Movement of T-lymphocyte cell surface CD43 is associated with both antigen activation of T-cell clones and chemokine induction of T-lymphocyte motility. Here, we demonstrate that CD43 movement away from the site of T-cell receptor ligation occurs in unprimed CD4+ T cells as well as T-cell clones. The T-cell receptor (TCR)-dependent movement of CD43 in unprimed T cells is associated with a polarized morphology and CD43 accumulation at the uropods of the cells, unlike that reported for primed T cells. The polarization of CD43 has a requirement for Src kinases and occurs in conjunction with lipid raft coalescence. Thymocytes and T-cell hybridomas, cells that have altered responses to TCR activation and lack lipid raft coalescence, do not polarize CD43 as readily as unprimed T cells. The movement of CD43 depends on the cholesterol biosynthetic pathway enzyme 3-hydroxy-3-methylglutaryl coenzyme A (HMG CoA) reductase. Blockade of this enzyme can specifically prevent CD43 redistribution without affecting cell shape polarization. The likely mechanism of this alteration in CD43 redistribution is through decreased protein prenylation because the cholesterol-dependent lipid rafts still coalesce on activation. These findings suggest that the polarization of cell shape, lipid raft coalescence, and CD43 redistribution on T-cell activation have signaling pathway distinctions. Dissecting out the relationships between various stages of molecular redistribution and lymphocyte activation may facilitate fine-tuning of immunologic responses.
Introduction
Although the T-cell receptor (TCR)–ligand interaction is critical for T-cell activation, other signals play a major role in T-cell activation.1,2 This requirement for non-TCR signals has been called the “2 signal model” with TCR ligation being “signal 1,” and the delivery of an additional costimulatory signal being “signal 2.”3-5 Although separate signaling pathways for costimulatory receptor-ligand pairs have been suggested to explain the enhancement of T-cell activation, a number of models have been proposed suggesting the costimulatory signal operates through enhancement of TCR-ligand interactions. One model states that “signal 1” correlates with inclusion or stabilization of TCR chains within specialized membrane compartments6referred to as rafts, DRMS, GEMS, or DIGS. These are sphingolipid cholesterol-rich membrane domains, which are associated with GPI-linked proteins, lck, LAT, ras, and other signaling molecules.7 These domains are generally small with an estimated diameter of 70 nm in unstimulated cell membranes.8 This “signal 1” activation is associated with protein phosphorylation and can signal for apoptosis. “Signal 2” correlates with coalescence of these small specialized membrane domains into a large domain at the T cell–antigen-presenting cell (APC) interface and is associated with lck SH3 interactions.6 CD28-mediated costimulation facilitates raft coalescence at the T cell-APC contact site.9 This “signal 2” activation is associated with interleukin (IL)-2 production, proliferation, and resistance to apoptosis in mature T cells. Ligation of costimulatory molecules CD28 and leukocyte function-associated antigen 1 (LFA-1) have also been shown to enhance actin-mediated transport of cell surface molecules toward the T cell–APC contact site.10 In addition to simply moving toward the T cell-APC–interface, the TCR and other cell surface molecules arrange into a supramolecular activation complex (SMAC) with a defined geometry.11,12 This leads to formation of an immunologic synapse13-15 with the APCs that may not be an absolute requirement for all T-cell activation16 but correlates with complete activation of CD4+ T cells.12
Thus, T-cell activation is a complex positional dance of varied receptor and signaling molecules. The timing and details of this molecular choreography are crucial in determining the varied outcomes of immune cell–antigen interactions. There is a complex cycle between molecular movements and signaling with each having effects on the other. In addition to T-cell interaction with antigen, T cells must control their motility and effector functions through overlapping signaling events. To dissect out these events, we have focused on the movement of CD43 during T-cell activation. The large size, extended structure, and negative charge of CD43 suggest it must be removed from the T cell–APC contact to prevent steric blockade of TCR-major histocompatibility complex (MHC)-peptide interactions.17CD43 may also negatively signal through its cytoplasmic domain18 adding to the need for its removal from the T cell–APC interface for full T-cell activation. As predicted, fluorescence microscopy data demonstrates CD43 is excluded from the T cell–APC contact zone using a T-cell clone.19
Chemokine-activated motile T cells are polarized and will also move CD43 away from their leading edge toward an extended structure at their trailing edge called the uropod.20 This CD43 movement may facilitate cell motility21 and regulate the recruitment of other leukocytes through their interaction with the uropod. Thus, in vivo peripheral T cells moving through tissues will be polarized and already have removed the potentially inhibitory CD43 from most of their membrane surfaces when they encounter antigen. Therefore, CD43 movement in unprimed nonpolarized T cells is the more relevant question for physiologic T-cell activation in vivo. To date, CD43 movement on T-cell activation has been evaluated only with a T-cell clone.19To evaluate unprimed T cells, we purified splenic CD4+ T cells and activated them with beads coated with anti-CD3 and anti-CD28. The anti-CD3 provided the TCR complex signal and anti-CD28 provided a costimulatory signal. Negatively charged sulfite beads were used to facilitate protein coating and simulate the negative charge of the APC cell surface. In studies of T-cell clones, activation through the TCR reorganized the microtubule organizing center (MTOC) to interface with the T cell–APC contact.22 This rounded the cells and disrupted the uropod, which contained the MTOC in migrating cells.23 Strikingly, when we activated unprimed CD4+ T cells, the rounded T cells elongated and formed uropods. We demonstrated redistribution of cell surface CD43 to the uropod. Thus, CD43 is not only moved away from the TCR contact site but toward the opposite cell pole. This may facilitate other contacts in addition to removing CD43-mediated inhibition at the initial contact site. Uropod formation by antigen activation of unprimed T cells may enhance cell recruitment and motility of newly activated T cells. This may favor activation of the best T-cell specificities for a newly encountered antigen.
Initial evaluation of thymocytes and T-cell hybridomas suggested a correlation between raft coalescence and polarization of T-cell shape and CD43. Src kinase blockade of unprimed CD4+ T cells also mimics the thymocyte behavior and abrogates polarization of both T-cell shape and CD43. However, the cell shape polarization and CD43 redistribution can be separated by further modulation of signaling using a 3-hydroxy-3-methylglutaryl coenzyme A (HMG CoA) reductase inhibitor. This inhibition of CD43 redistribution does not abolish raft coalescence suggesting the cholesterol depletion of rafts is not the mechanism for this effect. Therefore, it is likely that small G proteins that require HMG CoA reductase-dependent prenylation are required for CD43 polarization but unnecessary for cell shape polarization.
Materials and methods
T-cell activation (T cell–bead conjugation)
Surfactant-free sulfate latex beads (Interfacial Dynamics, Portland, OR) were coated with anti-CD3 (2C11, purified from ascites provided by Dr Ezio Bonvini, CBER, Bethesda, MD) and anti-CD28 (37.51, Becton Dickinson Pharmingen, San Diego, CA) at 50 μg/mL of each at 4°C. The T cell–bead conjugation and slide treatment were performed as published by Burkhardt19 with some modifications. Briefly, to approximate the size of the T cells, 6-μm beads were used. After overnight incubation with the antibodies, blocking, and washing, the beads were centrifuged with 2 × 105 cells in a volume of 100 μL (10 minutes, 450g), left for 15 minutes at 37°C, and allowed to adhere to glass slides coated with poly-l-lysine for an additional 20 minutes at 37°C.
Immunofluorescence staining and microscopy
The slides were fixed in 3% paraformaldehyde/phosphate-buffered saline (PBS) for 20 minutes and incubated with 50 mM NH4Cl/PBS for 5 minutes; the cells were permeabilized with 0.3% Triton X-100 for 1 minute or 3 minutes in the case of staining for lipid rafts. The cells were blocked with a solution of 0.01% saponin and 0.25% fish skin gelatin (Sigma, St Louis, MO) for 1 hour and stained with the indicated antibodies for 45 minutes. After five 5-minute washes with PBS, the slides were mounted with coverslips in mounting medium (Kirkegaard and Perry Laboratory, Gaithersburg, MD). CD43 was detected by phycoerythrin (PE)-conjugated affinity-purified antimouse CD43 (S7, Becton Dickinson Pharmingen) and for lipid raft visualization, fluorescein isothiocyanate (FITC)-labeled cholera toxin B subunit was used (Sigma). The immunofluorescence was recorded by cooled CCD camera (Photometrics Sensys, Tucson, AZ) mounted on a Zeiss Axioplan 2 microscope, equipped with narrow-band optical filters TR-1, TR-2, and TR-3 (Chroma, Brattleboro, VT). T cell–bead interaction sites were carefully examined by adjusting the plane of focus. For statistical analysis, cell scoring was performed by an observer blinded to the cell treatment group. Statistical comparisons were done using a 2-tailed ttest.
Mice
Female BALB/c mice were obtained from the National Cancer Institute (Frederic Cancer Research and Development Center, Frederick, MD). DO.11.10 TCR transgenic mice were generously provided by Dr Elizabeth Shores (CBER, Bethesda, MD). Eight-week-old to 4-month-old mice were used as a source of CD4+ spleen T cells, and 4- to 8-week-old mice as a source of CD4+CD8+thymocytes. The mice were used under a protocol approved by an animal care committee. The mice were maintained in pathogen-free conditions at the Association for Assessment and Accreditation of Laboratory Animal Care–accredited CBER animal care facility.
Cell isolation
Commercially available columns (R & D Systems, Minneapolis, MN) were used to purify CD4+ spleen T cells by negative selection. The negatively selected cell population obtained in this manner contained less that 2% to 3% Ia+ cells and less than 1% to 2% CD8+ T cells.
The CD4+CD8+ thymocytes were isolated by fluorescence-activated cell sorting. For sorting, thymocytes were stained with anti-CD8 FITC 53-6.7 (Becton Dickinson Pharmingen) using 1 μg antibody/106 cells. The sorted population was 99% CD8+ and 95% CD4+CD8+ as determined by analysis after sorting. Cell sorting was performed with a FACStar Plus cell sorter (Becton Dickinson, Mountain View, CA) equipped with an argon laser at 488 A (Coherent Innova 90) and using the Becton Dickinson software program Cell Quest. Live cells were identified based on forward scatter (FSC)/side scatter (SSC) profile. The CD8+ T cells were collected based on fluorescence. All procedures including staining and sorting were completed on wet ice.
Treatment of cells with PP2
Purified CD4+ splenic T cells were preincubated with 20 μM PP2 (Calbiochem, La Jolla, CA) in complete medium (RPMI, 10% fetal calf serum, 2 mM l-glutamine, nonessential amino acids, 1 mM penicillin/streptomycin, and 50 μM 2-mercaptoethanol) for 15 minutes, and then activated with beads as described above. Control cells were exposed to the same dimethyl sulfoxide concentration as the PP2-treated cells.
Treatment of cells with mevastatin and mevalonic acid lactone
CD4+ spleen T cells were treated with the inhibitor as published by Rosenshine.26 Briefly, purified cells were incubated with 50 μM mevastatin (Sigma) for 18 hours and then activated with beads. Mevalonic acid lactone (Sigma) was added at a final concentration of 1 mM in indicated experiments. Untreated cells used as a control were exposed to the same ethanol concentration as the treated cells.
T cell–APC conjugation
Conjugates were made with the OVA323-339-IAd–specific CD4+ T cells, purified as described above, from TCR transgenic DO.11.10 mice and UT139.B2 cells, used as APCs. The UT139.B2 cells (a generous gift from Dr Rolf König, University of Texas, Galveston, TX) are murine L cells transfected with IAd, intercellular adhesion molecule 1 (ICAM-1), and B7-1. The APCs were pulsed for 2 hours with 10 μg/mL OVA323-339, washed, and combined with the T cells at a 1:1 ratio. The conjugates were formed by centrifugation at 450g for 10 minutes, and the pellets were incubated at room temperature for 10 minutes longer. The cells were gently resuspended and allowed to adhere to glass slides coated with poly-l-lysine for an additional 20 minutes at 37°C. When indicated, T cells were treated with mevastatin as above.
Results
CD43 is polarized in activated primary lymphocytes but activated thymocytes and T hybridomas have decreased CD43 polarization
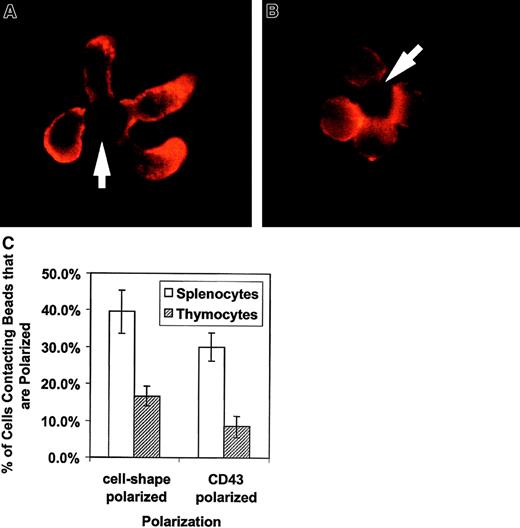
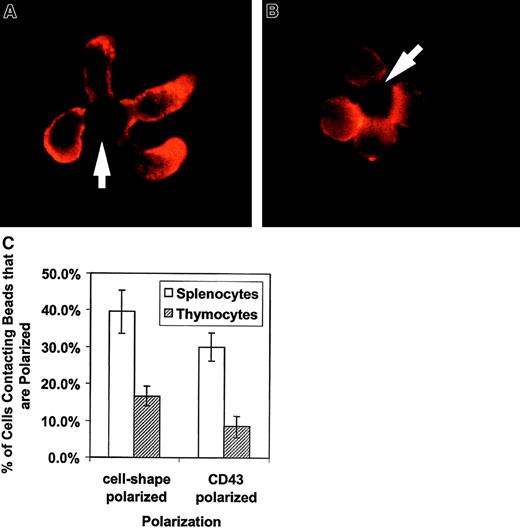
We evaluated the morphologic effect on T cells of APC-like negatively charged beads coated with anti-CD3 to stimulate the TCR complex and with anti-CD28 for costimulation. The cells were fixed and stained with anti-CD43 and evaluated by fluorescent microscopy. Figure1A shows an example of the effects of APC-like beads on purified unprimed splenic CD4+ T cells. The unprimed CD4+ T cells polarize their cell shape with a uropodlike projection away from the T cell–bead interface. CD43 also redistributes and concentrates in the uropod away from the T cell–bead interface. Thymocytes have an altered response to TCR ligation and undergo positive or negative selection rather than proliferation and cytokine production. This “signal 2”-deficient response correlates with a lack of raft coalescence at the site of TCR stimulus.27 To evaluate whether this altered response affects CD43 redistribution, we looked at cell polarization and CD43 redistribution in freshly explanted double-positive thymocytes. Figure1B has photographs of thymocytes purified by cell sorting and stained for CD43. The thymocytes have both diminished cell shape polarization and CD43 redistribution. This observation was verified by counting cell-bead clusters and scoring for polarization as shown in Figure1C.
Activation of primary T lymphocytes leads to a polarized morphology and CD43 redistribution but activation of thymocytes does not.
These results are representative of 3 independent experiments. (A) CD4+ spleen T cells were conjugated with beads coated with anti-CD3 and anti-CD28, fixed, and stained for CD43. The cells were analyzed by immunofluorescence microscopy as described in “Materials and methods.” They clearly change shape on activation and accumulate CD43 toward the opposite from the contact site end of the cell. The arrows indicate location of the beads. (B) Sorted thymocytes were activated with beads coated with anti-CD3 and anti-CD28, fixed, and stained for CD43. To obtain an enriched double-positive population of the thymocytes, cells were sorted by CD8+ selection. The activated thymocytes had a predominantly round shape without CD43 polarization. (C) Quantitation of thymocyte response to activation. The bars represent the cells polarized as a percentage of bead-conjugated cells from 3 experiments. The results for predominantly double-positive thymocytes (striped bar) and for CD4+ splenocytes (white bar) are shown. One to 2 slides with 80 to 120 bead-conjugated cells counted on each were evaluated in an experiment. The error bars represent 1 SD. The difference for cell shape polarization was significant at P < .0005 and the difference for CD43 polarization was significant at P < .00005. Original magnification × 1000.
Activation of primary T lymphocytes leads to a polarized morphology and CD43 redistribution but activation of thymocytes does not.
These results are representative of 3 independent experiments. (A) CD4+ spleen T cells were conjugated with beads coated with anti-CD3 and anti-CD28, fixed, and stained for CD43. The cells were analyzed by immunofluorescence microscopy as described in “Materials and methods.” They clearly change shape on activation and accumulate CD43 toward the opposite from the contact site end of the cell. The arrows indicate location of the beads. (B) Sorted thymocytes were activated with beads coated with anti-CD3 and anti-CD28, fixed, and stained for CD43. To obtain an enriched double-positive population of the thymocytes, cells were sorted by CD8+ selection. The activated thymocytes had a predominantly round shape without CD43 polarization. (C) Quantitation of thymocyte response to activation. The bars represent the cells polarized as a percentage of bead-conjugated cells from 3 experiments. The results for predominantly double-positive thymocytes (striped bar) and for CD4+ splenocytes (white bar) are shown. One to 2 slides with 80 to 120 bead-conjugated cells counted on each were evaluated in an experiment. The error bars represent 1 SD. The difference for cell shape polarization was significant at P < .0005 and the difference for CD43 polarization was significant at P < .00005. Original magnification × 1000.
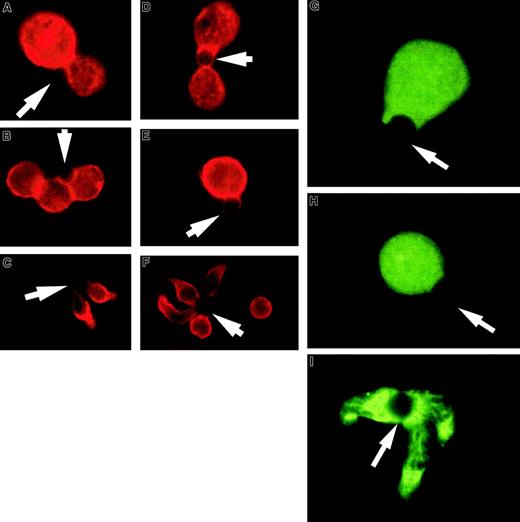
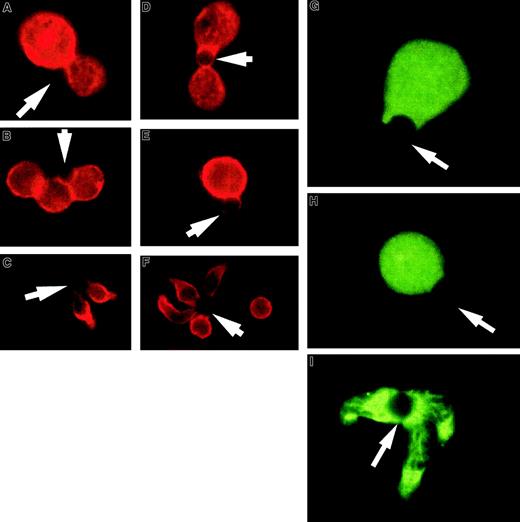
T-cell hybridomas, like thymocytes, often have a negative response to TCR activation. Figure 2A-F shows examples of the effect of APC-like beads on 2 T-cell hybridomas and purified unprimed splenic CD4+ T cells. The 3D9 hybridoma responds to class I–restricted peptide and the DO.11.10 hybridoma responds to class II–restricted peptide and expresses CD4. Although the staining of 2 T-cell hybridomas for CD43 expression revealed somewhat differing patterns, in both cases CD43 can be seen at the T cell–bead interface and the cells remain round without a uropodlike extension. Again the unprimed splenic CD4+ T cells polarize and redistribute CD43 into the uropod. Although the hybridomas did not polarize CD43, they were clearly activated on TCR engagement. Incubation of the T-cell hybridomas with APC-like beads led to a striking decrease in thymidine uptake (data not shown), consistent with the characteristic T-cell hybridoma response of apoptosis or growth inhibition.28 We also noticed that the hybridomas had fewer complexes available for examination on the slides. The same was noted for the thymocytes and could be explained by a shorter and less stable cell-bead complex (data not shown).
T-cell hybridoma activation does not lead to significant cell shape and CD43 polarization or lipid raft coalescence.
Hybridoma- or column-purified splenic T cells were conjugated with beads coated with anti-CD3 and anti-CD28, fixed, and stained for CD43. (A,D) Examples of 3D9 hybridoma-bead conjugation where CD43 does not modulate away from the interaction site. (B,E) Examples of D0.11.10 hybridoma-bead complexes. The red CD43 staining is clearly visible at the cell-bead contact area. (C,F) Unprimed T cells activated with the same beads. During the activation, the cells change shape and CD43 accumulates away from the bead contact site, toward the uropodlike structure. At the right image the cell not conjugated with the bead demonstrates the typical CD43 distribution on unstimulated cells. The arrows denote location of the beads. (G-I) T-cell hybridomas or column-purified spleen T cells were conjugated with beads coated with anti-CD3 and anti-CD28, fixed, and permeabilized as described in “Materials and methods.” Rafts were visualized with FITC-labeled cholera toxin B subunit. (G) An example of 3D9 hybridoma cell-bead conjugate. (H) An example of a D0.11.10 hybridoma cell-bead complex. Both photographs show dispersed noncoalescent lipid rafts after activation of the T-cell hybridomas. (I) Unprimed T cells activated at the same time show redistribution of lipid rafts and their coalescence at the cell-bead interface. The results are representative of at least 3 experiments. The arrows indicate location of the beads. Magnification × 1000.
T-cell hybridoma activation does not lead to significant cell shape and CD43 polarization or lipid raft coalescence.
Hybridoma- or column-purified splenic T cells were conjugated with beads coated with anti-CD3 and anti-CD28, fixed, and stained for CD43. (A,D) Examples of 3D9 hybridoma-bead conjugation where CD43 does not modulate away from the interaction site. (B,E) Examples of D0.11.10 hybridoma-bead complexes. The red CD43 staining is clearly visible at the cell-bead contact area. (C,F) Unprimed T cells activated with the same beads. During the activation, the cells change shape and CD43 accumulates away from the bead contact site, toward the uropodlike structure. At the right image the cell not conjugated with the bead demonstrates the typical CD43 distribution on unstimulated cells. The arrows denote location of the beads. (G-I) T-cell hybridomas or column-purified spleen T cells were conjugated with beads coated with anti-CD3 and anti-CD28, fixed, and permeabilized as described in “Materials and methods.” Rafts were visualized with FITC-labeled cholera toxin B subunit. (G) An example of 3D9 hybridoma cell-bead conjugate. (H) An example of a D0.11.10 hybridoma cell-bead complex. Both photographs show dispersed noncoalescent lipid rafts after activation of the T-cell hybridomas. (I) Unprimed T cells activated at the same time show redistribution of lipid rafts and their coalescence at the cell-bead interface. The results are representative of at least 3 experiments. The arrows indicate location of the beads. Magnification × 1000.
CD43 exclusion occurs with lipid raft aggregation and requires Src kinase activity
In addition to the lack of CD43 redistribution and shape polarization we observed in the thymocytes and hybridomas, thymocytes are unable to coalesce lipid rafts on activation.27 To correlate this with the lack of CD43 redistribution and polarization we have observed, we evaluated CD4+ T cells and T-cell hybridomas for raft coalescence on activation with APC-like beads. Figure 2G-I illustrates that the CD43 polarizing CD4+ T cells coalesce rafts on activation and the T-cell hybridomas do not.
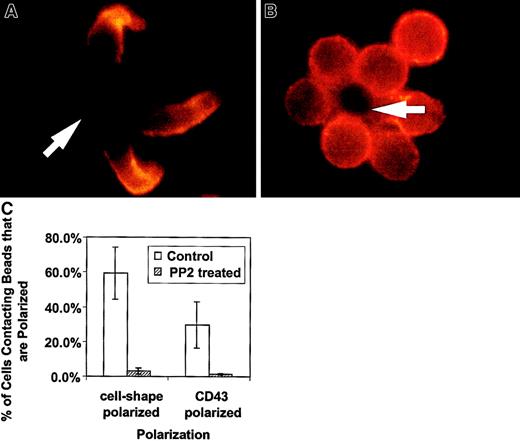
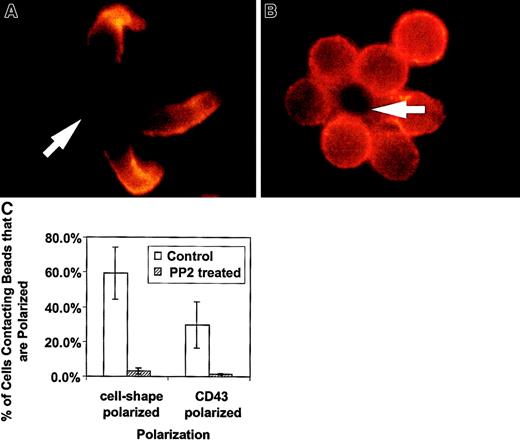
We have presented new data showing a decreased redistribution of CD43 in activated thymocytes. The lack of cell shape polarization in thymocytes is supported by the recent demonstration of deficient actin redistribution in freshly explanted thymocytes.27 The authors demonstrated that by bringing CD4 sequestered lck into the activating complex, thymocytes were then able to redistribute actin. To evaluate whether lck played a major role in the CD4+ T-cell shape and CD43 polarization we have observed, we treated unprimed CD4+ T cells with PP2, a specific inhibitor of Src kinases such as lck.29 This family of inhibitors does not block all of T-cell activation because activation-induced expression of IL-2 receptor is unaffected across a wide range of concentrations.29 In Figure3, examples of CD4+ T cell–bead clusters are shown with or without PP2 inhibitor treatment. We observed decreased cell shape and CD43 polarization in PP2-treated cells. Figure 3C contains quantification of this data by counting cell-bead clusters and scoring for polarization. Thus, the unprimed T-cell shape and CD43 polarization is dependent on a Src kinase and does not occur in double-positive thymocytes that sequester lck.30 31 It is of note that in experiments in which we purified double-positive thymocytes using anti-CD4, we observed more thymocyte polarization (data not shown). This suggests ligation of the cell surface molecule associated with lck in thymocytes begins to restore their polarization.
The Src kinase inhibitor, PP2, blocks the cell shape and CD43 polarization of activated primary T lymphocytes.
Purified CD4+ splenic T cells were preincubated with 20 μM PP2 in complete medium, then activated with beads coated with anti-CD3 and anti-CD28, fixed, and stained for CD43 as described in “Materials and methods.” (A) Control T cells untreated with PP2 inhibitor and activated at the same time as treated cells. The cells are clearly polarized and CD43 is redistributed away from the cell-bead contact site. (B) Cells treated with the inhibitor and activated. They are characterized by a round shape and lack CD43 redistribution. The results are representative of 2 experiments. The arrows indicate location of the beads. (C) Quantitation of cell shape and CD43 polarization of primary T lymphocytes treated with PP2. The bars represent the cells polarized as a percentage of bead-conjugated cells from 2 experiments. The results for PP2 treated (striped bar) and for control (white bar) T cells are shown. Two slides with 50 to 110 bead-conjugated cells counted on each were evaluated in an experiment. The error bars represent 1 SD. The difference for cell shape polarization was significant at P < .005 and the difference for CD43 polarization was significant atP < .05. Magnification × 1000.
The Src kinase inhibitor, PP2, blocks the cell shape and CD43 polarization of activated primary T lymphocytes.
Purified CD4+ splenic T cells were preincubated with 20 μM PP2 in complete medium, then activated with beads coated with anti-CD3 and anti-CD28, fixed, and stained for CD43 as described in “Materials and methods.” (A) Control T cells untreated with PP2 inhibitor and activated at the same time as treated cells. The cells are clearly polarized and CD43 is redistributed away from the cell-bead contact site. (B) Cells treated with the inhibitor and activated. They are characterized by a round shape and lack CD43 redistribution. The results are representative of 2 experiments. The arrows indicate location of the beads. (C) Quantitation of cell shape and CD43 polarization of primary T lymphocytes treated with PP2. The bars represent the cells polarized as a percentage of bead-conjugated cells from 2 experiments. The results for PP2 treated (striped bar) and for control (white bar) T cells are shown. Two slides with 50 to 110 bead-conjugated cells counted on each were evaluated in an experiment. The error bars represent 1 SD. The difference for cell shape polarization was significant at P < .005 and the difference for CD43 polarization was significant atP < .05. Magnification × 1000.
Blockade of HMG CoA reductase inhibits CD43 redistribution without inhibiting cell shape polarization
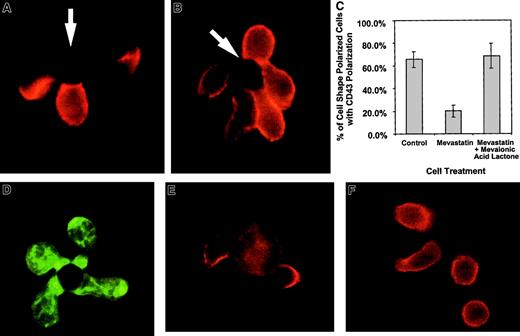
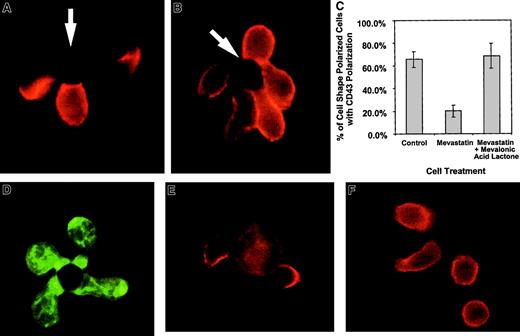
In the above studies, CD43 polarization and cell shape polarization correlate with each other. Work evaluating CD43 and cell shape polarization in migrating lymphocytes has also demonstrated correlation between cell shape and CD43 polarization.32,33Because small G proteins, such as rho, rac, and cdc-42, have been implicated in cytoskeletal movement, we evaluated the effects of these proteins on cell shape polarization and CD43 redistribution. Prenylation of small G proteins was prevented by blocking mevalonic acid synthesis using mevastatin, an inhibitor of HMG CoA reductase.34 Mevastatin was able to ablate the redistribution of CD43 without stopping cell shape polarization (Figure4). Panel C in Figure 4 quantifies similar data by enumerating the percentage of shape polarized cells that have redistributed CD43 to the uropod in the presence and absence of mevastatin. Supplying exogenous mevalonic acid restores the redistribution of CD43 (Figure 4C). Because in addition to blocking small G protein prenylation, statin inhibitors of HMG CoA reductase can block cholesterol synthesis, we demonstrate that cholesterol effects on lipid raft coalescence are not responsible for the mevastatin effect on CD43 redistribution. In Figure 4D, mevastatin-treated cells are able to coalesce lipid rafts on activation by APC-like beads. In addition, experiments performed with media containing cholesterol still reveal mevastatin inhibition of CD43 redistribution (Figure 4C and data not shown). Thus, altered prenylation of small G proteins such as rho, rac, and cdc-42 are the likely cause of the hysteresis between cell shape polarization and CD43 redistribution.
Mevastatin inhibits CD43 redistribution without inhibiting cell shape polarization.
Purified CD 4+ T cells were incubated with 50 μM mevastatin for 18 hours and then activated with beads coated with anti-CD3 and anti-CD28. The cells were fixed and stained for CD43 as described in “Materials and methods.” (A) An example of T cells untreated with mevastatin and activated with beads at the same time as mevastatin-treated cells. Two of the 3 cells conjugated with the bead on the photograph redistributed the CD43 away from cell-bead interaction site. (B) An example of cells treated with mevastatin. Although cell shape polarization was similar to the untreated cells, CD43 was not redistributed away from the cell-bead contact site. The arrows indicate location of the beads. (C) Quantitation of CD43 polarization in cell shape polarized cells treated with mevastatin. The bars represent the average percentage of cells with polarization of CD43 out of cells that have polarized shapes in conjunction with bead contact. Three slides, with 15 to 42 shape-polarized cells per slide, were counted and averaged and the error bars represent 1 SD. The difference between the mevastatin and mevastatin with mevalonic acid lactone groups was significant at P < .03. Three additional experiments, comparing mevastatin-treated and control groups, were averaged and showed a difference between the groups that was significant at P < .002. (D) Mevastatin-treated cells were conjugated with APC-like beads, fixed, permeabilized, and stained with FITC-labeled cholera toxin B subunit to detect rafts. (E-F) Purified DO.11.10 TCR transgenic CD4+ T cells were purified and combined with UT139.B2 APCs pulsed with the ovalbumin antigenic peptide, OVA323-339. The cells were fixed and stained for CD43 as described in “Materials and methods.” (E) T cell–APC interactions without mevastatin treatment. (F) T cell–APC interactions after T-cell treatment with 50 μM mevastatin for 18 hours. Similar results have been observed in 2 experiments. Magnification × 1000.
Mevastatin inhibits CD43 redistribution without inhibiting cell shape polarization.
Purified CD 4+ T cells were incubated with 50 μM mevastatin for 18 hours and then activated with beads coated with anti-CD3 and anti-CD28. The cells were fixed and stained for CD43 as described in “Materials and methods.” (A) An example of T cells untreated with mevastatin and activated with beads at the same time as mevastatin-treated cells. Two of the 3 cells conjugated with the bead on the photograph redistributed the CD43 away from cell-bead interaction site. (B) An example of cells treated with mevastatin. Although cell shape polarization was similar to the untreated cells, CD43 was not redistributed away from the cell-bead contact site. The arrows indicate location of the beads. (C) Quantitation of CD43 polarization in cell shape polarized cells treated with mevastatin. The bars represent the average percentage of cells with polarization of CD43 out of cells that have polarized shapes in conjunction with bead contact. Three slides, with 15 to 42 shape-polarized cells per slide, were counted and averaged and the error bars represent 1 SD. The difference between the mevastatin and mevastatin with mevalonic acid lactone groups was significant at P < .03. Three additional experiments, comparing mevastatin-treated and control groups, were averaged and showed a difference between the groups that was significant at P < .002. (D) Mevastatin-treated cells were conjugated with APC-like beads, fixed, permeabilized, and stained with FITC-labeled cholera toxin B subunit to detect rafts. (E-F) Purified DO.11.10 TCR transgenic CD4+ T cells were purified and combined with UT139.B2 APCs pulsed with the ovalbumin antigenic peptide, OVA323-339. The cells were fixed and stained for CD43 as described in “Materials and methods.” (E) T cell–APC interactions without mevastatin treatment. (F) T cell–APC interactions after T-cell treatment with 50 μM mevastatin for 18 hours. Similar results have been observed in 2 experiments. Magnification × 1000.
Because we used beads coated with anti-CD3 and anti-CD28 to mimic APCs in our experiments, we confirmed our results using unprimed TCR transgenic CD4+ T cells activated with cells bearing the appropriate MHC-peptide complexes. Figure4E shows examples of CD43 movement from the T cell–APC junction and toward a uropodlike structure. In the presence of mevastatin (Figure4F), cell shape polarization can occur in the absence of CD43 polarization. Thus, unprimed T cells activated by peptide-MHC complexes behave in a similar manner to bead-activated T cells.
Discussion
Two cell surface molecules, CD45 and CD43, have been reported to be excluded from the T cell–APC contact.19,35 CD45 is a large mucinlike tyrosine phosphatase that can dephosphorylate molecules involved in T-cell signal transduction and form a steric barrier to TCR-ligand interactions. CD43 is an abundant, large cell surface mucin found on T lymphocytes, neutrophils, bone marrow stem cells, and some B lymphocytes.36 In activated T cells and neutrophils, CD43 is removed by proteolytic cleavage and shedding.37,38 This observation along with the size, extended conformation, and negative charge of CD43 suggests CD43 forms a barrier that needs to be removed for cell-cell or cell-matrix interactions. Although CD43 may have a positive role in some lymphocyte interactions39 and anti-CD43 antibodies can augment lymphocyte function in some cases,32,40-42 a large body of data suggests CD43 has a negative effect on lymphocyte interactions. CD43-deficient T-cell lines were more susceptible to lysis by alloreactive T-cell clones than CD43+ cell lines.43 CD43-deficient T cells from knockout mice have a marked increase in their proliferative responses, enhancement of adhesion to immobilized soluble ICAM-1,44 and increased homing to secondary lymphoid organs.45 The negative effect of CD43 may be due to a mucin size and charge barrier to other interactions46 and specific signaling through interaction of CD43 with a ligand. Several laboratories have reported CD43-specific ligands and signaling pathways that may explain the inhibitory effect of CD43. CD43 was reported to bind to the ligands galectin-1,47 ICAM-1,48and MHC class 1 (MHC1).49 Additional evidence suggests that CD43-mediated inhibition may be due to negative signaling from the CD43 cytoplasmic domain.18 CD43 interacts with the cytoskeletal proteins ezrin and moesin50,51 and protein tyrosine kinases52 53 and these interactions may play a role in CD43 signaling. Therefore, the removal of CD43 from the T cell–APC contact can significantly enhance formation of an effective immunologic synapse and T-cell activation.
CD43 is redistributed on the cell surface of migrating T cells as well as antigen-activated T cells. On exposure to chemokines or cross-linking of surface ICAM-3 or CD43, T cells develop a polarized morphology with a leading edge containing chemokine receptors and a trailing edge with a projection called a uropod.23CD43 along with ICAM molecules and p-selectin glycoprotein ligand-1 accumulate in this uropod structure. This CD43 movement may facilitate the attachment and detachment of tethering integrins during cell motion. ICAM molecules and the cell MTOC are also localized with CD43 in the uropod and this concentration of molecules may regulate the recruitment of other leukocytes through interaction with the uropod.
It has been demonstrated that in antigen receptor activation of T-cell clones, the MTOC reorganizes to interface with the T cell–APC contact22 and the uropod may be disrupted leading to cell rounding.23 Although chemokine activation of motility and antigen receptor activation of T-cell functions both redistribute CD43, in chemokine activation the redistribution is much more striking and associated with cell shape polarization. In fact, chemokine and antigen receptor activation oppose each other in MTOC localization and cell shape polarization. In vivo it is likely that primed motile T cells reach antigen-containing tissues through chemokine signals and the leading edge of the T cells that will encounter APCs will already have redistributed CD43 away from the contact site. The need for antigen receptor-mediated CD43 redistribution is more apparent for unprimed rounded T cells because their uniform CD43 expression is much more likely to hinder T cell–APC interaction at the primary site of contact in lymphoid tissue. To study this, we evaluated antigen receptor activation of purified unprimed CD4+ T cells. Anti-CD3 provided the TCR complex signal and anti-CD28 provided a costimulatory signal. These antibodies were coated on negatively charged sulfite beads to simulate the negative charge of the APC cell surface. We found that this activation led not only to exclusion of CD43 from the T cell-bead contact site as seen with a T-cell clone19 but to redistribution of CD43 from a large fraction of the cell surface into a uropod structure away from the contact site. This combination of cell shape polarization and CD43 redistribution is generally associated with induction of cell motility in previously activated CD45 RO+ T-cell populations.20 Our data suggest that unprimed T cells develop cell characteristics suitable for motility and a uropod for cell recruitment on antigen stimulation. Memory T cells may need to decrease their motility-oriented polarization and uropod-mediated recruitment on activation. Memory T cells often have a specific phenotype related to effector response. The delivery of this response often requires MTOC and Golgi realignment and the type of cell recruitment appropriate for the effector phenotype may be specified through the secretion of selected cytokines. In contrast, the initial responses of unprimed T cells are more likely to promote expansion, mobilization to the appropriate lymphoid tissue compartment, and general recruitment rather than a directed specific effector response limited to the antigen-containing tissue. In support of this, we observed this cell shape change with unprimed transgenic T cells activated with peptide–MHC-bearing APC. The generation of a motile polarized phenotype by naı̈ve T cells has also been shown by others using transgenic T cells activated with antigen and APCs.54 Therefore, this observation is unlikely to be limited to the APC-like beads used in our study.
Comparison of T-cell hybridomas and thymocytes with unprimed CD4+ T cells illustrates differences in both cell shape polarization and CD43 movement on TCR ligation. Both cell shape and CD43 polarization depend on Src kinases because blockade of these kinases abrogate both changes. CD43 movement occurs with lipid raft coalescence, which is also associated with costimulatory signals in T-cell activation. To evaluate the relationship of cell shape polarization, lipid rafts and CD43 redistribution, we inhibited the HMG CoA reductase enzyme. In addition to cholesterol synthesis, this pathway provides mevolanate for prenylation of proteins including the small G proteins rho, rac, and cdc42.34 These proteins play a key role in cytoskeletal changes in T cells.55,56Mevastatin, an HMG CoA reductase inhibitor, is able to reverse CD43 polarization without altering cell shape polarization in unprimed CD4+ T cells. This mevastatin effect is also observed with TCR transgenic T cells activated with antigen and APCs. Cholesterol depletion by HMG CoA reductase inhibitors is unable to destroy the association of raft-associated proteins with detergent-insoluble fractions in biochemical fractionation of rafts.57 In our experiments, mevastatin does not disrupt lipid raft coalescence and cholesterol-containing media does not reverse the effect of mevastatin. This suggests the mechanism of mevastatin inhibition of CD43 polarization is through altered protein prenylation.
Cell surface CD43 redistribution is associated with T-cell clone activation by antigen and by chemokine induction of T-lymphocyte motility. The movement of CD43 may allow effective formation of the immunologic synapse and facilitate rapid motility and leukocyte recruitment. We demonstrate for the first time that CD43 movement away from the site of TCR ligation occurs in unprimed CD4+ T cells as well as in T-cell clones. This movement of CD43 is associated with a polarized morphology and CD43 accumulation at the uropods of the T cells. This suggests that unprimed T cells may have different motility and recruitment responses on activation than do more mature cells.
These changes are not seen in either thymocytes or T-cell hybridomas. It is of note that T-cell hybridomas can undergo TCR-mediated signaling for apoptosis and cytokine production despite a lack of CD43 redistribution. CD43 redistribution may be relevant to cell fate determination and required for full signaling by normal T lymphocytes, which require a stable immunologic synapse. It is possible that dispersed TCR ligand interactions in thymocytes or T-cell hybridomas occur through transient or localized CD43 movements that would not be detected by fluorescent or possibly even by confocal microscopy. Variability in the detection of CD45 exclusion from T cell–APC contact sites suggests that the sensitivity of different forms of microscopy may play a role in the evaluation of molecular movement.19 35
The polarization of cell shape morphology and CD43 have a requirement for Src kinase activity and are generally associated with the coalescence of raft lipid domains. Furthermore, the cell shape polarization, lipid raft coalescence, and CD43 redistribution can be separated by further modulation of signaling using an HMG CoA reductase inhibitor. The blockade of this enzyme provides the first example of cell morphology polarization in the absence of CD43 redistribution. Statin inhibitors of HMG CoA reductase are used clinically for reduction of cholesterol, but they have been reported to have benefits beyond reduction of cholesterol.58 59 Subtle effects on the behavior of lymphocytes and other inflammatory cells may play a role in these additional benefits.
These results suggest that the molecular redistribution steps of T-cell activation have intricate pathway distinctions. Understanding the relationships between the stages of molecular redistribution and the steps in lymphocyte activation may facilitate subtle modulation of immunologic responses.
We thank Drs Ezio Bonvini and Elizabeth Shores for their critical review of this manuscript. We thank Dr Kathryn Stein for her support, Howard Mostowsky for sorting of thymocytes, Dr John Kappler for providing the D0.11.10 hybridoma, and Dr Paul Gottleib for providing the 3D9 hybridoma. We thank Dr Elizabeth Shores for providing the DO.11.10 transgenic mice and Dr Rolf Konig for providing the UT139.B2 cell line.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Steven Kozlowski, Center for Biologics Evaluation and Research (CBER), Food and Drug Administration, 29 Lincoln Dr, Bldg 29B-3NN08, HFM-561, Bethesda, MD 20892; e-mail:kozlowski@cber.fda.gov.