H-Tdr after 3 days of culture. Similar results were obtained in 5 independent experiments.
Dose dependence of the suppressor activity.
Suppressor activity of anergic T cells was found to be dose dependent. Different numbers of anergic CD4+ (in the ratios indicated) were restimulated with allogeneic DCs (1 × 104) (generated from the same donor used for the primary culture) in the presence of syngeneic activated CD4+ T cells (1 × 105) stimulated with mature DCs. Proliferation of anergic T cells alone were indicated by an asterisk. Proliferation of T cells was determined by addition of 3H-Tdr after 3 days of culture. Similar results were obtained in 5 independent experiments.
These experiments demonstrate that anergic T cells induced by coculture with IL-10–treated DCs suppressed the activation of T cells in an alloantigen-specific manner and showed no unspecific bystander inhibition of the immune response.
Antigen-specific suppression of cocultured T cells is induced by anergic T cells
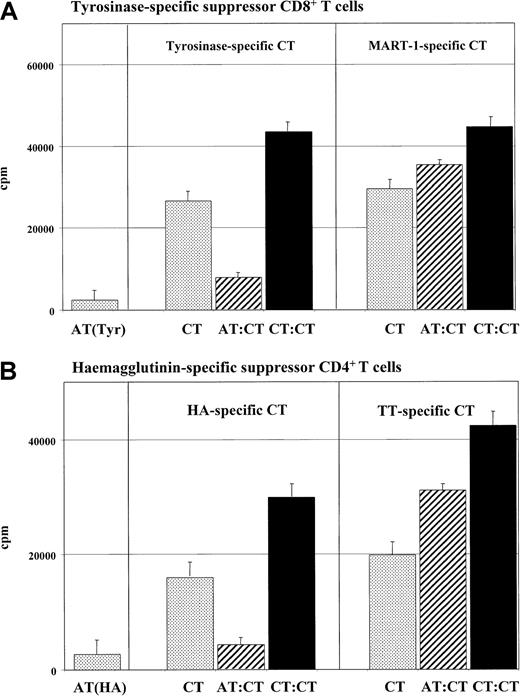
In our previous studies, we demonstrated that IL-10–treated DCs induce anergic T cells in peptide-specific models of T-cell activation. Thus, we assessed whether these anergic T cells are able to inhibit the activation of peptide-specific T cells. Anergic T cells (5 × 104) were incubated with syngeneic activated T cells (5 × 104) of the same specificity and stimulated with mature DCs of the same donor. In contrast to control experiments with activated T cells, inhibition of proliferation of cocultured syngeneic activated tyrosinase-specific CD8+ T cells (Figure 3A) or HA-specific CD4+ T cells (Figure 3B) was observed after coculture with anergic T cells.
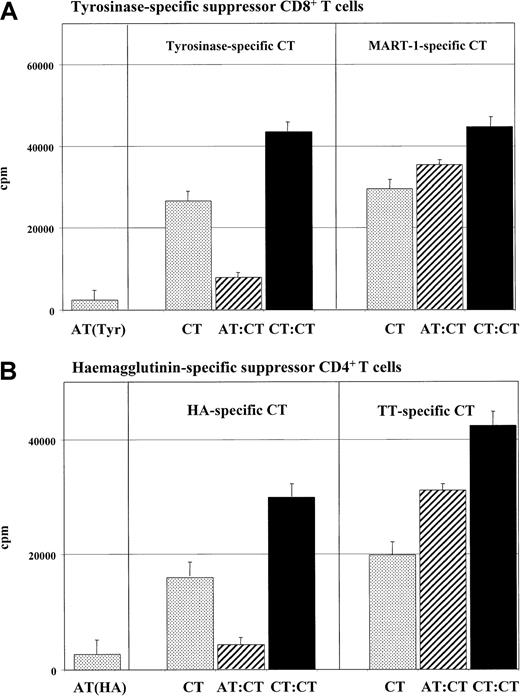
Peptide-specific suppression of cocultured T cells induced by anergic T cells.
Tyrosinase-specific CD8+ T-cell lines or HA-specific CD4+ T cells of the HA1.7 clone were cultured and restimulated as described in “Materials and methods.” For anergy induction, these cells were cocultured with IL-10–treated DCs of HLA-A.2 (tyrosinase) or HLA-DR1+ (HA1.7) donors. Subsequently, (5 × 104) anergic tyrosinase-specific CD8+ T cells (ATs) (panel A) or CD4+ T cells of the clone HA1.7 (ATs) (panel B) were cocultured with (5 × 104) syngeneic T cells (CTs) of the same specificity and restimulated with syngeneic mature DCs (1 × 104) (pulsed with the specific antigen) (hatched bars). A coculture of control activated (tyrosinase- or HA-specific) T cells (1 × 105) (CTs) with mature DCs served as controls (black bars). To test the peptide specificity, control CD8+MART-1 or TT CD4+ T cells (5 × 104) were cocultured with anergic syngeneic tyrosinase CD8+ or anergic syngeneic HA-specific CD4+ T cells (5 × 104) and stimulated with syngeneic mature DCs (1 × 104). As controls, anergic (ATs) or activated (Tyr or MART CTs) (5 × 104) T cells were stimulated with syngeneic (5 × 103) DCs in an MLR (dotted bars). T-cell proliferation was measured after 3 days of incubation and an additional 16-hour pulse with 3H-Tdr (37 kBq/well) by means of a liquid scintillation counter. Similiar results were measured in 5 independent experiments.
Peptide-specific suppression of cocultured T cells induced by anergic T cells.
Tyrosinase-specific CD8+ T-cell lines or HA-specific CD4+ T cells of the HA1.7 clone were cultured and restimulated as described in “Materials and methods.” For anergy induction, these cells were cocultured with IL-10–treated DCs of HLA-A.2 (tyrosinase) or HLA-DR1+ (HA1.7) donors. Subsequently, (5 × 104) anergic tyrosinase-specific CD8+ T cells (ATs) (panel A) or CD4+ T cells of the clone HA1.7 (ATs) (panel B) were cocultured with (5 × 104) syngeneic T cells (CTs) of the same specificity and restimulated with syngeneic mature DCs (1 × 104) (pulsed with the specific antigen) (hatched bars). A coculture of control activated (tyrosinase- or HA-specific) T cells (1 × 105) (CTs) with mature DCs served as controls (black bars). To test the peptide specificity, control CD8+MART-1 or TT CD4+ T cells (5 × 104) were cocultured with anergic syngeneic tyrosinase CD8+ or anergic syngeneic HA-specific CD4+ T cells (5 × 104) and stimulated with syngeneic mature DCs (1 × 104). As controls, anergic (ATs) or activated (Tyr or MART CTs) (5 × 104) T cells were stimulated with syngeneic (5 × 103) DCs in an MLR (dotted bars). T-cell proliferation was measured after 3 days of incubation and an additional 16-hour pulse with 3H-Tdr (37 kBq/well) by means of a liquid scintillation counter. Similiar results were measured in 5 independent experiments.
To test the antigen/peptide specificity of this suppression, the anergic T cells were cocultured with syngeneic T cells specific for a second antigen (tyrosinase-specific T cells were cocultured with MART-1–specific T cells; HA-specific T cells, with TT-specific T cells). An unrestricted T-cell response was measured when syngeneic tyrosinase-specific anergic T cells and activated MART-1–specific CD8+ T cells (Figure 3A) or anergic HA-specific and TT-specific CD4+ T cells (Figure 3B) were cocultured in these experiments.
These results suggest that the supression of anergic T cells in our system is not restricted to alloantigen-specific interactions but is also relevant for antigen-specific and peptide-specific interactions. No antigen-unspecific bystander inhibition is mediated by anergic T cells induced by IL-10–treated DCs.
Cell-to-cell contact is required for the suppressive function of anergic T cells
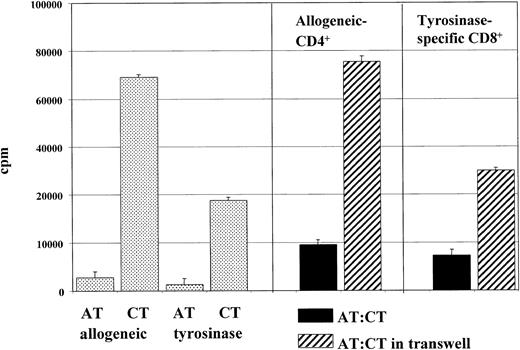
The observed immunosuppressive effect of anergic T cells could be mediated by surface molecules and/or by soluble mediators. To analyze the underlying mechanisms involved, transwell experiments were performed (Figure 4). Alloreactive anergic CD4+ T cells were either added directly to cocultures of activated control CD4+ T cells or placed in transwell chambers in the same well and activated with allogeneic mature DCs generated from the same donor used for induction of anergy. The semipermeable polycarbonate membrane allows the free exchange of soluble factors but excludes direct cell contact of the 2 cell populations. As shown in Figure 4, coculture experiments with syngeneic anergic and activated control T cells demonstrate a suppressed proliferation of cocultured control T cells. In contrast, separation of CD4+ T-cell populations in transwell chambers completely abrogated this immunosuppressive effect of anergic T cells. Alloreactive CD4+ T cells proliferated in the presence of allogeneic mature DCs, whereas control anergic T cells with low proliferative capacity showed only weak rates of expansion. In Figure4, similiar results were shown in experiments with anergic tyrosinase-specific CD8+ T cells cocultured with syngeneic tyrosinase-specific CD8+ T cells.
Need for cell-to-cell contact for suppressor activity of anergic T cells.
Cell-to-cell contact was found to be necessary for suppresor activity of anergic T cells. Transwell experiments were done in 24-well plates. Anergic alloantigen-specific 106 T cells (ATs) or anergic peptide (tyrosinase)–specific 105 T cells (ATs) were stimulated with (105 or 104) mature DCs (loaded with the specific antigen). Additionally, 106 allogeneic activated control (CTs) or 105 peptide-specific control T cells (CTs) were either added directly to the cultures of anergic T cells (ATs) or placed in transwell chambers (0.4 μm) (Millicell) (Millipore) in the same well with (105 or 104) mature DCs (primed with the specific antigen). After 3 days of culture, activated T cells (105 per well) were transferred to 96-well plates in triplicate. Proliferation was measured after an additional 16-hour pulse with 3H-Tdr by means of a liquid scintillation counter. Similar results were obtained in 5 independent experiments.
Need for cell-to-cell contact for suppressor activity of anergic T cells.
Cell-to-cell contact was found to be necessary for suppresor activity of anergic T cells. Transwell experiments were done in 24-well plates. Anergic alloantigen-specific 106 T cells (ATs) or anergic peptide (tyrosinase)–specific 105 T cells (ATs) were stimulated with (105 or 104) mature DCs (loaded with the specific antigen). Additionally, 106 allogeneic activated control (CTs) or 105 peptide-specific control T cells (CTs) were either added directly to the cultures of anergic T cells (ATs) or placed in transwell chambers (0.4 μm) (Millicell) (Millipore) in the same well with (105 or 104) mature DCs (primed with the specific antigen). After 3 days of culture, activated T cells (105 per well) were transferred to 96-well plates in triplicate. Proliferation was measured after an additional 16-hour pulse with 3H-Tdr by means of a liquid scintillation counter. Similar results were obtained in 5 independent experiments.
Suppression requires T-cell stimulation by APCs
Next, we assessed whether the presence of antigen-specific DCs is essential during the process of suppression. Coculture experiments of anergic alloantigen-specific CD4+ or tyrosinase-specific CD8+ T cells and activated control T cells were performed with DCs generated from the same donor used for anergy induction or without APCs. To exclude an inhibitory effect of the anergic T cells during the second restimulation, T cells were subsequently rescued and cultured without additional stimulation (DCs or IL-2) for 2 to 3 days to remove the anergic T cells as it has been demonstrated that anergic T cells undergo rapid apoptosis without further activation. To determine the time point of the initiation of the second restimulation, simultaneous experiments with isolated populations of control anergic or activated T cells (stimulated with DCs or without APCs) were performed, and the viability of the T cells was analyzed as described in “Materials and methods” (data not shown). The remaining T cells were restimulated with mature DCs, and the T-cell proliferation was measured after 2 to 3 days.
No suppressor activity was observed if no dendritic cells (Figure5) or DCs generated from an unrelated second donor (data not shown) were used in the coculture of anergic and activated control T cells. These results indicate that an antigen-specific stimulation is necessary for the suppression of the T-cell response, but do not distinguish between an effect of the APCs on the induction of the regulatory properties of the anergic T cells and an effect of the APCs on the activation of the target T cells to become susceptible to the suppressor capacities of the anergic T cells.
Effect of antigen-specific stimulation on suppressor activity of anergic T cells.
Suppressor activity of anergic T cells was found to require antigen-specific stimulation. Anergic alloantigen-specific CD4+ (1 × 105) or tyrosinase-specific CD8+ T cells (5 × 104) (ATs) were cocultured with syngeneic activated T cells (CTs) (1 × 105 or 5 × 104) and stimulated with allogeneic DCs (2 × 104 or 5 × 104) used for the induction of anergy or without APCs. Subsequently, the T cells were rescued, cultured for 4 to 5 days without additional stimulation (DCs or IL-2) to remove the anergic T cells, and then cocultured with allogeneic DCs to test the suppressor activity as described in detail in “Materials and methods.” Proliferation was measured after an additional 16-hour pulse with 3H-Tdr by means of a liquid scintillation counter. One of 3 representative experiments is shown.
Effect of antigen-specific stimulation on suppressor activity of anergic T cells.
Suppressor activity of anergic T cells was found to require antigen-specific stimulation. Anergic alloantigen-specific CD4+ (1 × 105) or tyrosinase-specific CD8+ T cells (5 × 104) (ATs) were cocultured with syngeneic activated T cells (CTs) (1 × 105 or 5 × 104) and stimulated with allogeneic DCs (2 × 104 or 5 × 104) used for the induction of anergy or without APCs. Subsequently, the T cells were rescued, cultured for 4 to 5 days without additional stimulation (DCs or IL-2) to remove the anergic T cells, and then cocultured with allogeneic DCs to test the suppressor activity as described in detail in “Materials and methods.” Proliferation was measured after an additional 16-hour pulse with 3H-Tdr by means of a liquid scintillation counter. One of 3 representative experiments is shown.
Anergic T cells display increased surface and intracellular expression of the CTLA-4 molecule
Activated T cells are characterized by the expression of typical surface molecules. To compare anergic CD4+ T lymphocytes induced by IL-10–treated DCs with activated control CD4+ T cells, kinetic studies of various surface molecules were performed.
Figure 6A shows the results of FACS analysis of anergic alloantigen-specific CD4+ T cells obtained 48 hours after initiation of a restimulation experiment. After restimulation with mature DCs as described in “Materials and methods,” anergic CD4+ T cells down-regulated activation markers such as CD11a, CD25, CD28, and CD69 compared with activated T lymphocytes previously activated by mature DCs in the primary culture. No differences were observed in the expression of CD4, CD8, CD122 (beta chain of the IL-2 receptor), CD95, or CD95L (data not shown). However, increased surface expression of the CTLA-4 molecule was observed (Figure 6A).
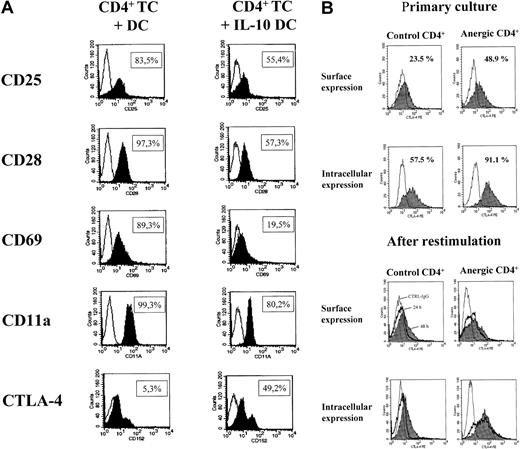
Expression of activation markers and CTLA-4 on anergic cells.
Anergic T cells were found to down-regulate the expression of activation markers and display an increased expression of CTLA-4. Fluorescence-activated cell sorter (FACS) analysis was performed 48 hours after initiation of a restimulation experiment (panel A). Alloantigen-specific CD4+ anergic T cells were generated by stimulation with allogeneic IL-10–treated DCs, and activated CD4+ T cells were stimulated with allogeneic mature DCs. Subsequently, the T cells were restimulated with mature DCs and stained as described in “Materials and methods.” Kinetic studies of the surface and intracellular staining of the CTLA-4 molecule were performed during primary culture and after restimulation with mature DCs as described in “Materials and methods.” CD4+ T cells were analyzed during primary culture (day 5) and at 24 and 48 hours after restimulation with mature DCs (panel B).
Expression of activation markers and CTLA-4 on anergic cells.
Anergic T cells were found to down-regulate the expression of activation markers and display an increased expression of CTLA-4. Fluorescence-activated cell sorter (FACS) analysis was performed 48 hours after initiation of a restimulation experiment (panel A). Alloantigen-specific CD4+ anergic T cells were generated by stimulation with allogeneic IL-10–treated DCs, and activated CD4+ T cells were stimulated with allogeneic mature DCs. Subsequently, the T cells were restimulated with mature DCs and stained as described in “Materials and methods.” Kinetic studies of the surface and intracellular staining of the CTLA-4 molecule were performed during primary culture and after restimulation with mature DCs as described in “Materials and methods.” CD4+ T cells were analyzed during primary culture (day 5) and at 24 and 48 hours after restimulation with mature DCs (panel B).
To address the role of the CTLA-4 molecule in the suppressor activity of anergic T cells, additional experiments were performed to analyze the surface and intracellular expression of the CTLA-4 molecule, which is known to be involved in down-regulation of T-cell function and in tolerance induction. Compared with activated CD4+ T cells stimulated with mature DCs, anergic CD4+ T cells are characterized by increased surface and intracellular expression of the CTLA-4 molecule after primary culture (day 5) and 24 and 48 hours after restimulation with mature DCs (Figure6B).
CTLA-4 is involved in the suppressor activity of anergic T cells
The molecule CTLA-4 is known to be involved in the down-regulation of T-cell activation and tolerance induction. Since we detected an increased expression of the CTLA-4 molecule on anergic CD4+T cells in our system, we wanted to investigate the role of the CTLA-4 molecule during immune suppression mediated by anergic T cells in experiments with neutralizing antibodies.
Results demonstrated that blocking antibodies to the CTLA-4 molecule restored the T-cell activity up to 70% in experiments performed with anergic alloantigen-specific CD4+ T cells (Figure 7).
Role of CTLA-4, B7-1, and B7-2 in the suppressor activity of anergic T cells.
CTLA-4 and B7-2 were found to be involved in the suppressor activity of anergic T cells. Alloantigen-specific anergic CD4+ (1 × 105) T cells were cocultured with an equivalent number of activated control T cells (1 × 105) (CTRL) of the same donor and stimulated with mature DCs (2 × 104) from the same DC donor as used during induction of anergy (hatched bars). A coculture of syngeneic control CD4+ T cells (1 × 105) (CTRL) with mature DCs served as controls (black bars). The mAb to CTLA-4, B7-1, and B7-2 or control antibody was added to the coculture experiments as described in “Materials and methods.” T-cell proliferation was measured after 3 days of incubation and an additional 16-hour pulse with 3H-Tdr (37 kBq per well) by means of a liquid scintillation counter. One of 4 independent experiments is shown.
Role of CTLA-4, B7-1, and B7-2 in the suppressor activity of anergic T cells.
CTLA-4 and B7-2 were found to be involved in the suppressor activity of anergic T cells. Alloantigen-specific anergic CD4+ (1 × 105) T cells were cocultured with an equivalent number of activated control T cells (1 × 105) (CTRL) of the same donor and stimulated with mature DCs (2 × 104) from the same DC donor as used during induction of anergy (hatched bars). A coculture of syngeneic control CD4+ T cells (1 × 105) (CTRL) with mature DCs served as controls (black bars). The mAb to CTLA-4, B7-1, and B7-2 or control antibody was added to the coculture experiments as described in “Materials and methods.” T-cell proliferation was measured after 3 days of incubation and an additional 16-hour pulse with 3H-Tdr (37 kBq per well) by means of a liquid scintillation counter. One of 4 independent experiments is shown.
Additionally, to test the importance of the costimulatory pathways mediated by B7-1/B7-2 molecules during the suppressor activity of anergic T cells, blocking mAbs to the costimulatory molecules B7-1 or B7-2 were used. As shown in Figure 7, blocking of the B7-2 pathway inhibited the suppressor activity of alloantigen-specific anergic CD4+ T cells. In contrast, no effect was observed when mAbs to the B7-1 molecule or control antibodies were used. Similar results were obtained for anergic peptide-specific T cells cocultured with activated T cells of the same specificity (data not shown).
In control experiments, blocking antibodies to B7-1, B7-2, and CTLA-4 were added to control CD4+ T cells stimulated with mature DCs. As described previously, antibodies to B7-1/B7-2 blocked the stimulatory capacity of APCs, whereas anti–CTLA-4 mAb inhibited the negative regulation induced by activation of the CTLA-4 pathway (Figure7).29 30
Soluble mediators are not involved in the suppressor activity of anergic T cells
Immunosuppressive cytokines might be involved in the suppressor activity of anergic T cells. Therefore, we assessed the cytokine pattern of T cells after restimulation with mature DCs by ELISA. Compared with activated control CD4+ and CD8+ T cells, anergic CD4+ or CD8+ T cells showed a markedly reduced secretion of the cytokines IL-2 and IFN-γ, but little or no production of IL-4 or IL-10, as described before (Table1).16 After coculture of anergic and syngeneic tyrosinase-specific CD8+ T cells, a similar cytokine pattern was measured (Table 1). These data indicate blocking of TH1/TC1 cytokine production without a shift to a TH2/TC2 pattern in anergic and cocultured T cells (Table 1).
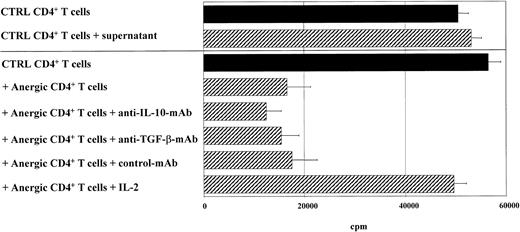
The inhibitory effect of anergic T cells on the proliferation of syngeneic T cells of the same specificity might be a result of unknown soluble inhibitory factors produced by anergic T cells, which were not detectable by the conventional ELISAs used. To test this hypothesis, transfer experiments with supernatants of anergic alloantigen-specific CD4+ or CD8+ T cells were performed. These supernatants were added to syngeneic (alloantigen-specific) control CD4+ or CD8+ T cells additionally stimulated with mature DCs generated from the same donor used for anergy induction (Figure 8). Addition of supernatants harvested from anergic T cells did not mimic the suppressor activity of anergic T cells, indicating that soluble mediators such as cytokines cannot mediate the suppressor function of anergic T cells (Figure 8). Further experiments showed that high doses of IL-2, but not blocking antibodies to IL-10 or TGF-β, overcame the suppressor activity of anergic T cells (Figure 8). Similar results were obtained when peptide-specific T cells were used (data not shown).
Effect of soluble mediators on the suppressor activity of anergic T cells.
Soluble mediators were found to be not essential for the suppressor activity of anergic T cells. Alloantigen-specific anergic CD4+ T cells (1 × 105) were cocultured with an equal number of activated control T cells (1 × 105) (CTRL) and stimulated with mature DCs (1 × 104) from the same donor used during induction of anergy. A coculture of control syngeneic CD4+ T cells (1 × 105) (CTRL) with DCs served as controls. Blocking antibodies to IL-10, TGF-β, and IL-2 (100 U/mL) were added to coculture experiments. In some experiments, supernatants of anergic T cells were harvested and added to coculture of syngeneic control T cells. T-cell proliferation was measured after 3 days of incubation and an additional 16-hour pulse with3H-Tdr (37 kBq per well) by means of a liquid scintillation counter.
Effect of soluble mediators on the suppressor activity of anergic T cells.
Soluble mediators were found to be not essential for the suppressor activity of anergic T cells. Alloantigen-specific anergic CD4+ T cells (1 × 105) were cocultured with an equal number of activated control T cells (1 × 105) (CTRL) and stimulated with mature DCs (1 × 104) from the same donor used during induction of anergy. A coculture of control syngeneic CD4+ T cells (1 × 105) (CTRL) with DCs served as controls. Blocking antibodies to IL-10, TGF-β, and IL-2 (100 U/mL) were added to coculture experiments. In some experiments, supernatants of anergic T cells were harvested and added to coculture of syngeneic control T cells. T-cell proliferation was measured after 3 days of incubation and an additional 16-hour pulse with3H-Tdr (37 kBq per well) by means of a liquid scintillation counter.
Discussion
In our study, we demonstrate that human anergic T cells induced by IL-10–treated DCs display suppressor activity in an antigen-specific manner. In both the allogeneic and a peptide-specific system, CD4+ and CD8+ T cells inhibited the proliferation and activity of cocultured T cells. This suppressor activity required cell-to-cell contact and antigen-pulsed DCs and was not transferred by soluble mediators.
The induction of antigen-specific tolerance is critical for the prevention of autoimmunity and maintenance of immune homeostasis. The ability of the immune system to distinguish between self and nonself and between innocuous and harmful antigens is controlled by mechanisms of central and peripheral tolerance.1-5 Several mechanisms, including induction of clonal deletion, cell death, and anergy, are well characterized.2-5 In addition, active inhibition by regulatory or suppressor T cells is also crucial for maintaining tolerance in the periphery.31
At present, the term “regulatory” T cells is used to describe a variety of T-cell subsets with regulatory properties based on the expression of surface molecules, cytokine production, and functional assays.31 One of the best-characterized populations of murine regulatory CD4+ T cells is defined by a constitutive expression of the alpha chain of the IL-2 receptor (CD25).32 These cells arise from the thymus of naive mice, and their function and activation are thought to be essential for the maintenance for self-tolerance and control of autoimmunity.33 They compose 5% to 10% of the peripheral CD4+ T cells in mice and suppress immune responses in vivo and in vitro via non–antigen-specific mechanisms.32,34Recently, it was demonstrated that regulatory CD4+CD25+ T cells with similiar properties also exist in the human system.35-39
A subset of regulatory CD4+ cells was developed by addition of IL-10 to primary murine T-cell culture and was termed Tr (regulatory)-1 cells.40 Tr1 cells are distinct from TH1/TH2 cells in that they produce high levels of IL-10, moderate amounts of TGF-β, little IL-2, and no IL-4. Tr1 cells proliferate poorly after TCR-mediated stimulation and suppress immune responses in vivo and in vitro.40,41 It was shown that suppression is primarily mediated by IL-10 and TGF-β.40,41 In contrast, other investigators demonstrated that the suppressor activity is cytokine independent, but mediated through direct cell-to-cell contact.42 These discrepancies might be due to different subpopulations of Tr1 cells but could also be related to the differences in the experimental design. Furthermore, CD4+ T cells called TH3 are characterized as regulatory T cells in the model of oral tolerance. After feeding low doses of nominal or self-antigens, the development of regulatory T cells is induced, mediating their suppressor function via TGF-β secretion.43
A role for DCs in the induction of peripheral tolerance has been proposed by several studies.25,26,44,45 Most of these studies support the notion that the maturation state of DCs and/or subtype of DCs are fundamental in the induction of peripheral tolerance.46,47 Recently, it was shown that immature human DCs may control peripheral tolerance by inducing the differentation of human nonproliferating Tr-like (regulatorylike) cells with regulatory properties in vitro and in vivo.47,48 The CD4+T cells induced by immature DCs in vitro and CD8+ T cells induced in vivo appear to share key properties with Tr cells in that they both produce high amounts of IL-10 and suppress the activation of cocultured T cells in an antigen-nonspecific fashion.48 49The suppressor activity requires cell-to-cell contact, but immunsuppressive cytokines such as IL-10 and TGF-β and the induction of CTLA-4 pathway appear to be dispensable for the suppressor function of the T cells.
In contrast to regulatory Tr or Tr-like cells induced by immature DCs or CD4+/CD25+ T cells, anergic T cells induced by IL-10–treated human DCs suppress the activity of cocultured T cells in an antigen-specific mechanism as demonstrated in allogeneic and peptide-specific systems. Additionally, anergic suppressor T cells show no production of imunosuppressive cytokines such as IL-10 or TGF-β. Furthermore, the suppressor activity is not mediated by other soluble mediators as demonstrated by ELISA and blocking experiments. However, in agreement with other populations of regulatory T cells, cell-to-cell contact is required for the induction of the suppressor activity of the anergic T cells.
In further contrast to CD4+/CD25+ T cells in the murine and human system, the anergic T cells in our study express reduced surface levels of CD25 as demonstrated by FACS analysis, and depletion of the CD4+/CD25+ T-cell subpopulation did not inhibit the suppressor activity of the anergic T cells (unpublished data, 2001).
In our hands, the suppressor T cells induced by coculture with IL-10–treated DCs display high surface and extracellular expression of the CTLA-4 molecule. Evidence exists that CTLA-4 can act as a negative regulator of T-cell activation.49 CTLA-4 deficiency or blockade induces or exacerbates autoimmunity, enhances tumor immunity, or prevents induction of immunologic tolerance.50-52 In agreement with these results, blocking of the CTLA-4 signaling on anergic T cells induced by IL-10–treated DCs inhibits the suppressor activity of the T cells, pointing toward an important role of the CTLA-4 pathway for the suppressor function of anergic T cells in our system. Recent studies have shown that regulatory T cells stimulated via CTLA-4 predominantly secrete TGF-β, suggesting a role for this immunosuppressive cytokine in the suppressor function of the T-cell population. Our experiments demonstrate that neither TGF-β nor other immunosuppressive cytokines are involved in the suppressor activity of the anergic T cells induced by IL-10–treated DCs.
Additionally, IL-10–treated DCs can also induce anergic CD8+ suppressor T cells. Compared with CD4+ T cells, anergy among CD8+ T cells is less well characterized, although anergic CD8+ T cells have been induced in vivo and in vitro.53-55 Furthermore, similiar to CD4+ Tr cells, CD8+ Tr cells can be isolated in vitro after multiple stimulations and are known to suppress antigen-specific responses by down-regulation of CD86 expression on APCs.56 Furthermore, CD8+ suppressor cells, which regulate the immune responses via immunosuppressive cytokines (IL-4, IL-5, or IL-10), were identified in vivo in the murine model of low zone tolerance or in patients suffering from leprosy.57,58 Anergic CD8+ T cells induced by IL-10–treated DCs inhibit the proliferation of cocultured CD8+ T cell in an antigen-specific manner without any effect on cocultured DCs as observed by analyzing the accessory capacity and the expression of surface molecules on DCs after coculture with anergic T cells (unpublished data). Furthermore, similar to anergic CD4+ T cells, anergic CD8+ T cells with suppressor activity do not release immunosuppressive cytokines. The concept that anergic CD4+ T cells can exert regulatory effects as suppressor cells was demonstrated in vivo and in vitro.25,26 59-62 More importantly, to the best of our knowledge, this study is the first report to show that human anergic CD8+ T cells have suppressor properties.
Our observation that IL-10–treated DCs induce anergic CD4+/CD8+ T cells with antigen-specific suppressor activity opens new therapeutic perpectives for the use of DCs in vivo. Induction of anergic T cells might be exploited therapeutically for suppression of cellular immune responses in allergic or autoimmune diseases with identified (auto-) antigens.
The authors thank Dr H. Jonuleit, Dr E. von Stebut, and Dr T. Tüting for critical reading of the manuscript.
Supported by a grant from the German Research Foundation (SFB548/B6 and A6).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Kerstin Steinbrink, Department of Dermatology, University of Mainz, Langenbeckstr 1, D-55131 Mainz, Germany; e-mail:steinbrink@hautklinik.klinik.uni-mainz.de.