Abstract
Human primary immunodeficiency diseases are experiments of nature characterized by an increased susceptibility to infection. In many cases, they are also associated with troublesome and sometimes life-threatening autoimmune complications. In the past few years, great strides have been made in understanding the molecular basis of primary immunodeficiencies, and this had led to more focused and successful treatment. This review has 3 aims: (1) to highlight the variety of autoimmune phenomena associated with human primary immunodeficiency diseases; (2) to explore how primary immunodeficiencies predispose patients to autoimmune phenomena triggered by opportunistic infections; and (3) to consider the rationale for the current treatment strategies for autoimmune phenomena, specifically in relation to primary immunodeficiency diseases. Reviewing recent advances in our understanding of the small subgroup of patients with defined causes for their autoimmunity may lead to the development of more effective treatment strategies for idiopathic human autoimmune diseases.
Introduction
This review is limited largely to immunodeficiency diseases in which the molecular basis of the condition is understood, except in the case of common variable immunodeficiency (CVID) and selective IgA deficiency (SIgAD), which are the most common primary immunodeficiency diseases and which are often associated with autoimmune phenomena. Most primary immunodeficiency diseases have been reviewed recently, and we therefore will not here provide a comprehensive account of all the features of each condition; rather, we will focus on the interrelation among human primary immunodeficiency and autoimmunity. Recent developments in our understanding of the pathogenesis of multigenic autoimmune diseases, including the role of HLA genes and the thymus, were reviewed elsewhere and are not discussed here.1-4 The use of bone marrow transplantation (BMT) in the treatment of severe primary immunodeficiencies, a procedure that may either precipitate or ameliorate autoimmunity, was recently extensively reviewed.5
The basis of autoimmunity in primary immunodeficiency diseases and methods of classification
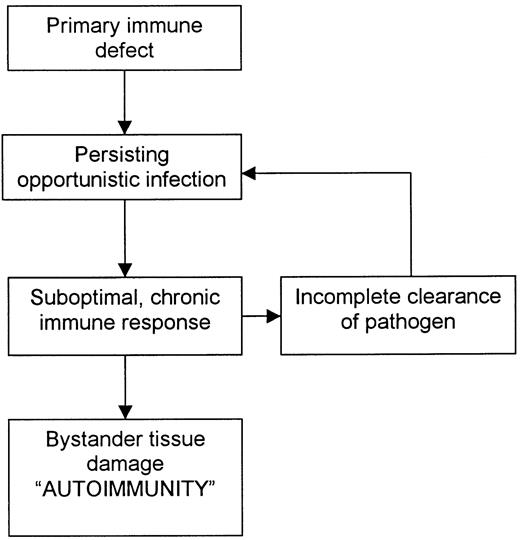
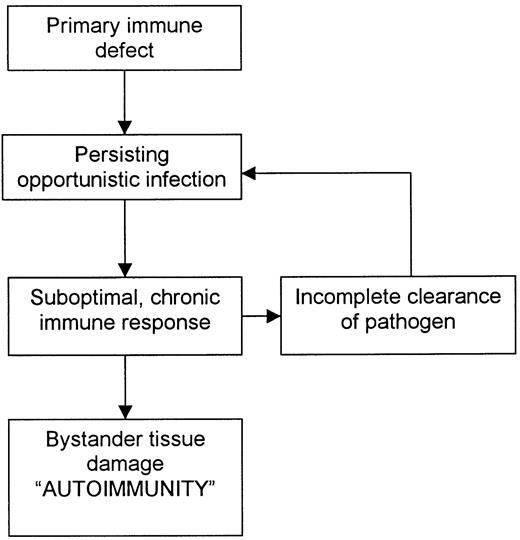
For many primary immunodeficiency diseases, the basis of the autoimmunity is the inability of the host to eradicate microbial pathogens and their antigens completely through the usual immune pathways. The result is a compensatory, often exaggerated and chronic inflammatory response by less effective alternative immune pathways, which damage not only infected cells but also surrounding tissue (Figure 1). Thus, in many affected patients, autoimmunity is not a breakdown of tolerance to self-antigens; rather, it is tissue damage incurred as the host attempts to rid itself of foreign immunogens.6 7
Common mechanism of autoimmunity in several primary immunodeficiency diseases.
Classification of autoimmune phenomena in primary immunodeficiency diseases is problematic. One method of classification is based on the underlying genetic defect. This results in a long list that does little to help clinicians plan treatment strategy (Table 1). An alternative classification system is based on the proposed immune pathway causing the autoimmunity (Table 2). This may seem more logical because it indicates the part of the immune system that must be suppressed to control the problem. However, suppression of compensatory immune pathways may not only decrease the inflammation causing the autoimmunity but may also leave the patient open to overwhelming sepsis and death. Furthermore, the problem is likely to recur after the immunosuppression has been reduced because the underlying primary immunodeficiency has not been corrected. A third method of classification—and that used here—is to organize autoimmune phenomena according to which functional component of the immune system is defective (Table 3). This method is based on the idea that treatment should ideally focus on replacing the defective immune component. For many severe primary immunodeficiency diseases, BMT is currently the only effective treatment. Specific gene therapy is still in its infancy.
Specific primary immunodeficiency diseases associated with autoimmunity
Phagocyte disorders
Chronic granulomatous disease.
Chronic granulomatous disease (CGD) consists of a group of X-linked and autosomal recessive disorders of neutrophil production of nicotinamide adenine dinucleotide phosphate oxidase that affect 1 in 200 000 children. In these disorders, neutrophils are incapable of completely eradicating phagocytosed catalase-positive bacteria and fungi. Patients have recurrent bacterial and fungal (especially Aspergillus) infections that may affect any part of the body, including the skin, lungs, liver, and bone. Death is usually caused by respiratory failure. The average patient survives to the age of 20 years.8 9
Along with infective complications, chronic inflammation of the gut (similar to Crohn disease) is a common (occurring in 50% of patients) although less well-recognized cause of morbidity in patients with CGD. Gastrointestinal symptoms of abdominal pain, diarrhea, and malabsorption respond variably to immunomodulatory agents (prednisolone, cyclosporin A, and interferon-γ), and there is an increased risk of reactivating latent infection.10-12Inflammation can occur anywhere from mouth to anus. Widespread granuloma formation and fibrosis may result in stomatitis and oral ulcers; esophagitis associated with dysphagia, dysmotility, and obstruction; gastric outlet obstruction and eosinophilic gastritis; intestinal villous atrophy or granulomatous colitis; and liver fibrosis and cirrhosis. Chronic inflammation in other systems, such as the lungs, may produce fibrosis and cor pulmonale. Specific pathogens are usually not isolated. The pathogenesis of this chronic inflammation is unknown, but one possibility is that the inability to eradicate bacterial and fungal immunogens completely promotes a chronic inflammatory reaction that destroys surrounding tissue.13Carriers of the defective genes involved may not be entirely asymptomatic: 10% of X-linked recessive kindred and 3% of autosomal recessive kindred have family members with discoid lupus.9
Because of the poor long-term prognosis in CGD, early BMT is now being recommended by many specialist centers if a matched sibling donor is available.14 Although gene therapy for CGD has been attempted, it has not been successful.
Leukocyte adhesion molecule deficiency.
Leukocyte adhesion molecule deficiency (LAD) is due to defects in integrin family adhesion molecules (CD18) that are essential for binding of neutrophils to the endothelial surface as a prerequisite to infiltration into inflamed tissues. Consequently, patients with LAD have high circulating neutrophil counts, resulting in severe, recurrent life-threatening infections associated with a lack of pus formation.15 Persistence of bacterial antigens and the inability of neutrophils to escape from the circulation may produce a persistent leukocytoclastic vasculitis. Early death can be averted only by BMT.
Major histocompatibility complex class I deficiency (bare lymphocyte syndrome type I)
Major histocompatibility complex class I (MHC I) deficiency is rare and has a variable clinical phenotype that ranges from totally asymptomatic to a condition similar to that resulting from severe combined immunodeficiency (SCID).16 Mutations in the genes coding for the transporter associated with antigen presentation (TAP) have been found in patients with this disorder. TAP mediates the translocation of foreign peptide from the proteasome into the endoplasmic reticulum so that it can combine with HLA-I molecules, an essential step in the presentation of MHC I–peptide complexes on the cell surface. Patients deficient in TAP have reduced cell-surface expression of MHC I. Immunity to viruses appears intact, with normal antibody titers, but chronic recurrent bacterial sinopulmonary infections are a major problem and patients have a course similar to those of patients with cystic fibrosis and ciliary dyskinesia. Chronic pulmonary infection may cause a reactive cutaneous leukocytoclastic vasculitis and a polyarthritis.16 Necrotizing granulomatous inflammation of the nose and skin may resemble manifestations of Wegener granulomatosis or lethal midline granuloma.17 Histologic assessment of the granuloma shows large numbers of activated natural killer (NK) cells. Unlike patients with Wegener granulomatosis, patients with MHC I deficiency do not have glomerulonephritis or proteinase 3–antineutrophil cytoplasmic antibodies. Immunosuppressive therapy using steroids and cyclophosphamide worsens the clinical condition because it dampens the host immune response to the chronic infection. Care of these patients should include regular chest physiotherapy and antibiotic therapy.
Antibody production appears to be intact in MHC I deficiency, since patients have normal viral titers and a polyclonal hypergammaglobulinemia. Cellular immunity also appears to be unaffected: NK-cell–mediated and T-cell–mediated immunity to viruses is normal and skin testing using purified tuberculin may yield positive results. Thus, it is not known why patients with this disorder are susceptible to pyogenic bacterial infections.
NK-cell disorders
Chediak-Higashi syndrome and Griscelli syndrome.
These syndromes are autosomal recessive diseases characterized by partial oculocutaneous albinism, a mild predisposition to pyogenic infections, and in Chediak-Higashi syndrome (CHS), abnormally large granules in many different cell types.18 Griscelli syndrome is differentiated from CHS by the presence of pathognomonic light and electron microscopical features in skin and hair and the absence of consistent granulocyte abnormalities. Mutations in the large lysosomal trafficking regulator gene that lead to truncation of the protein cause CHS.19,20 Mutations in the guanosine triphosphate (GTP)–binding protein RAB27A, which is involved in cytotoxicity and cytolytic granule exocytosis pathways, occur in Griscelli syndrome.21
Susceptibility to infection in patients with these syndromes may be due to defects in NK- and T-cell–mediated cytotoxicity, especially to herpes viruses, as well as defects in chemotaxis and the bactericidal capacity of granulocytes and monocytes. The accelerated phase of the disease, which is characterized by virus-induced hemophagocytic lymphohistiocytosis (HLH), has never been observed in animal models, possibly because the Epstein-Barr virus (EBV) is specific to humans.22 Many children with CHS or Griscelli syndrome will die young, largely as a result of EBV-triggered HLH, unless treated with BMT.
Familial hemophagocytic lymphohistiocytosis.
Familial hemophagocytic lymphohistiocytosis (FHL) is a rare, rapidly fatal autosomal recessive immune disorder characterized by uncontrolled activation of T cells and macrophages and overproduction of inflammatory cytokines. The disease is due to a defect in the perforin gene.23 Perforins are a class of proteins present in the secretory granules of NK and T lymphocytes that mediate cytotoxicity by polymerizing to form pores in target-cell membranes in a way similar to that of a structurally related protein, complement factor C9. Although FHL is considered a NK-cell disorder, its pathogenesis involves dysfunction of T cells. Incomplete clearance of triggering viruses results in a persistent, exaggerated inflammatory response with tissue destruction. After control of the HLH with immunosuppressive drugs, the treatment of choice is BMT.24 25
X-linked lymphoproliferative disease.
X-linked lymphoproliferative disease (XLP), previously called Duncan disease,26 is due to a defect in SLAM-associated protein.27 28 The disease is characterized by an inability to mount an effective immune response to EBV. EBV-driven B-cell lymphoproliferative disease develops in 30% of patients and is usually fatal.
Alternatively, persistent infection may be associated with an ineffectual but damaging T-cell–mediated inflammatory response. If generalized, the result is virus-associated HLH (58%); if more localized to the infected lymphocytes, aplastic anemia (3% of patients) or isolated hypogammaglobulinemia (31%) may develop.29 Three percent of boys have an exaggerated T-lymphocyte response to EBV-infected B cells lodging in lung tissue and vessel walls, resulting in pulmonary lymphomatoid granulomatosis associated with a lymphoid vasculitis.30,31A similar T-cell reaction has been observed in boys with Wiskott-Aldrich syndrome (WAS).32 Aggressive combination chemotherapy with or without radiotherapy33 and interferon-α2b therapy34 have produced encouraging improvements in survival of patients with lymphomatoid granulomatosis. Anti–B-cell monoclonal antibodies that destroy B cells—some of which are infected with EBV—have also been used with some success to treat this condition.35
The immune mechanisms underlying the clinical features of XLP are becoming clearer. Defective NK-cell and cytotoxic T-lymphocyte activity due to aberrant activity of the cytoplasmic lymphocyte activation proteins 2B4 and SLAM result in persistence of the EBV virus that leads to serious clinical disease.36-38 Reduced NK-cell activity has been found in other patients with unusually severe or prolonged EBV infections.39-42 Augmented activation of NK cells by recombinant interleukin 2 has been used successfully to treat patients with chronic EBV infection,43 although it has not so far been used in XLP.
Complement deficiencies
Deficiencies in components of complement are rare, with the most common being a deficiency in C2 that occurs in 1 in 20 000 people. Only deficiencies in the earlier components of the classical pathway (C1q, C1r, C1s, C2, and C4) have been linked to autoimmune diseases.45,46 One percent of patients with systemic lupus erythematous (SLE) have defects in their complement pathway. C1q deficiency occurred with SLE in 38 of 41 cases (92%) reported. Among such patients, SLE tends to be more severe, the age of onset is earlier (median age, 7 years), and a preponderance of male patients is observed. Patients with deficiencies in C1r and C1s that often occur together and C4 deficiencies have a similar propensity to SLE (60%-75%). Homozygous C2 deficiency is associated with SLE, SLE-like disease, or immune complex disease in 50% of cases. Heterozygous complement deficiencies do not produce an increased prevalence of autoimmunity. A deficiency of mannose-binding protein, which cleaves C4 and C2 when bound to antibody in the same way as activated C1s, has been linked to an increase in autoimmune disorders (arthritis, idiopathic thrombocytopenic purpura [ITP], enteropathy, pernicious anemia, and vitiligo) when associated with CVID.47 The exact mechanism by which complement deficiencies cause autoimmunity is unknown. The fact that it is C1q that binds to the Fc component of IgG or IgM and apoptotic cells suggests that the lack of interaction of complement with immunoglobulin and these cells may prevent the cells' clearance, thereby leading to production and buildup of circulating autoantibodies.48 49
T-cell disorders
Omenn syndrome.
Omenn syndrome is a form of SCID associated with ineffectual T-lymphocyte responses to all infections. Patients have overwhelming viral, bacterial, fungal, or parasitic infections leading to death in infancy. The disorder is caused by mutations in recombination-activating genes (RAG) 1 and 2 that result in a partial absence of the protein product.50,51 RAG is involved in immunoglobulin and T-cell–receptor gene recombination and thus the generation of immune diversity. Defective development of lymphocytes causes a systemic autoimmune reaction (with lymphadenopathy, splenomegaly, erythroderma, autoimmune hepatic dysfunction and failure, and encephalopathy) that is similar to HLH and that characterizes Omenn syndrome.52 53 Children with Omenn syndrome will die unless treated with BMT. Dysfunctional T-lymphocyte activity is controlled by administering a combination of high-dose steroids, antithymocyte globulin, and cyclosporin A (HLH protocol) before transplantation.
Autoimmune polyendocrinopathy-candidiasis-ectodermal dystrophy (autoimmune polyglandular syndrome type 1).
Autoimmune polyendocrinopathy-candidiasis-ectodermal dystrophy (APECED) is a rare autoimmune disease that primarily affects the endocrine glands.54 The typical triad for APECED is hypoparathyroidism (85% of patients), primary adrenocortical failure (72%), and chronic mucocutaneous candidiasis (100%). Gonadal failure (60%), diabetes mellitus (18%), and pernicious anemia (13%) may also occur. Ectodermal manifestations include dystrophy of the dental enamel (77%) and nails (52%). The most life-threatening complications are oral squamous cell carcinoma and fulminant autoimmune hepatitis. The disease prevalence is especially high in Finland, among Iranian Jews, and in the Sardinian population.
Mutations in the autoimmune regulator (AIRE) gene cause this organ-specific human autoimmune disease.55 It was suggested that through transcriptional regulation, AIRE is involved in the negative selection or anergy induction of self-reactive lymphocytes in the thymus.56-59
Patients with chronic mucocutaneous candidiasis (CMC) have similar problems with Candida infections and autoimmune polyendocrinopathies but no ectodermal dysplasia. The underlying gene defect in CMC is unknown.60
Autoimmune lymphoproliferative syndrome.
Patients with type I Autoimmune lymphoproliferative syndrome (ALPS; Canale-Smith syndrome) have a defect in Fas, a member of the tumor necrosis factor (TNF) superfamily.61,62 A mutation in caspase 10, another cellular component of the same apoptotic pathway, has been found in patients with type II ALPS.63 ALPS does not produce an overt increase in propensity to infections. The principal clinical features are chronic benign lymphoproliferation (lymphadenopathy and splenomegaly) and autoimmune disease (especially autoimmune cytopenias, although other organ-specific autoimmune phenomena also occur).62,64 The precise trigger for the clinical features of the disease is still unknown, but common childhood herpes virus infections (human herpesvirus 6, cytomegalovirus, and EBV infections) that induce a lymphoproliferative response though cleared normally may be involved.65
The prognosis for children with Fas deficiency is generally good unless they have the very rare homozygous form of the disease, for which early BMT is recommended. The heterozygous form tends to improve spontaneously with age and without treatment. Patients and their families appear to have an increased risk of both Hodgkin (51-fold increase) and non-Hodgkin (14-fold increase) lymphomas.66
CD40 ligand deficiency (hyper-IgM syndrome).
CD40 ligand (CD40L), like Fas, is a member of the TNF superfamily, involved in T-cell–mediated cytolysis and T-cell–dependent, B-cell antibody responses. It is the gene underlying the X-linked form of hyper-IgM syndrome.67 Defects in the activation-inducing cytidine deaminase gene cause an autosomal recessive form of the disorder.68 Hyper-IgM syndrome was initially characterized as a humoral immunodeficiency with a delay in the class switching from IgM production (normal or high levels of serum IgM) to immunoglobulin molecules of other classes (low IgG, IgA, and IgE levels) associated with sinopulmonary infections.69Pneumocystis carinii pneumonia (PCP) is common in patients with hyper-IgM syndrome but is not characteristic of humoral immunodeficiencies. The increased prevalence of PCP is not related to the humoral immune abnormalities but is due to the inability of T lymphocytes to induce apoptosis of pulmonary epithelium infected with Pneumocystisorganisms.70
A principal cause of death in patients with hyper-IgM syndrome who reach adolescence or young adulthood is liver failure due to sclerosing cholangitis.71,72 The mechanism for this organ-specific autoimmune phenomenon has been described. Cryptosporidiumgastroenteritis is common in these patients, and Cryptosporidiumparasites may also ascend the biliary tree and infect bile duct epithelium. The inability of T lymphocytes to induce destruction of infected cells results in persistence of the pathogen, chronic inflammation, and subsequently, sclerosing cholangitis.73In contrast to the increase in destructive cellular immune responses, patients with CD40L deficiency have a reduction in self-reactive antibodies, a finding that illustrates the essential role of functional interactions between CD40 and CD40L in the selection of natural self-reactive B-cell repertoires.74
The long-term prognosis for patients with hyper-IgM syndrome is poor; 50% of boys with the disorder are dead by the age of 20 years. Definitive treatment with BMT in early childhood is recommended.75 Children who undergo BMT after liver damage occurs have a significantly poorer outcome post-BMT, largely because of graft-versus-host disease in the diseased liver that results in liver failure and often death if liver transplantation is not done.76 77
MHC class II deficiency.
MHC II deficiency consists of a group of rare autosomal recessive conditions caused by mutations in 4 genetic complementation groups or regulatory proteins involved in MHC II gene expression.78All cells derived from bone marrow, as well as thymic epithelium critical for the maturation of CD4+ T cells, have no MHC class II expression and a resultant CD4+ T lymphopenia. Patients with MHC class II deficiency usually die in infancy of overwhelming infections, especially disseminated viral infections.79-81 BMT is the only curative therapy, although success rates are lower than for SCID and the CD4+T lymphopenia persists.82 As with CD40L deficiency, incomplete clearance of parasites can lead toCryptosporidium-associated sclerosing cholangitis (up to 16% of cases). Autoimmune cytopenias also occur in up to 19% of patients.83
Wiskott-Aldrich syndrome.
WAS is a rare X-linked immunodeficiency disorder caused by a maturational defect affecting both lymphocytes and platelets. Death in childhood (mean age, 11 years) is due to hemorrhage (23% of patients), infection (44%), or EBV-driven lymphomas (26%).84 Eczema is common (81%); autoimmune problems occur in children with the more severe phenotype (40%) and are associated with an increased risk of EBV-related lymphoid cancer (P < .001). In a review, Sullivan et al85 observed that 15 of 20 nonperitransplantation cases of malignant disease (75%) occurred in patients with a history of autoimmune disease. Conversely, malignant disease ultimately developed in 25% of patients with a history of autoimmune disease but in just 5% of patients without such a history. These data suggest that EBV, which underlies malignant disease in patients with WAS, may also trigger the autoimmunity. The most common autoimmune problems are hemolytic anemia and vasculitis, followed by renal disease and arthritis. In some boys, clinical features similar to those in Kawasaki disease are observed.86 SLE, Sjögren syndrome, autoimmune endocrinopathies, and sarcoidosis do not occur.85
The gene responsible for WAS (WAS protein) is expressed in all hematopoietic stem cell–derived lineages and may play a role in the regulation of the actin-cytoskeleton system by interacting directly with the Ρ-like GTPase cdc42.87 88 The immune problems in WAS are not static, and there is a continual deterioration in immune function.
Symptomatic treatment with intravenous immunoglobulin, splenectomy, steroids, and antibiotic prophylaxis should be used only as a holding measure.85,89 It is clear that BMT is the treatment of choice and should be done before patients reach school age and have cumulative tissue damage due to infection and autoimmune disease.86
Common variable immunodeficiency.
CVID is the most common symptomatic primary antibody deficiency syndrome. It is characterized clinically by recurrent sinopulmonary and gastrointestinal infections. Chronic bronchiectasis leading to respiratory failure is a common cause of premature death. Many patients require immunoglobulin-replacement therapy, prophylactic antibiotic treatment, or both.
CVID is not a single disease but an idiopathic group of diseases characterized by various degrees of defective antibody production, ranging from isolated defects in the production of antibody to carbohydrate antigens to almost complete absence of all immunoglobulin subclasses.90 Leaky phenotypes of SCIDs and the molecular defects listed in Table 4 are sometimes found in patients presenting with clinical features suggestive of CVID. A greater understanding of the molecular genetic background of primary immunodeficiency disorders will lead to a smaller proportion of patients being considered to have CVID and a larger proportion given a diagnosis of a defined primary immunodeficiency disease. This will allow more accurate advice to be given on the possible complications and likely natural history of a patient's condition.
Although the hallmark of CVID is defective antibody production, in many cases, B-cell dysfunction is due to a lack of help from defective T lymphocytes, since in vitro tests of isolated B-cell function yield normal results in 75% of cases. More than half of patients with CVID have abnormal T-cell numbers or defective proliferation to mitogens.
Autoimmune diseases, particularly chronic inflammatory bowel disease, autoimmune cytopenias such as thrombocytopenia and hemolytic anemia, and rheumatoid arthritis, are common in patients with CVID.91,92 Inflammatory bowel disease associated with chronic diarrhea and sometimes malabsorption, failure to thrive, or protein-losing enteropathy, occurs in about 30% of patients and most often affects the large bowel, although it can affect the stomach or small bowel.93,94 If the stomach is involved, associated atrophic gastritis and pernicious anemia may occur. The inflammatory bowel disease may have histologic features of celiac disease, Crohn disease, or acute graft-versus host disease, with villous atrophy, apoptotic bodies in crypts, and lymphocyte infiltration. In up 16% of patients with CVID, cancer develops, particularly gastric adenocarcinoma and small-bowel lymphoma that arise in the presence of severe atrophic gastritis and nodular lymphoid hyperplasia, respectively.95 96
Liver disease with persistently elevated levels of liver enzymes develops in 20% of patients with CVID. In some cases, liver disease is due to viral hepatitis, although with screening of immunoglobulin-replacement agents for hepatitis viruses, this should now be rare. In other cases, the cause is unknown. Granuloma or mild inflammatory changes in the portal tracts are often found at liver biopsy.97
Autoimmune disease in patients with CVID may be associated with particular HLA and mannose-binding protein genotypes.98Steroids and other immunosuppressive agents frequently used to treat autoimmune diseases should be used with caution in patients with CVID because such drugs will further increase the patients' risk of infection.
About 10% of patients with CVID have multisystem, noncaseating granulomatous disease. CVID-associated granulomatous disease can affect solid organs, skin, gut, lymph nodes, and spleen, and it may be confused with idiopathic sarcoidosis,99,100 CGD, or Crohn disease. Patients with granulomatous disease often have clinical evidence of lymphoproliferation (splenomegaly and lymphadenopathy),101 and laboratory testing frequently shows an expansion of CD8+ T lymphocytes that may underlie the inflammatory reaction.102 The lungs (interstitial lung disease) and gut (chronic inflammatory disease) are the principal sites affected. Specific HLA types may be associated with granulomatous disease in CVID. Functional polymorphisms in the TNF gene in the HLA class III region related to increased TNF production are associated with granuloma in patients with CVID.98
Isolated IgA deficiency or SIgAD.
SIgAD is the most common form of primary immunodeficiency disease in the Western world (1 in 600), although it is less common in the Japanese (1 in 18 000) and Chinese (1 in 4000) populations.103 Familial inheritance of either SIgAD or CVID occurs in about 20% of cases, and CVID may develop from SIgAD, suggesting that at least in some cases, SIgAD and CVID may be part of a spectrum of diseases caused by common, not-yet-identified genetic factors.94 Most people with SIgAD are asymptomatic and do not have an increased risk of infection. Patients with recurrent infections, particularly respiratory and gastrointestinal infections, often have other antibody defects and are therefore more appropriately classified as having CVID.
Patients with SIgAD have an increased risk of autoimmune phenomena, especially systemic autoimmune diseases such as SLE (1%-5% of patients) and rheumatoid arthritis (2%-4% of patients).104 There is also a clear link between SIgAD and celiac disease. The prevalence of SIgAD in patients with celiac disease is 2.6%, which represents a 10- to 16-fold increase over that in the general population.105 An increased risk of other organ-specific autoimmune diseases, such as insulin-dependent diabetes mellitus, myasthenia gravis, and autoimmune thyroiditis, has been reported, but the evidence for such associations is insufficient, and larger studies are required before definite conclusions can be drawn.
A high prevalence of autoantibodies (especially rheumatoid factor, anticardiolipin, and antinuclear antibodies) not associated with any clinical disease occurs in patients with SIgAD.104Antibodies against ingested food antigens are also common. Anti-IgA antibodies, especially those of the IgG subclass, are found in 9% to 44% of patients with complete, but not partial, SIgAD. Although reactions to blood products in patients with SIgAD are uncommon (1 in 30),106 anti-IgA antibodies increase the risk of severe or even fatal reactions to blood, plasma, and agents used in immunoglobulin-replacement therapy.107 There is no correlation between the titer of anti-IgA antibodies of the IgG and IgM class and the severity of transfusion reactions. The risk of anaphylaxis may be related to the production of antibodies of the IgE isotype.108 Blood products should be administered with caution to patients with SIgAD, and intravenous immunoglobulin products with low levels of IgA (< 1 mg/L) are often recommended.109
There are several theories for why patients with SIgAD have an increased risk of autoimmunity. IgA on mucosal surfaces may bind to environmental antigens, promoting their removal and preventing development of secondary T-cell–mediated inflammatory responses. Certain HLA alleles and haplotypes (eg, HLA-A1, HLA-B8, and HLA-DR3) associated with autoimmune diseases are also associated with SIgAD.110 Production of IgA is known to be strictly T-cell dependent, and some patients with SIgAD have CVID and overt abnormalities in T-cell regulation. T-cell dysfunction may be responsible for both SIgAD and the autoimmunity.104
DiGeorge syndrome.
Cytokine storms
Hereditary febrile disorders.
Some rare disorders are characterized by recurrent episodes of fever and autoimmune phenomena. Familial Mediterranean fever (FMF) is an autosomal recessive disorder that chiefly affects people from the Mediterranean basin and is caused by mutations in the pyrin-marenostrin (MEFV) gene.113 MEFV, a member of a highly conserved group of nuclear transcription factor genes, is expressed in neutrophils, eosinophils, and monocytes114 and is thought to provide inhibitory signals that may decrease inflammatory responses by these cells.115 FMF is characterized clinically by recurrent episodes of fever, polyserositis, and arthritis, which occurs in almost half of the patients and is usually monoarticular. Up to 47% of patients have Henoch-Schönlein purpura with a leukocytoclastic vasculitis, and 9% have polyarteritis nodosa.116-118 In one series, 12 of 16 patients had high levels of antistreptolysin O titer, suggesting a possible role for streptococcal infections in triggering the vasculitis.117 Amyloidosis develops in 25% of patients. Colchicine is the treatment of choice for both prophylaxis and acute exacerbations of FMF.
TNF-receptor (TNFR)–associated periodic syndrome (TRAPS) is an autosomal dominant condition caused by missense mutations in the gene encoding type I TNFR.119 The mutations may produce an inability to cleave the extracellular domain of the TNFR, thereby leading to a persistent, exaggerated response to TNF. Patients with TRAPS may have fever, polyserositis, arthritis, rash, and conjunctivitis; amyloidosis occurs in 25%. Clinical improvement in TRAPS has been observed after infusions of p75 TNFR–Fc fusion proteins.118
Hyperimmunoglobulinemia D and periodic fever syndrome, in which patients have fever, polyserositis, arthritis, lymphadenopathy, and cutaneous vasculitis, is due to mutations in the gene encoding mevalonate kinase.120 Mevalonic kinase catalyzes conversion of mevalonic acid to 5-phosphomevalonic acid during biosynthesis of cholesterol and nonsterol isoprene compounds, but the mechanism by which mutations in the gene cause inflammation is unknown. Nonsteroidal anti-inflammatory agents may partly control symptoms.
Translating progress in our understanding of autoimmunity associated with primary immunodeficiency disease to idiopathic autoimmune conditions
It is currently assumed that autoimmunity is a breakdown in self-tolerance. However, advances in our understanding suggest that autoimmunity associated with primary immunodeficiency diseases is not simply defective self-tolerance; rather, it is the inability of an inherently defective immune system to eradicate persisting microbial immunogens. Persistence of an antigen results in chronic, ineffective, damaging immune responses (Table 2). Herpes viruses are the archetypal persisting infectious agents that, even in individuals with essentially normal immunity, intermittently escape from normal immune control and produce symptomatic disease. It is therefore not surprising that incomplete eradication or relapse of EBV seems to trigger autoimmune phenomena. Examples include virus-associated HLH in CHS, Griscelli syndrome, FHL, and XLP; lymphomatoid vasculitis in XLP and lymphomatoid vasculitis; and possibly other vasculitides in WAS. Most animals are resistant to EBV, and the classic virus-associated HLH of the NK-cell disorders does not occur, limiting the usefulness of animals as models for these diseases. Boys with X-linked agammaglobulinemia have no B lymphocytes and therefore cannot be chronically infected with EBV. Autoimmunity is not a feature of this B-cell disorder.
This review highlighted the great variety of genetic defects that may predispose an individual to autoimmunity (Table 1). Mutated genes are involved in a variety of cellular functions. In some cases, it is difficult to reconcile the underlying genetic defect (eg, MEFV and mevalonate kinase) with the clinical disease, and therefore candidate-gene screening approaches may not always be useful. The fact that mutations in a variety of genes may result in almost identical clinical phenotypes (eg, HLH in XLP and FHL and ALPS in Fas and caspase-10 deficiency) is likely to complicate population studies aimed at determining genetic predisposition for specific autoimmune syndromes. Studies in animals will help explain why a particular autoimmune disease develops in a particular inbred strain, but results should be applied cautiously to human disease.
Despite these difficulties, studies of autoimmunity in primary immunodeficiency diseases and of their underlying molecular defects have allowed clinicians to predict the natural history of these conditions and to optimize clinical management. In our pediatric immunology practice, we have created strong collaborative links with clinical colleagues in other specialties, including rheumatology, gastroenterology, and particularly hematology, as well as with scientists in laboratories around the world. This has led to a diagnosis in several patients and their relatives, some of whom were thought to have had an “idiopathic” autoimmune disease for many years. Strengthening of links and communication among clinicians in a variety of specialties and development of collaborative research with basic scientists has and will continue to provide the best means for translating the advances in understanding autoimmunity in primary immunodeficiency diseases to idiopathic autoimmunity.
References
Author notes
P. D. Arkwright, Academic Unit of Child Health, First Floor, St Mary's Hospital, Hathersage Road, Manchester, M13 0JH, United Kingdom; e-mail: peter_arkwright@lineone.net.