Leukocyte traffic into lymph nodes and sites of inflammation is guided by L-selectin. Experiments performed in vitro and with gene-deleted mice suggest that CD34 recognizes L-selectin if decorated by 6-sulfo sialyl Lewis x (sLex) saccharides and the MECA-79 epitope. However, very little is known about glycosylation of human L-selectin ligands. We report here on matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI-TOF MS) profiles of N- and O-linked oligosaccharide fractions from human tonsillar endothelial CD34. All detected O-glycans were sialylated; some were also monosulfated or monosulfated and monofucosylated. If a given CD34-glycan may carry all requirements for L-selectin recognition, that is, both 6-sulfo-sLex and MECA-79 epitopes, only one O-glycan fraction, O-9, SA2Hex3HexNAc3- Fuc1(SO3)1, meets the criteria. A candidate structure is SAα2-3Galβ1-4(Fucα1-3)(6-sulfo)GlcNAcβ1-3Galβ1-3(SAα2-3Galβ1-4GlcNAcβ1-6)GalNAc. However, if sulfo sLex glycans are supplemented with separate sulfated, nonfucosylated O-glycans, saccharides in O-6, O-8, or O-9, putatively carrying MECA-79 epitopes, could form multiglycan binding epitopes for L-selectin.
Introduction
High-endothelial venules (HEV) are the sites of leukocyte entry into the lymph nodes from the blood circulation. L-selectin on leukocytes recognizes its ligands on HEV, such as CD34 and other members of a heterogenous group of mucin-type glycoproteins forming the peripheral node addressins (PNAd).1,2 In vitro experiments and results derived from gene-deleted mice suggest that the proper recognition of L-selectin requires at least the presence of the following posttranslational decorations: α2,3sialylated galactose as well as α1,3fucosylated and 6-sulfated N-acetylglucosamine in the form of 6-sulfo sLex epitopes in the ligand glycoproteins.2-7 Furthermore, the HEVs express the MECA-79 epitope, which resides in the PNAd complex and has recently been shown to recognize a core 1 O-glycan Galβ1-4(sulfo-6)GlcNAcβ1-3Galβ1-3GalNAc.8-10 The 6-sulfo sLex and MECA-79 epitopes are not commonly expressed in other locations of the vascular endothelium under normal conditions.2 However, these glycans are rapidly induced in the endothelium at sites of inflammation, where they probably participate in recruitment of the inflammatory leukocytes into the inflammed tissue.11-13
The L-selectin–binding glycans are synthesized by various glycosyltransferases. As the repertoire of these enzymes shows remarkable interspecies variability, the data obtained from murine experiments cannot be directly extrapolated to human settings. So far, very little is known of the human endothelial L-selectin ligands, except that they contain sLex, 6-sulfo sLex, and MECA-79 epitopes.8,14,15 Therefore, we have initiated research on human leukocyte extravasation,16 and report here on the glycosylation of CD34 in human tonsillar HEV.
Study design
Purification of CD34
Human tonsils were obtained from tonsillectomies performed at the Department of Otorhinolaryngology, Helsinki University Central Hospital, after the institutional review board had approved the study protocol. The tonsils used for this study were all removed due to hyperplasia, and the patients had no signs of infections or inflammations at the time of their operations. CD34 was isolated from tonsillar lysates by chromatography on immobilized wheat germ agglutinin (WGA) and anti-CD34 mAb581 as described.8 The purity of the sample was verified by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) followed by silver staining and MALDI-TOF MS fingerprinting.
Glycan preparation
N-glycans were liberated on-blot with the Flavobacterium meningosepticum N-glycosidase F from CD34 blotted on polyvinylidenefluoride (PVDF) membrane. The liberated N-glycans were passed through a BondElut C18 column (Varian, Palo Alto, CA) and desalted by gel filtration HPLC. The blot was subjected to reductive β-elimination and liberated O-glycan alditols were isolated by gel filtration high-performance liquid chromatography (HPLC).
Matrix-assisted laser desorption/ionization time-of-flight mass spectrometry
MALDI-TOF MS was performed with a Biflex mass spectrometer (Bruker Daltonics, Bremen, Germany). Analysis was performed in negative- and positive-ion linear delayed-extraction mode, using 2,4,6-trihydroxyacetophenone (THAP; Sigma-Aldrich Finland, Helsinki, Finland) as the matrix.17,18 External calibration was performed with THAP matrix dimer and sialyl Lewis x beta-methylglycoside (Toronto Research Chemicals, Ontario, Canada). (3-sulfo)Galβ1-4(Fucα1-3)GlcNAc (Calbiochem, San Diego, CA), and SAα2-3Galβ1-4(Fucα1-3)GlcNAcβ1-3Galβ1-4GlcNAc17gave equivalent matrix assisted laser desorption ionization-time of light mass spectrometry (MALDI) responses, implying that sialylated and sulfated glycans were analyzed with comparable efficiency (data not shown). In mass spectra of the CD34 glycans, only peaks with signal-to-noise ratios above 2 were taken into account.
Results and discussion
In the present study, endothelial CD34 was isolated from human tonsils, the N- and O-linked glycans were released separately from the protein, and the oligosaccharides were analyzed and quantified with gel filtration HPLC and MALDI-TOF MS. N-glycans were mostly complex-type and contained 1-4 sialic acid residues (Table1). No signs of neutral N-glycans were detected in positive-ion mode MALDI-TOF MS. No functions in leukocyte traffic are currently known for N-glycans of CD34.
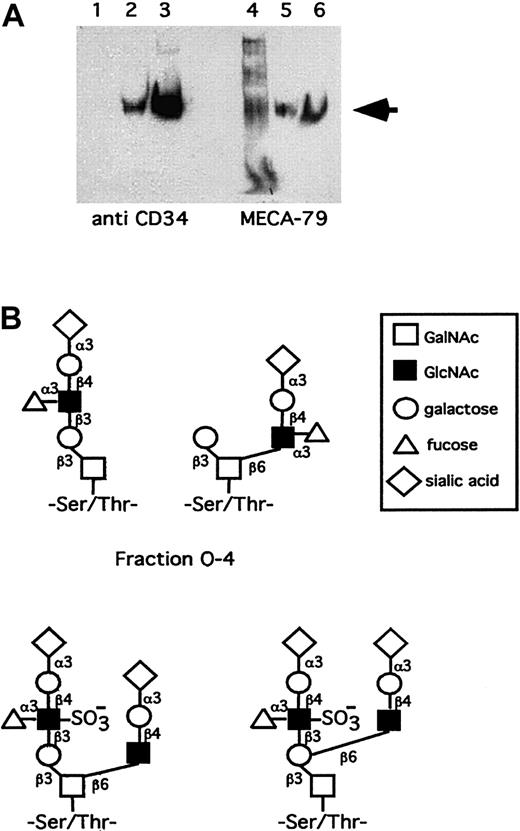
Western blot shows that human tonsillar endothelial CD34 reacts with MECA-79 (Figure 1A). The molar amounts of O-glycans were 7-fold higher compared with N-glycans (Table 1). The procedures for isolating CD34, releasing glycans from it, and the purification of glycans for analysis leaves open that some structures may be lost during this processing. However, currently we cannot make any valid statements of such putative glycans.
Isolation and characterization of CD34 and its glycans.
(A) Western blot shows that human tonsillar endothelial CD34 reacts with MECA-79. Lanes 1 and 4 are after the WGA column, lanes 2 and 5 after the CD34 mAB 581 column, and lanes 3 and 6 after desalting and concentration. The arrow indicates the CD34 at 105 kd. (B) Proposal for structures of human tosillar high endothelial CD34 O-glycans of the sLex containing fraction O-4 and sulfated sLex containing fraction O-9. The latter putatively bears both 6-sulfo-sLex and MECA-79 epitopes.
Isolation and characterization of CD34 and its glycans.
(A) Western blot shows that human tonsillar endothelial CD34 reacts with MECA-79. Lanes 1 and 4 are after the WGA column, lanes 2 and 5 after the CD34 mAB 581 column, and lanes 3 and 6 after desalting and concentration. The arrow indicates the CD34 at 105 kd. (B) Proposal for structures of human tosillar high endothelial CD34 O-glycans of the sLex containing fraction O-4 and sulfated sLex containing fraction O-9. The latter putatively bears both 6-sulfo-sLex and MECA-79 epitopes.
The recovered glycans represented core 1 and core 2 structures, but most likely not core 3-7 branches as the number of hexoses (Hex) was equal to the number of N-acetylhexosamines (HexNAc). Acidic but not neutral O-glycans were detected in human tonsillar endothelial CD34. Monosialylated, disialylated, monosialylated-monofucosylated, disialylated-monosulfated, and disialylated-monosulfated-monofucosylated O-glycans were observed (Table 1). It remains to be explained why the relatively large fractions, O-5 and O-7, representing 21% and 5% respectively, do not undergo sulfate-independent fucosylation to generate sLex epitopes on human endothelial CD34, although in vitro fucosylation with endothelial α1,3fucosyltransferases prefer nonsulfated over sulfated N-acetyllactosamine acceptors.6
Recombinant soluble CD34 expressed in Chinese hamster ovary (CHO) cells has been decorated with bivalent 6-sulfo sLex glycans10 and was shown to be several-fold more effective in mediating L-selectin–dependent lymphocyte rolling than the 2 variants lacking one or the other sulfo sLex branch.10However, none of the human CD34 glycans revealed 2 sulfates or 2 fucoses in the present experiments, suggesting that in the tonsillar repertoire of human CD34 only one sulfo sLex epitope may be present in one glycan. Previously, the glycans from a murine endothelial L-selectin ligand, GlyCAM-1, have been shown to carry 6 or 6′-sulfo sLex in the core 2 branch,2 19 but similar sulfated heptasaccharides were not present among the O-glycans of human high endothelial CD34.
A prerequisite for L-selectin binding is the presence of fucose on the ligand.2-7 As the fraction O-4 contains fucose, 2 putative glycans containing the sLex structure have been depicted in Figure 1B. The most interesting O-glycan fraction in human tonsillar CD34 is O-9 as it is the only one that is both sulfated and fucosylated. It may contain the nonasaccharideSAα2-3Galβ1-4(Fucα1-3)(6-sulfo)GlcNAcβ1-3Galβ1-3(SAα2-3Galβ1-4GlcNAcβ1-6)GalNAc(Table 1, Figure 1B), which represents both a 6-sulfo-sLex–reactive (underlined) and a MECA-79–reactive saccharide10 (italics) in a partially overlapping form. Another version is also possible, where the sialylated N-acetyllactosamine is linked to the 6-position of the proximal galactose on the core 1 chain (Figure 1B). If the MECA-79 and 6-sulfo-sLex epitopes were not present in a single glycan, they could be organized in clusters.20 The 6-sulfo-sLex saccharides from O-9, representing several structures isomeric to the nonasaccharides of Figure 1B would be supplemented in these clusters by separate MECA-79 glycans (containing the Galβ1-4(6-sulfo)GlcNAcβ1-3Galβ1-3GalNAc10) from O-6 and/or O-8 and/or O-9, forming multiglycan binding determinants for lymphocyte L-selectin.
The sulfated O-glycans O-6, O-8, and O-9 of Table 1 represented together about 4% of the total O-glycans. It has been estimated that the HEV-derived CD34 represents one fourth of the total tonsillar CD34.8 This implies that as much as 16% of the O-glycans in HEV-derived CD34 may be sulfated. The mucin-rich areas of the CD34 glycoprotein might contain O-glycans in such a high density that 2 or several copies of the L-selectin recognizing sulfated O-glycans, belonging to fractions O-6, O-8, and O-9, could be properly spaced to act in a functionally multivalent manner to ligate advantageously with the clustered L-selectin molecules at the tips of lymphocyte microvilli.21 Our previous ex vivo work in the rat allograft rejection model, showing that lymphocyte L-selectin adheres much more efficiently to multivalent sLex epitopes than to monovalent ones,22-24 is in agreement with this suggestion.
We thank Gunilla Rönnholm and Leena Penttilä for technical assistance.
Supported by grants from the Academy of Finland, Technology Development Center of Finland, Emil Aaltonen Foundation, Tampere; Finnish Heart Association, Sigrid Juselius Foundation, Helsinki; Roche Organ Transplant Research Fund (ROTRF), and the HUCH Research Fund.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Risto Renkonen, Biomedicum and Haartman Institute, PO Box 63 (Haartmaninkatu 8), SF-00014 University of Helsinki, Helsinki, Finland; e-mail: risto.renkonen@helsinki.fi.