Abstract
Dendritic cells (DCs) play a pivotal role in the generation of virus-specific cytotoxic T-cell responses, but some viruses can render DCs inefficient in stimulating T cells. We studied whether infection of DCs with human cytomegalovirus (HCMV) results in a suppression of DC function which may assist HCMV in establishing persistence. The effect of HCMV infection on the phenotype and function of monocyte-derived DCs and on their ability to mature following infection with an endothelial cell–adapted clinical HCMV isolate were studied. HCMV infection induced no maturation of DCs; instead, it efficiently down-regulated the expression of surface major histocompatibility complex (MHC) class I, CD40, and CD80 molecules. Slight down-regulation of MHC class II and CD86 molecules was also observed. Lipopolysaccharide (LPS)–induced maturation of infected DCs was strongly inhibited, as indicated by lower levels of surface expression of MHC class I, class II, costimulatory, and CD83 molecules. The down-regulation or inhibition of these surface markers occurred only in HCMV antigen-positive DCs. DCs produced no interleukin 12 (IL-12) and only low levels of tumor necrosis factor alpha (TNF-α) upon HCMV infection. Furthermore, cytokine production upon stimulation with LPS or CD40L was significantly impaired. Inhibition of cytokine production did not depend on viral gene expression as UV-irradiated HCMV resulted in the same effect. Proliferation and cytotoxicity of T cells specific to a recall antigen presented by DCs were also reduced when DCs were HCMV infected. This study shows that HCMV inhibits DC function, revealing a powerful viral strategy to delay or prevent the generation of virus-specific cytotoxic T cells.
Introduction
Dendritic cells (DCs) are the most potent professional antigen-presenting cells (APCs) playing a central and unique role in the generation of primary T-cell responses.
Whereas virus infections in general stimulate DC maturation and lead to the generation of efficient antiviral T-cell responses, some viruses have developed mechanisms to interfere with the normal functions of DCs. Human immunodeficiency virus (HIV), measles virus, herpes simplex virus-1 (HSV-1), and vaccinia virus, for example, are all known to cause defects in DC maturation and function which consequently lead to impaired antiviral T-cell responses.1-4
The major risk factor for HCMV infection in bone marrow transplant patients is their pre-existing seropositive status.5 Delayed reconstitution of the antiviral cellular immune response in these patients is often associated with HCMV infection and progressive disease with poor prognosis. CD8+T cells are the main cell type controlling HCMV infection as proven by their successful adoptive transfer preventing viraemia and HCMV disease in bone marrow transplant recipients.6 HCMV infection is also associated with serious secondary bacterial or fungal infections in immunosuppressed patients,7,8 although during the early phase of acute HCMV infection, transiently suppressed cellular immune responses9,10 and immunologic abnormalities of healthy individuals11 were also reported. HCMV has been shown to interfere with antigen presentation in fibroblasts and endothelial cells12 by (1) expressing viral proteins which interfere with the MHC class I antigen–presenting pathways (US3, US2, US11); (2) modulating antigen procession of the HCMV immediate early 1 (IE1) protein (pp65); and (3) preventing peptide transport through TAP proteins (US6). However, little is known about whether the induction phase of HCMV-specific T-cell responses is also affected. From the few studies that looked at HCMV infection of monocytes, macrophages, and DCs,13-15 only one seemed to establish a link between HCMV infection of macrophages and impaired proliferation of CD4+T cells upon stimulation with these macrophages.15
We hypothesized that similar to its effects in fibroblasts and endothelial cells, HCMV also inhibits the expression of MHC class I molecules in DCs. Although this inhibitory effect alone would probably be sufficient to prevent or impair the generation of virus-specific primary T-cell responses, other important features such as the expression of costimulatory molecules, the production of proinflammatory cytokines, and the antigen-presenting function of monocyte-derived DCs were also studied following HCMV infection. We show that HCMV impairs all these properties of DCs. The viral inhibition may contribute not only to the successful establishment of latency by HCMV but also to the immunosuppression observed in association with the infection.
Patients, materials, and methods
Donors
HCMV seropositive and seronegative healthy laboratory volunteers were included in this study. Ethical approval was obtained and informed consent was provided according to the Declaration of Helsinki. HCMV serostatus was determined by a high sensitivity IgG enzyme-linked immunosorbent assay (ELISA) (Department of Medical Microbiology and Public Health Laboratory, University Hospital of Wales, Cardiff, United Kingdom). The HLA types for T-cell assays were determined by microlymphocyte cytotoxicity assay (Welsh Blood Transfusion Service, Cardiff, United Kingdom) or by polymerase chain reaction–sequence-specific primers (PCR-SSP).16
Cell lines and tissue culture reagents
Human foreskin fibroblasts (HFFs) were cultured in minimum essential medium (MEM; Sigma, St Louis, MO) supplemented with 10% fetal calf serum (FCS; PAA Laboratories, Linz, Austria), 100 IU/mL penicillin, 100 μg/mL streptomycin, 2 mM L-glutamine, 25 mM Hepes buffer, sodium pyruvate, and nonessential amino acids. HFFs were used for experiments between passages 10 and 30. B lymphoblastoid cell lines (BLCLs) were prepared according to the standard method by infecting peripheral blood mononuclear cells (PBMCs) with Epstein-Barr virus (EBV)–containing B95.8 cell supernatant. The tCD40L cell line, which is a murine L fibroblast line transfected with human CD40L, was kindly provided by M. Rowe (University of Wales, Cardiff, United Kingdom). The BLCLs and tCD40L cells were cultured in complete RPMI (RPMI-1640; Sigma; supplemented as above except for nonessential amino acids) containing 10% FCS.
Viruses
HCMVTB40/E is an endothelial cell–adapted HCMV strain isolated from a bone marrow transplant recipient. It was kindly provided by Dr Christian Sinzger (Tübingen, Germany).17 HFFs were grown to about 90% confluence and infected with a low multiplicity of infection (MOI) (0.1-1 plaque-forming unit [PFU]/cell) of HCMVTB40/E in 4 mL of MEM for 1 to 2 hours at 37°C. The virus was then removed and the cells cultured in MEM plus 10% FCS until the first cytopathic effects appeared (4-7 days). Supernatants of infected HFFs were collected daily, or every second day, until the cytopathic effects became advanced. Supernatants were centrifuged at 1200g for 10 minutes to remove cells and debris and the supernatant was stored at −70°C in small aliquots. In some experiments a concentrated virus stock was used which was obtained from cell-free supernatant by ultracentrifugation at 80 000g for 70 minutes at room temperature in a Beckman L8-M ultracentrifuge using an SW41 rotor (Beckman Instruments, Fullerton, CA). The pellet containing viral particles from 20 mL supernatant was resuspended in 1 mL complete RPMI. Viral titres, determined from the TCID50values (dilution of virus required to infect 50% of cultures) on HFF cells, grown in 48-well plates for 2 to 3 weeks, varied between 5 × 106/mL and 5 × 108/mL. HCMVTB40/E was inactivated at room temperature by a 2-minute UV irradiation (253 nm, 30 W/G30 T8 tube by Philips, Almelo, The Netherlands).
Influenza A (AX/31) virus was kindly provided by Dr D. B. Thomas and Dr J. Skehel (National Institute for Medical Research, London, United Kingdom). The virus has been grown in the allantoic cavity of embryonated chicken eggs. The viral hemagglutination titer, determined by chicken red cell agglutination, was 1000 hemagglutinating units (HAU)/50 μL. The cell lines and the virus stocks were negative for contamination with mycoplasma as determined by a mycoplasma PCR kit (VenorGem; Minerva Biolabs GmbH, Berlin, Germany).
Generation and culture of monocyte-derived DCs
PBMCs were isolated by Ficoll-Histopaque (Sigma) density gradient centrifugation of heparinized peripheral blood of healthy donors. DCs were generated from blood monocytes according to standard methods.18 Briefly, the adherent fraction of PBMCs was obtained after a 1 hour plastic adherence step (6-well plates, 15 × 106 PBMCs/well) (Nunc, Naperville, IL) in complete RPMI containing 1% FCS. The nonadherent cells were removed and the adherent cells were cultured in complete RPMI containing 10% FCS, 50 ng/mL human recombinant granulocyte macrophage–colony-stimulating factor (GM-CSF) (Leucomax; Novartis Pharmaceuticals, East Hanover, NJ) and 500 U/mL human recombinant IL-4 (BD Pharmingen, San Diego, CA). Nonadherent and loosely adherent DCs were collected and used in the experiments after 4 to 7 days in culture. More than 90% of the cells were of DC phenotype (CD1a+, HLA-DR+, CD14−, CD80+) after gating on size and side scatter parameters by fluorescence activated cell sorting (FACS) to exclude lymphocytes. Maturation of DCs was induced by 1 μg/mL LPS (Sigma) in the presence of 100 ng/mL interferon gamma (IFN-γ) (Peprotech, Rocky Hill, NJ) and 50 ng/mL TNF-α (R&D, Minneapolis, MN) at 0.5 × 106 to 1 × 106 DCs/mL in complete RPMI containing 10% FCS.
Infection of DCs
DCs were counted and either infected with HCMVTB40/Eat MOI = 1-10 PFU/cell or mock infected with MEM plus 10% FCS for 3 hours or overnight as indicated in the figure legends. After removal of the virus or MEM, DCs were washed and cultured in complete RPMI containing 10% FCS and 50 ng/mL GM-CSF for a total of 24 or 48 hours. For infection with influenza, 105 DCs were incubated with 5 HAU influenza A (AX/31) in a total volume of 200 μL for 10 minutes on ice followed by 30 minutes at 37°C. DCs were washed once in serum-free complete RPMI and cultured in complete RPMI containing 10% AB serum (Sigma) for 6 hours before using them in the experiments.
Flow cytometry
To assess expression of surface molecules on DCs, 1 × 105 to 2 × 105 DCs were labeled for 40 minutes at 4°C with antibodies at 1 μg per sample or as recommended by the manufacturer. In order to detect viral antigens, intracellular staining of DCs, labeled as above for surface molecules or unlabeled, was carried out. DCs were fixed with 4% paraformaldehyde for 20 minutes at room temperature and permeabilized with 0.025% Triton X-100 for 20 minutes at room temperature. Nonspecific binding was blocked with 2% mouse serum for 10 minutes at room temperature followed by labeling with fluorescein isothiocyanate (FITC)– or phycoerythrin (PE)–conjugated antibodies for 1 hour at 37°C. Cytokine production by LPS-stimulated or infected DCs was detected by adding 1 μL/mL GolgiPlug (BD Pharmingen) to DCs 2 hours following infection or LPS treatment and culturing the cells for a further 18 to 20 hours. DCs were fixed, permeabilized, and blocked as above and HCMV pp52-FITC antibody was added together with either IL-12–PE or TNF-α–PE antibody for 1 hour at 37°C. The cells were analyzed on a FACSCalibur (BD Pharmingen) using CellQuest 3.1 software. The following antibodies were used for labeling: HCMV p52 early antigen-specific FITC-conjugated antibody (CCH2; Dako, Carpinteria, CA), anti-MHC class I/CyChrome, anti–HLA-DR/CyChrome, anti–CD80/CyChrome, anti–CD83/PE, anti–IL-12/PE and anti–TNF-α/PE (all from BD Pharmingen), anti–CD86/TriColour and anti-CD120b (TNFRII, 75 kd)/RPE (Caltag, Burlingame, CA), anti-CD40 (Diaclone, Besançon, France), PE-conjugated mouse IgG1 (Immunotech, Luminy, France), and PE-conjugated rabbit anti–mouse IgG F(ab')2 (STAR 12A; Serotec, Oxford, United Kingdom).
Preparation of protein extracts and immunoblotting
Immature DCs were either mock infected, infected with HCMVTB40E, or UV-irradiated HCMVTB40E was added. After 24 hours, the cells were either left unstimulated or were stimulated with LPS in the presence of IFN-γ and TNF-α, as above, for 24 hours. Cells were washed in phosphate-buffered saline. For nuclear extracts the cell pellet of 3 × 105 to 5 × 105 DCs per group was lysed in nuclear extraction buffer and an equal volume of 2x gel sample buffer was added to the clarified lysate. The proteins were separated by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to polyvinylidenefluoride (PVDF) for immunoblotting using an alkaline phosphatase chemiluminescent detection protocol. RelB and p50 were detected using rabbit polyclonal antibodies, kindly provided by Dr Nancy Rice (Frederick, MD). The polyclonal actin-specific antibody was purchased from Sigma.
Generation of cytotoxic T lymphocytes in vitro, cytotoxicity and proliferation assays
Nonadherent PBMCs were kept frozen in liquid N2 and later used as a T-cell source. T-cell stimulation was carried out in complete RPMI containing 10% AB serum (Sigma). DCs were either (1) infected with influenza for 6 hours; (2) infected with both HCMVTB40/E (total of 48 hours) and influenza (6 hours); or (3) mock infected as described earlier. T-cells were added to DCs at a ratio of 10:1 at the concentration of 1.5 × 106nonadherent PBMCs plus 1.5 × 105 DCs per mL and cultured in 12-well trays at 3 mL/well for 7 days prior to testing the cytotoxic T lymphocyte (CTL) activity in a standard51Cr-release assay. Autologous BLCs were used as target cells. They were labeled with 135 μCi (5 MBq)51Cr/106 cells for 1 hour at 37°C. BLCs were washed twice and incubated either with 50 μM HLA-A2–restricted influenza M1 58-66 peptide in 5 μL dimethyl sulfoxide (DMSO) or with 5 μL/mL DMSO without peptide for another hour. Targets were plated out at 3 × 103 BLCs/well. T cells, cultured for 7 days with DCs, were washed, counted, and added at gradually decreasing numbers to target cells, starting at a 40:1 effector-target (E/T) ratio, in triplicates. The cells were incubated for 4 hours at 37°C in 200 μL final volume per well. A 25-μL aliquot of the supernatant from each well was transferred into wells of 96-well soft plates and mixed with 130 μL Betaplate scintillation fluid. Radioactivity was measured on a β-plate counter (1450 Microbeta; Wallac, Milton-Keynes, United Kingdom). The lysis of targets from each well was calculated as follows: (experimental release − spontaneous release) / (maximum release − spontaneous release) × 100, and expressed as percent specific lysis. Spontaneous release and maximum release of51Cr were determined by incubating target cells either with medium only or with 5% Triton X-100. T-cell proliferation was measured after stimulation of the nonadherent PBMCs with DCs generated and infected as described above. A quantity of 105 autologous nonadherent PBMCs per well was coincubated with 5 × 103, 2.5 × 103, 1.25 × 103, 6.25 × 102, or 3.1 × 102 DCs/well in triplicates. 3H-thymidine (Amersham Pharmacia, Piscataway, NJ) was added at 0.5 μCi/well (0.018 MBq) on day 5 of the culture for 16 hours. The cells were harvested onto fibroglass filtermats and 3H-thymidine incorporation was measured by a Wallac 1450 Microbeta β-plate counter.
Statistical analysis
Statistical analysis was performed using the Studentt test. Differences were considered statistically significant withP < .05.
Results
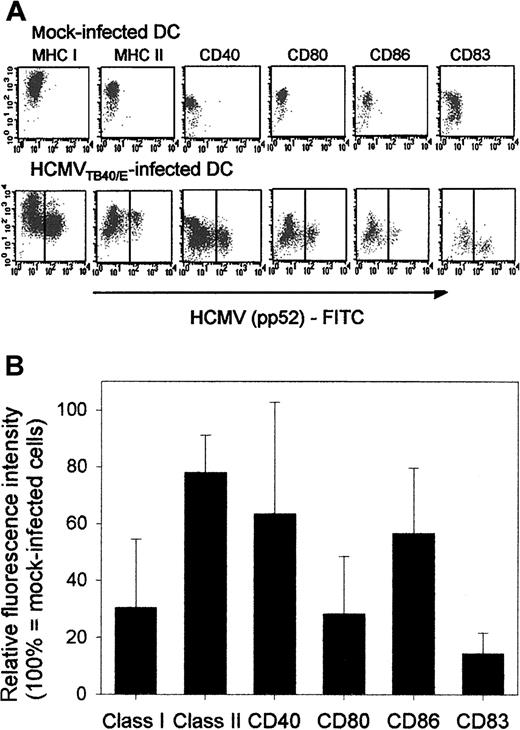
HCMV infection does not induce maturation of DCs: the expression of cell-surface molecules, especially that of MHC class I, CD40, and CD80, is decreased on DCs following infection. Viral infections can either induce or suppress the maturation and functional activation of DCs. To study the effect of HCMV on DCs, we first compared the expression of surface markers on DCs infected with HCMVTB40/E (Figure 1A, second row) with that on mock-infected DCs (Figure 1A, first row). The infection rate of DCs varied between 30% and 70% in the experiments and thus it was necessary to analyze HCMV Ag-positive DCs (Figure 1A, second row, right side of dot plots) separately from Ag-negative DCs (Figure 1A, second row, left side of dot plots). The summary of results from 3 to 4 repeated experiments (Figure 1B) shows that the relative fluorescence intensity (RFI) of surface MHC class I molecules on Ag-positive DCs is only 20% of that on mock-infected DCs. The RFI of the expression of CD40 and CD80 molecules (Figure 1B) was also less than 50%. The expression of MHC class II and CD86 molecules was also weaker on Ag-positive than on mock-infected DCs, whereas CD83 expression was low on mock-infected DCs and remained low following infection (Figure 1A). The level of expression of all the molecules mentioned above was at least as high or higher on the Ag-negative DCs (Figure 1A, second row, left side of dot plots) than on mock-infected DCs (Figure 1A, first row), indicating that in the absence of viral infection bystander DC activation by binding of viral products or soluble factors may occur. Higher level of TNFRII expression on Ag-positive than on Ag-negative DCs indicated that the down-regulation of surface molecules was not due to a general shut-down of protein synthesis by the virus (data not shown).
HCMV infection does not induce maturation of DCs and modifies the expression of cell-surface molecules on immature DCs.
The effect of HCMV infection on the expression of MHC class I, MHC class II, costimulatory molecules, and CD83 was investigated by FACS analysis on immature DCs. DCs were generated in the presence of GM-CSF and IL-4. On day 5 of the culture they were either mock infected or were infected with HCMVTB40/E (MOI = 10). Immunofluorescent labeling of cell-surface molecules and intracellular staining of fixed and permeabilized cells with FITC-conjugated HCMV pp52 antigen were carried out 2 days after infection as described in “Patients, materials, and methods.” (A) A representative experiment showing the original FACS data of the expression of cell-surface molecules on mock-infected (first row) or HCMV-infected (second row) immature DCs. The Y axes represent the mean fluorescence intensity (MFI) of surface molecules indicated above the dot plots and on the X axes the MFI of the HCMV pp52-specific antibody is shown. Throughout this paper, DCs from HCMV-infected cultures were described either as HCMV Ag negative (left side of dot plots in the second row) or HCMV Ag positive (right side of dot plots in the second row) representing uninfected and infected DCs, respectively. (B) The relative fluorescence intensity (RFI) of surface molecules on Ag-positive DCs are shown. Means and standard deviations were calculated from 3 to 4 experiments. Each data point represented the RFI of a given surface molecule (M) on HCMV Ag-positive DCs, calculated as: (MFI ofM on HCMV Ag-positive DCs / MFI of M on mock-infected DCs) × 100.
HCMV infection does not induce maturation of DCs and modifies the expression of cell-surface molecules on immature DCs.
The effect of HCMV infection on the expression of MHC class I, MHC class II, costimulatory molecules, and CD83 was investigated by FACS analysis on immature DCs. DCs were generated in the presence of GM-CSF and IL-4. On day 5 of the culture they were either mock infected or were infected with HCMVTB40/E (MOI = 10). Immunofluorescent labeling of cell-surface molecules and intracellular staining of fixed and permeabilized cells with FITC-conjugated HCMV pp52 antigen were carried out 2 days after infection as described in “Patients, materials, and methods.” (A) A representative experiment showing the original FACS data of the expression of cell-surface molecules on mock-infected (first row) or HCMV-infected (second row) immature DCs. The Y axes represent the mean fluorescence intensity (MFI) of surface molecules indicated above the dot plots and on the X axes the MFI of the HCMV pp52-specific antibody is shown. Throughout this paper, DCs from HCMV-infected cultures were described either as HCMV Ag negative (left side of dot plots in the second row) or HCMV Ag positive (right side of dot plots in the second row) representing uninfected and infected DCs, respectively. (B) The relative fluorescence intensity (RFI) of surface molecules on Ag-positive DCs are shown. Means and standard deviations were calculated from 3 to 4 experiments. Each data point represented the RFI of a given surface molecule (M) on HCMV Ag-positive DCs, calculated as: (MFI ofM on HCMV Ag-positive DCs / MFI of M on mock-infected DCs) × 100.
HCMV infection of DCs inhibits LPS-induced DC maturation. As shown in Figure 1, HCMV does not induce maturation of immature DCs, unlike, for example, adenoviruses or dengue virus. The ability of immature DCs to begin the differentiation process into mature DCs in response to certain proinflammatory cytokines (TNF-α, IFN-γ), or to bacterial products (LPS) and probably to other stimuli is crucial in the generation of antiviral immune responses and in combating bacterial infections. Therefore, we investigated the effect of HCMV infection on the maturation of DCs by first infecting DCs with HCMVTB40/E and then inducing maturation with LPS in the presence of IFN-γ and TNF-α. Despite the strong activation signal, HCMV Ag-positive DCs failed to up-regulate MHC class I, MHC class II, costimulatory, and CD83 molecules (Figure2A, second row, right side of dot plots) to the same level that mock-infected (Figure 2A, first row) or Ag-negative DCs did (Figure 2A, second row, left side of dot plots). The greatest inhibitory effect on up-regulation can be observed with MHC class I, CD80, CD86, and CD83 molecules (Figure 2B; RFI of these molecules on HCMV Ag-positive DCs was 31%, 30%, 53%, and 17%, respectively) whereas MHC class II expression was inhibited to a lesser extent (RFI 77%, Figure 2B). Down-regulation of the CD40 molecules upon infection seemed to vary more among individuals. As UV-inactivated HCMV did not inhibit LPS-induced DC maturation, measured by the surface expression of MHC class I, class II, CD40, CD86, and CD83 molecules (data not shown), our results indicate that active viral infection by HCMV of immature DCs is inhibiting DC maturation.
HCMV infection of DCs inhibits LPS-induced up-regulation of cell-surface molecules.
The effect of HCMV infection on the LPS-induced expression of MHC class I, MHC class II, costimulatory molecules, and CD83 on DCs was investigated by FACS analysis. DCs were generated in the presence of GM-CSF and IL-4. On day 5 of the culture they were either mock infected or infected with HCMVTB40/E (MOI = 10). After 48 hours, LPS was added in the presence of IFN-γ and TNF-α for 24 hours. Immunofluorescent labeling of cell-surface molecules and intracellular staining of fixed and permeabilized cells with FITC-conjugated HCMV pp52 antigen were carried out as described in “Patients, materials, and methods.” (A) A representative experiment showing original FACS data of the expression of cell-surface molecules on mock-infected (first row) or HCMV-infected (second row) DCs following LPS treatment. The Y axis represents the mean fluorescence intensity (MFI) of surface molecules as indicated above the dot plots, and the X axis indicates the binding of HCMV pp52-specific antibody. (B) The relative fluorescence intensity (RFI) of surface molecules on HCMV Ag-positive DCs following LPS treatment are shown. Means and standard deviations were calculated from 3 experiments. RFI was calculated as in Figure 1, except that the 100% expression was represented by LPS-treated mock-infected DCs.
HCMV infection of DCs inhibits LPS-induced up-regulation of cell-surface molecules.
The effect of HCMV infection on the LPS-induced expression of MHC class I, MHC class II, costimulatory molecules, and CD83 on DCs was investigated by FACS analysis. DCs were generated in the presence of GM-CSF and IL-4. On day 5 of the culture they were either mock infected or infected with HCMVTB40/E (MOI = 10). After 48 hours, LPS was added in the presence of IFN-γ and TNF-α for 24 hours. Immunofluorescent labeling of cell-surface molecules and intracellular staining of fixed and permeabilized cells with FITC-conjugated HCMV pp52 antigen were carried out as described in “Patients, materials, and methods.” (A) A representative experiment showing original FACS data of the expression of cell-surface molecules on mock-infected (first row) or HCMV-infected (second row) DCs following LPS treatment. The Y axis represents the mean fluorescence intensity (MFI) of surface molecules as indicated above the dot plots, and the X axis indicates the binding of HCMV pp52-specific antibody. (B) The relative fluorescence intensity (RFI) of surface molecules on HCMV Ag-positive DCs following LPS treatment are shown. Means and standard deviations were calculated from 3 experiments. RFI was calculated as in Figure 1, except that the 100% expression was represented by LPS-treated mock-infected DCs.
Mature DCs are relatively resistant to infection by HCMV. Immature DCs capture antigens in the periphery, and during maturation DCs gradually lose their ability to take up antigens by phagocytosis. However, they still may be susceptible to viral infections which could impair DC function. We mimicked this scenario by exposing DCs first to maturation signals provided by LPS, TNF-α and IFN-γ and then infecting them with HCMV 24 hours later. The control immature DCs were infected with HCMV in a dose-dependent manner, the proportion of HCMV early antigen-positive DCs reaching 38% when the virus was added at 10 MOI (Figure 3A). By contrast, only 1.4% of LPS-matured DCs were susceptible to infection at the same MOI of the virus (Figure 3B), and further increase of infectious virus up to MOI = 50 and MOI = 100 also failed to significantly increase the proportion of infected mature DCs (data not shown). Although the proportion of infected DCs was low following LPS treatment, FACS analysis of these DCs revealed lower levels of cell-surface MHC class I, costimulatory, and CD83 molecules than in mock-infected LPS-treated DCs (not shown). As the phenotype of DCs which became infected after treatment with LPS resembled the phenotype of Ag-positive immature DCs, it would be difficult to determine whether (1) up-regulation of maturation-associated molecules was inhibited due to the infection with the virus or (2) these DCs represent a small population of cells that remained immature with immature phenotypes and were more susceptible to infection with HCMV.
LPS-matured DCs are relatively resistant to infection.
The effect of LPS-induced DC maturation on the infection rate of DCs by HCMV was studied by FACS analysis. DCs were generated in the presence of GM-CSF and IL-4. On day 5 of the culture the cells were either left untreated (A) or LPS was added in the presence of IFN-γ and TNF-α. After 24 hours the cells were infected with decreasing amounts of infectious HCMVTB40/E (MOI = 10, 5, 1, and 0) for 48 hours. Immunofluorescent labeling of DCs in each group was carried out, first with CyChrome-conjugated HLA class II antibody (MFI shown on Y axis), followed by intracellular staining of fixed and permeabilized cells with FITC-conjugated HCMV pp52 antigen, as described in “Patients, materials, and methods.” The numbers in the upper right quadrants represent HCMV Ag-positive, HLA class II positive cells. A representative of 3 repeated experiments is shown.
LPS-matured DCs are relatively resistant to infection.
The effect of LPS-induced DC maturation on the infection rate of DCs by HCMV was studied by FACS analysis. DCs were generated in the presence of GM-CSF and IL-4. On day 5 of the culture the cells were either left untreated (A) or LPS was added in the presence of IFN-γ and TNF-α. After 24 hours the cells were infected with decreasing amounts of infectious HCMVTB40/E (MOI = 10, 5, 1, and 0) for 48 hours. Immunofluorescent labeling of DCs in each group was carried out, first with CyChrome-conjugated HLA class II antibody (MFI shown on Y axis), followed by intracellular staining of fixed and permeabilized cells with FITC-conjugated HCMV pp52 antigen, as described in “Patients, materials, and methods.” The numbers in the upper right quadrants represent HCMV Ag-positive, HLA class II positive cells. A representative of 3 repeated experiments is shown.
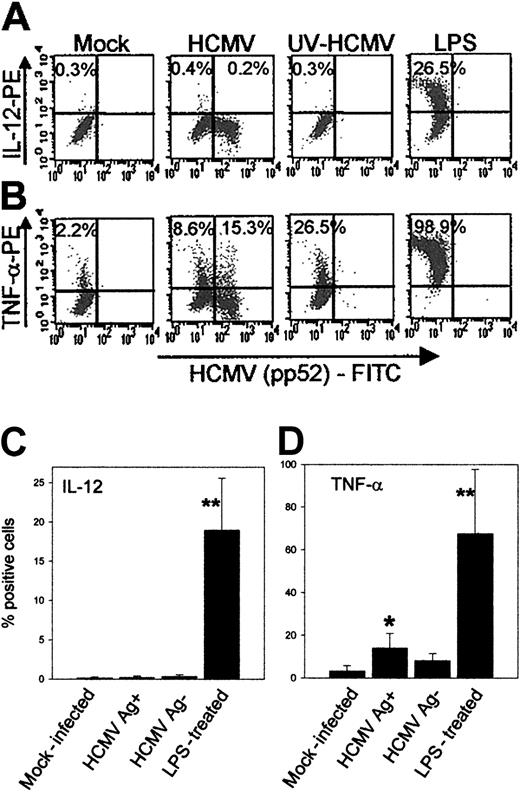
DCs produce no IL-12 and only low levels of TNF-α following infection with HCMV. An important function of DCs during viral and bacterial infections is the production of proinflammatory cytokines, such as IL-12 and TNF-α. IL-12 enhances the proliferation and cytolytic activity of NK cells and memory T cells at the site of infection and regulates Th1 cell development in lymphoid organs.19TNF-α mediates IL-12 production, DC maturation, and migration.20 Some pathogens are known to be efficient inducers of IL-12 and TNF-α production by DCs, but the effect of HCMV on the production of these cytokines has not been studied. Here we show that Ag-positive DCs do not produce IL-12 (0.2% vs 0.3% by mock-infected DCs) (Figure 4A, first and second panel; Figure 4C) within the first 24 hours following infection, whereas 18.9 ± 6.6% LPS-treated DCs produced IL-12 (Figure 4C, n = 3). UV-inactivated HCMV was also ineffective in the stimulation of IL-12 production (Figure 4A, third panel). The proportion of DCs producing TNF-α was much higher (67.6 ± 29%, n = 4, Figure 4D; also Figure 4B) than that of DCs producing IL-12 following LPS treatment. A low but statistically significant proportion (14 ± 6.7%, Figure 4D) of the Ag-positive DCs produced TNF-α following HCMV infection. However, viral replication did not seem to be important for the stimulation of TNF-α production, as Ag-negative DCs (8.1 ± 3.3%, Figure 4D) and those that were exposed to UV-inactivated HCMV (26.5%, Figure 4B, third panel) also produced TNF-α. To exclude the possibility that the production of IL-12 and TNF-α peaked later than the first 24 hours following infection with HCMV, intracellular cytokine staining experiments, identical to those shown in Figure 4A-B, were carried out 2 and 3 days after infection. No IL-12 production was detected by HCMV-infected Ag-positive or Ag-negative cells on either day, and the proportion of TNF-α–producing DCs decreased to background level by day 2 and day 3 after infection (data not shown).
HCMV infection of DCs induces no IL-12 and only a low level of TNF-α production.
Cytokine production by DCs following HCMV infection was measured by intracellular staining of cytokines in brefeldin A–treated, fixed, and permeabilized DCs by FACS analysis as described in “Patients, materials, and methods.” DCs were generated as described in the presence of GM-CSF and IL-4. On day 5 of the culture they were either mock infected or infected with HCMVTB40/E (MOI = 10), or UV-irradiated HCMV was added (MOI = 10), or LPS was added in the presence of IFN-γ and TNF-α. After 2 hours the medium was removed, the cells were washed once, and fresh complete medium containing GM-CSF and brefeldin A, in the form of GolgiPlug, was added. The cells were incubated for 22 hours and immunofluorescent labeling of fixed and permeabilized cells with FITC-conjugated HCMV pp52-specific antibody and either PE-conjugated IL-12 specific antibody or PE-conjugated TNF-α–specific antibody was carried out as described. (A) A representative experiment showing the original FACS data of the IL-12 production, or (B) TNF-α production by DCs following mock infection (first panel); infection with HCMV (second panel); treatment with UV-inactivated HCMV (third panel); LPS treatment (fourth panel). The Y axis represents the mean fluorescence intensity (MFI) of IL-12 or TNF-α and the X axis shows the MFI of the HCMV pp52-specific antibody. The numbers represent the proportions of cytokine-producing DCs. In the HCMV-infected group the cytokine production by the HCMV Ag-negative and by the HCMV Ag-positive DCs was calculated separately. (C) The summary of IL-12 production and (D) TNF-α production by DCs following HCMV infection. Means and standard deviations were calculated from 4 experiments. Significant differences to the mock-infected control are indicated as follows: *P < .05, **P < .01.
HCMV infection of DCs induces no IL-12 and only a low level of TNF-α production.
Cytokine production by DCs following HCMV infection was measured by intracellular staining of cytokines in brefeldin A–treated, fixed, and permeabilized DCs by FACS analysis as described in “Patients, materials, and methods.” DCs were generated as described in the presence of GM-CSF and IL-4. On day 5 of the culture they were either mock infected or infected with HCMVTB40/E (MOI = 10), or UV-irradiated HCMV was added (MOI = 10), or LPS was added in the presence of IFN-γ and TNF-α. After 2 hours the medium was removed, the cells were washed once, and fresh complete medium containing GM-CSF and brefeldin A, in the form of GolgiPlug, was added. The cells were incubated for 22 hours and immunofluorescent labeling of fixed and permeabilized cells with FITC-conjugated HCMV pp52-specific antibody and either PE-conjugated IL-12 specific antibody or PE-conjugated TNF-α–specific antibody was carried out as described. (A) A representative experiment showing the original FACS data of the IL-12 production, or (B) TNF-α production by DCs following mock infection (first panel); infection with HCMV (second panel); treatment with UV-inactivated HCMV (third panel); LPS treatment (fourth panel). The Y axis represents the mean fluorescence intensity (MFI) of IL-12 or TNF-α and the X axis shows the MFI of the HCMV pp52-specific antibody. The numbers represent the proportions of cytokine-producing DCs. In the HCMV-infected group the cytokine production by the HCMV Ag-negative and by the HCMV Ag-positive DCs was calculated separately. (C) The summary of IL-12 production and (D) TNF-α production by DCs following HCMV infection. Means and standard deviations were calculated from 4 experiments. Significant differences to the mock-infected control are indicated as follows: *P < .05, **P < .01.
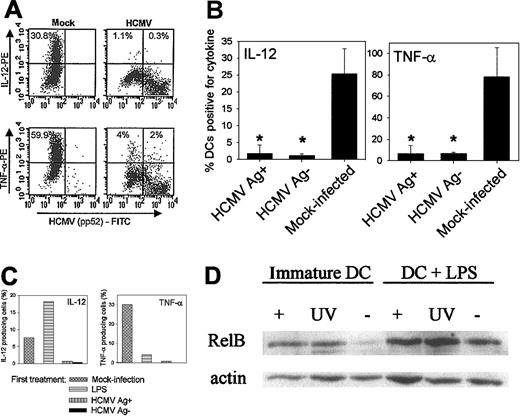
HCMV-infected DCs remain unresponsive to further stimulation to produce cytokines. Some pathogens stimulate DCs to produce IL-12 with early kinetics followed by a refractory period when DCs become unresponsive to further stimulation to produce cytokines.21 This can lead either to some degree of immunosuppression, similar to that observed in IL-12R–deficient patients,22 or to protection of the host against the pathogenic events of prolonged inflammation.21 On the other hand, interaction with certain pathogens or with their products “conditions” DCs to be susceptible to second signals such as T-cell encounter (DC40-CD40L interaction) which then leads to the production of high levels of IL-12.23 HCMV did not induce an early peak of IL-12 production and only a low level of TNF-α was produced following infection (Figure 4). We studied whether the interaction of DCs with HCMV influenced the ability of these cells to produce IL-12 and TNF-α upon a secondary stimulation. DCs were mock infected or infected with HCMVTB40/E for 48 hours before stimulation with LPS or with CD40L, and intracellular cytokine production was detected by FACS analysis. A representative experiment showing the original data (Figure5A) and results calculated from repeated experiments (Figure 5B; n = 3) show that HCMV-infected DCs, 48 hours after infection, produced significantly reduced levels of IL-12 and TNF-α upon stimulation with LPS (Figure 5B), compared with mock-infected DCs. Cytokine production was inhibited both in Ag-positive and in Ag-negative DCs, suggesting that this inhibitory effect does not require active viral replication.
HCMV-infected DCs are refractory to produce IL-12 or TNF-α when stimulated with LPS or CD40L.
Cytokine production by mock-infected or HCMVTB40/E-infected DCs upon stimulation with LPS or CD40L was measured by FACS analysis. (A) A representative experiment showing IL-12 and TNF-α production by DCs upon stimulation with LPS. DCs were either mock infected (first panel) or were infected with HCMVTB40/E (MOI = 10) for 48 hours and then stimulated with LPS/IFN-γ/TNF-α for 22 hours in the presence of GolgiPlug for the last 19 hours. The Y axis represents the MFI of IL-12 or TNF-α and the X axis shows the MFI of the HCMV pp52-specific antibody. The numbers express the proportion of cytokine-producing cells. (B) The summary of 3 experiments, showing the mean and standard deviation of cytokine-producing mock-infected and HCMV-infected (Ag-positive or Ag-negative) DCs following LPS stimulation. Significant differences to the mock-infected control are indicated as *P < .05. (C) IL-12 and TNF-α production by DCs following stimulation with CD40L. Mock-infected DCs were either left unstimulated or were prestimulated with LPS/IFN-γ/TNF-α. One group of DCs was infected with HCMVTB40/E (MOI = 10 PFU/cell) for 48 hours. DCs were then stimulated with CD40L-expressing fibroblasts at DC/Fb ratio = 10:1 in the presence of IFN-γ (100 ng/mL) for 22 hours. GolgiPlug was added for the last 21 hours of the incubation. The results are expressed as percent of DCs positive for the studied cytokine. A representative of 2 similar experiments is shown. (D) HCMV induces up-regulation of nuclear NF-κB in DCs. Immunoblotting of nuclear NF-κB proteins is shown from DCs following HCMV infection. DCs on day 5 of culture in GM-CSF and IL-4 were either mock infected (-), infected with HCMV (MOI = 10) (+), or UV-inactivated virus was added (UV) for 24 hours. DCs were then either treated with LPS/IFN-γ/TNF-α (right panel, +LPS) for 24 hours or were left untreated (left panel, immature DCs). Nuclear extracts were analyzed for RelB expression by separation of the proteins on a 10% SDS-PAGE gel followed by electrotransfer to PVDF membranes and incubation with polyclonal antibodies against RelB and actin.
HCMV-infected DCs are refractory to produce IL-12 or TNF-α when stimulated with LPS or CD40L.
Cytokine production by mock-infected or HCMVTB40/E-infected DCs upon stimulation with LPS or CD40L was measured by FACS analysis. (A) A representative experiment showing IL-12 and TNF-α production by DCs upon stimulation with LPS. DCs were either mock infected (first panel) or were infected with HCMVTB40/E (MOI = 10) for 48 hours and then stimulated with LPS/IFN-γ/TNF-α for 22 hours in the presence of GolgiPlug for the last 19 hours. The Y axis represents the MFI of IL-12 or TNF-α and the X axis shows the MFI of the HCMV pp52-specific antibody. The numbers express the proportion of cytokine-producing cells. (B) The summary of 3 experiments, showing the mean and standard deviation of cytokine-producing mock-infected and HCMV-infected (Ag-positive or Ag-negative) DCs following LPS stimulation. Significant differences to the mock-infected control are indicated as *P < .05. (C) IL-12 and TNF-α production by DCs following stimulation with CD40L. Mock-infected DCs were either left unstimulated or were prestimulated with LPS/IFN-γ/TNF-α. One group of DCs was infected with HCMVTB40/E (MOI = 10 PFU/cell) for 48 hours. DCs were then stimulated with CD40L-expressing fibroblasts at DC/Fb ratio = 10:1 in the presence of IFN-γ (100 ng/mL) for 22 hours. GolgiPlug was added for the last 21 hours of the incubation. The results are expressed as percent of DCs positive for the studied cytokine. A representative of 2 similar experiments is shown. (D) HCMV induces up-regulation of nuclear NF-κB in DCs. Immunoblotting of nuclear NF-κB proteins is shown from DCs following HCMV infection. DCs on day 5 of culture in GM-CSF and IL-4 were either mock infected (-), infected with HCMV (MOI = 10) (+), or UV-inactivated virus was added (UV) for 24 hours. DCs were then either treated with LPS/IFN-γ/TNF-α (right panel, +LPS) for 24 hours or were left untreated (left panel, immature DCs). Nuclear extracts were analyzed for RelB expression by separation of the proteins on a 10% SDS-PAGE gel followed by electrotransfer to PVDF membranes and incubation with polyclonal antibodies against RelB and actin.
IL-12 and TNF-α production upon CD40L binding following HCMV infection were also studied. Mock-infected, unstimulated DCs or DCs prestimulated with LPS produced IL-12 and TNF-α upon binding CD40L on transfected fibroblasts (Figure 5C, first and second columns), which confirms that IL-12 production is higher if DCs encounter both a microbial product and CD40L, whereas TNF-α is produced mainly upon stimulation of DCs with pathogens. However, HCMV-infected DCs produced neither IL-12 nor TNF-α upon stimulation with CD40L (Figure 5C, last 2 columns). Cytokine production was inhibited in both the Ag-positive and Ag-negative DC populations, suggesting again that the viral inhibition of cytokine production did not depend upon the active replication of the virus.
HCMV induces changes in the level of nuclear NF-κB proteins in DCs. Cytokine production by DCs is controlled by NF-κB nuclear factors which, with the exception of p65, are markedly up-regulated in uninfected DCs upon LPS treatment.24 We examined whether the inhibition of cytokine production was possibly due to a viral interference of the nuclear accumulation of NF-κB. The expression of RelB and p50 proteins in the nuclear fraction of DCs, which were mock infected or infected with HCMV or to which UV-inactivated HCMV was added, was studied before or after LPS treatment. Both infectious and UV-inactivated HCMV caused increased levels of RelB in the nucleus of immature DCs (Figure 5D, left panel, lanes “+” and “UV” vs “–”). LPS treatment of DCs resulted in the accumulation of nuclear RelB in mock-infected, HCMV-infected, and in UV-inactivated virus-treated DCs (Figure 5D, right panel). Similar results were obtained with p50 antibody (not shown). The results show that although HCMV and its viral products cause an early nuclear translocation of NF-κB factors in immature DCs which is followed by further NF-κB accumulation upon LPS treatment, the increase in the level of NF-κB proteins is not sufficient to result in cytokine production by these DCs.
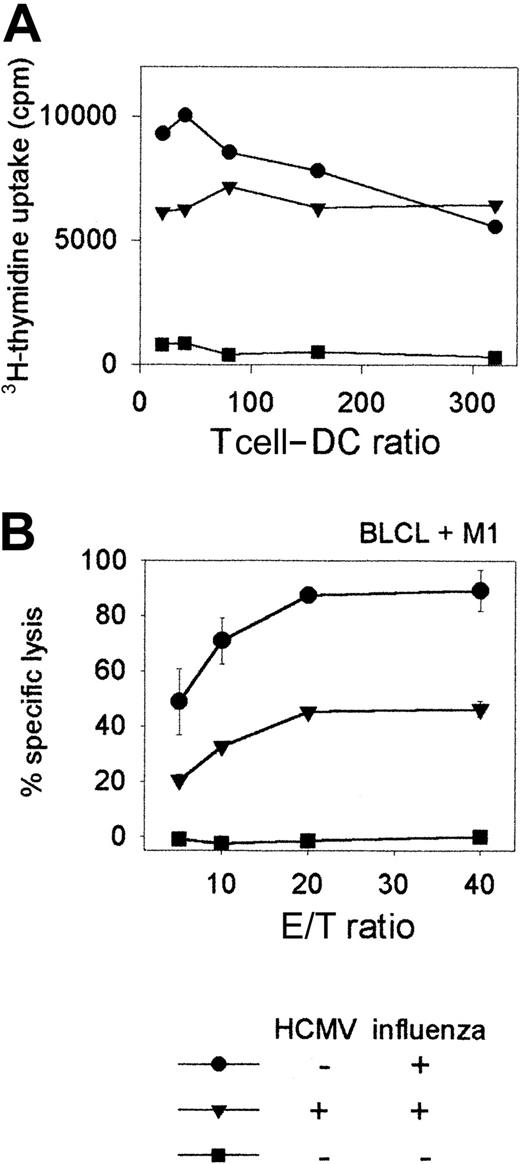
T-cell proliferation and cytotoxicity are impaired following stimulation with HCMV-infected DCs. The consequences of DC infection by HCMV, such as interference with the expression of DC surface molecules and inhibition of maturation and cytokine production, probably have the main impact on the generation of primary antiviral T-cell responses. In the absence of an in vitro model to prime naı̈ve T cells, we investigated whether HCMV-infected DCs were impaired in their ability to induce MHC class I–mediated T-cell responses to a recall antigen in vitro (Figure 6). Secondary CD8+ T-cell responses do not require costimulation and thus the outcome of such an experiment would mainly be influenced by the down-regulation of MHC class I molecules on infected DCs. Immature DCs from an HCMV seronegative donor were infected with HCMVTB40/E (MOI = 10) for 48 hours and then incubated with influenza virus for 6 hours prior to adding autologous T cells. Influenza-specific T-cell proliferation at day 5 following stimulation was reduced when HCMV-infected DCs were used as APCs, compared with proliferation induced with mock infected DCs (Figure 6A). A CTL assay, with T cells from day 7 cultures against M1 peptide–pulsed autologous BLCL targets revealed that HCMV-infected DCs had a much-reduced ability to stimulate cytotoxic T-cell responses to influenza virus (Figure 6B, triangles) than did mock-infected DCs (Figure 6B, circles), confirming that antigen presentation by DCs and their ability to stimulate a secondary T-cell response are impaired following HCMV infection. The results for (A) and (B) are representative of 3 similar experiments.
T-cell proliferation and cytotoxicity are impaired following stimulation with HCMV-infected DCs.
(A) Influenza virus–specific T-cell proliferation is shown following stimulation of T cells with HCMV-infected DCs. 105 T cells per well were stimulated at different T cell–DC ratios either with HCMV-infected or mock-infected DCs which were also infected with influenza as described in “Patients, materials, and methods.” T-cell proliferation was measured by adding 3H-thymidine to the cells in the last 16 hours of a 5-day in vitro culture. Means of3H-thymidine uptake (cpm) are shown from triplicate wells. (B) Influenza virus–specific T-cell cytotoxicity is shown using HCMV-infected or mock-infected DCs as stimulators. Immature DCs (day 7) from an HCMV-seronegative HLA-A2+ donor were either infected with HCMVTB40/E (MOI = 10) (▴) or were mock infected (●) for 48 hours and then both groups of DCs were incubated with influenza virus for 6 hours prior to adding them to autologous T cells (T cell–DC ratio = 10:1) in bulk cultures, as described in “Patients, materials, and methods.” Mock-infected DCs (■) to which influenza virus was not added served as controls to detect nonspecific T-cell stimulation. CTL assay was carried out 7 days later against influenza M1 peptide-pulsed autologous BLCL target cells or unpulsed target cells. Lysis of unpulsed targets was significantly lower by each group of T cells than that of peptide-pulsed targets, and is not shown. Means and standard deviations of percentage specific lysis from triplicate wells are shown.
T-cell proliferation and cytotoxicity are impaired following stimulation with HCMV-infected DCs.
(A) Influenza virus–specific T-cell proliferation is shown following stimulation of T cells with HCMV-infected DCs. 105 T cells per well were stimulated at different T cell–DC ratios either with HCMV-infected or mock-infected DCs which were also infected with influenza as described in “Patients, materials, and methods.” T-cell proliferation was measured by adding 3H-thymidine to the cells in the last 16 hours of a 5-day in vitro culture. Means of3H-thymidine uptake (cpm) are shown from triplicate wells. (B) Influenza virus–specific T-cell cytotoxicity is shown using HCMV-infected or mock-infected DCs as stimulators. Immature DCs (day 7) from an HCMV-seronegative HLA-A2+ donor were either infected with HCMVTB40/E (MOI = 10) (▴) or were mock infected (●) for 48 hours and then both groups of DCs were incubated with influenza virus for 6 hours prior to adding them to autologous T cells (T cell–DC ratio = 10:1) in bulk cultures, as described in “Patients, materials, and methods.” Mock-infected DCs (■) to which influenza virus was not added served as controls to detect nonspecific T-cell stimulation. CTL assay was carried out 7 days later against influenza M1 peptide-pulsed autologous BLCL target cells or unpulsed target cells. Lysis of unpulsed targets was significantly lower by each group of T cells than that of peptide-pulsed targets, and is not shown. Means and standard deviations of percentage specific lysis from triplicate wells are shown.
Discussion
In this paper, we demonstrate that HCMV interferes with the maturation and antigen-presenting function of DCs. Following HCMV infection of DCs in vitro, we observed down-regulation of MHC class I molecules on immature and on LPS-treated DCs; inhibition of DC maturation, as indicated by the lack of up-regulation of MHC class II and costimulatory molecules (CD40, CD80, CD86) and CD83 on DCs; impaired proinflammatory cytokine production (IL-12 and TNF-α) and impaired antigen-presenting function, as measured by decreased CTL activity and T-cell proliferation following stimulation of T cells with HCMV-infected DCs.
HCMV decreases the expression of MHC class I molecules on fibroblasts12 and on endothelial cells25 and partially downregulates surface expression of MHC class I molecules on human macrophages26 by multiple mechanisms. The finding of down-regulation of MHC class I molecules on DCs is in agreement with these studies. Our results indicate that MHC class I down-regulation is an important viral mechanism not only in avoiding CTL killing of infected target cells but also in inhibiting, or at least delaying, the generation of virus-specific primary T-cell responses.
Constitutive MHC class II expression is restricted to B cells, monocytes, and thymic epithelial cells, whereas IFN-γ induces MHC class II expression on some other cell types. IFN-γ–induced MHC class II expression is impaired in HCMV-infected endothelial cells due to inhibition of the Jak/STAT pathway.27 Furthermore, HCMV gpUS2 causes degradation of 2 essential MHC class II proteins (HLA-DR-α and DM-α),28 which leads to impairment of antigen-presentation to CD4+ T cells. We studied both the constitutive expression of MHC class II on immature DCs following HCMV infection and the effect of infection on up-regulation of MHC class II by LPS/IFN-γ/TNF-α treatment. Unlike the strong inhibitory effect of HCMV on MHC class I expression in DCs, constitutive expression of MHC class II was only slightly impaired by HCMV. Induction of MHC class II up-regulation with LPS in the presence of IFN-γ and TNF-α was also only partially inhibited in HCMV-infected DCs. One possible explanation for the moderate MHC class II down-regulation observed by us is that class II molecules may become down-regulated with different kinetics from those of MHC class I; thus, a longer incubation after infection may have revealed more profound effects.
This is the first study to examine the effect of HCMV infection on the expression of costimulatory molecules on DCs. We demonstrate here that HCMV infection causes down-regulation of CD40, CD80 and, to a lesser extent, of CD86 molecules on immature DCs. Ligation of CD40 molecules on DCs by the CD40L on T cells normally results in the terminal differentiation of DCs. The interaction strongly increases the expression of MHC class I, class II, and costimulatory molecules on DCs and the production of cytokines (IL-12, TNF-α, IL-8) that are necessary for full activation, differentiation, and cytokine production of naı̈ve CD4+ T cells.29,30 In the absence of CD4+ T cells and therefore of CD40L-mediated help, the generation of memory CTL is also often compromised.31 Lack of costimulation via the CD80 and/or CD86 and CD28 interaction after TCR triggering may result in anergy instead of activation of T cells, marked by their inability to proliferate and to produce cytokines.32 We found that both CD80 and CD86 expression remain at low levels in HCMV-infected DCs upon stimulation with LPS. This might have a profound effect on the priming of HCMV-specific T cells either by causing anergy and/or apoptosis or by delaying the development of virus-specific primary T-cell responses, allowing enough time for the virus to reach the site of latency. T-cell unresponsiveness/anergy and an increased rate of T-cell apoptosis have in fact been reported in HCMV-infected transplant patients.33
The production of TNF-α by activated DCs plays an important role in the maturation and migration of DCs and in the induction of proliferation and cytolytic activity of NK and T cells. On the other hand, increased levels of TNF-α have been shown to up-regulate IE enhancer/promoter activity in undifferentiated monocytic cells34 and in the lungs of latently infected mice.35 In our experiments only a small proportion of DCs produced TNF-α following HCMV infection, and it was not sufficient to drive the differentiation of infected DCs. Although we have not studied the effect of TNF-α on HCMV replication in DCs, it is possible that TNF-α had a stimulatory effect on HCMV IE gene expression but not on DC differentiation. In this study we also showed that infection of DCs with HCMV does not induce IL-12 production, which may lead to the lack of stimulation of NK and memory T cells at the site of infection. DCs also remain unresponsive to subsequent stimulation with LPS or with CD40L to produce IL-12 and TNF-α. Lack of IL-12 production, especially in the presence of viral IL-10,36 could cause a Th2/Th1 shift. Our observations may help in explaining the lack of Th1-type responses and the significantly up-regulated Th2-type cytokine production in bone marrow transplant recipients with HCMV-associated fatal interstitial pneumonitis.37
DCs represent an ideal target for pathogens because interference with the maturation process and function of DCs can have severe and multiple consequences for T-cell and B-cell responses. Measles virus is one of the best known examples. Although the virus induces maturation of DCs,38 it inhibits IL-12 production by activated DCs, resulting in impaired capacity to stimulate T cells and causing transient but profound immunosuppression.2Other viruses, such as HSV-1 and vaccinia,3,4 inhibit the maturation of DCs, resulting in impaired development of efficient antiviral T-cell immunity. The severe secondary bacterial or fungal infections7,8 following acute HCMV infection in immunosuppressed patients might be due to viral interference with DC function. Our results show that HCMV-infected DCs do not mature upon additional signals such as LPS and that there is a nearly complete inhibition of IL-12 and TNF-α production upon secondary stimulation with LPS or with CD40L. This could lead to impaired DC functions, resulting in a reduced ability to stimulate CTL responses, which we also found in our study. The activation of cytotoxic and helper effector memory T-cell responses, unlike the activation of naı̈ve or central memory T-cell responses, is independent from costimulation by CD80 and/or CD86 binding.39 HCMV infection of DCs in vivo is likely to result in suboptimal stimulation of the latter types of helper and cytotoxic T cells, too, and thus it would be more profound than the in vitro findings presented here.
The inhibition of DC maturation and function by HCMV and the low level of cell-surface expression of MHC class I molecules on DCs at any stage of their maturation seem to contradict the relatively high frequencies of T cells specific for HCMV antigens reported by several authors.40-43 Our previous report on cross-presentation of HCMV antigens by uninfected DCs to CD8+ T cells offers a likely explanation of how the immune system bypasses the inhibitory effects of HCMV.44 Our model described uninfected DCs acquiring viral antigens from infected fibroblasts, but it is probable that cross-presentation of viral antigens occurs between uninfected DCs and any infected cell, such as endothelial cells and even DCs. Cross-presentation may play a much more important role in the generation of virus-specific T-cell responses than previously thought.
Down-regulation of MHC class I molecules and the inhibition of up-regulation of maturation-associated molecules on DCs requires active viral infection because UV-irradiated HCMV did not have any inhibitory effect. Soluble inhibitory factors produced by the virus or by infected DCs can also be excluded because HCMV Ag-negative DCs from cultures containing infected DCs showed signs of bystander stimulation instead of inhibition, probably due to phagocytosed viral particles and or cellular debris. The search for viral genes responsible for the observed inhibition of cell-surface molecule expression on DCs is hampered by the fact that (1) the well-characterized laboratory strain of HCMV (AD169), which is used extensively by virologists, does not infect DCs17,44; and (2) recombinant adenovirus vectors, which would be useful for HCMV gene expression studies, cause maturation of DCs.45 The binding of HCMV or its purified ligands (gB and gH) to monocytes was shown to be sufficient in initiating nuclear translocation of NF-κB and activation of cellular factors leading to IL-1β production.46 We also observed accumulation of nuclear NF-κB factors in DCs which encountered either infectious or UV-inactivated HCMV. However, nuclear NF-κB accumulation did not lead to cytokine production in virus-treated DCs, suggesting a selective regulatory or inhibitory effect of NF-κB activity by HCMV. Finally, the straight correlation between cellular differentiation and permissiveness for productive HCMV infection, found in monocytes/macrophages,47 was not observed in DCs. We found that mature DCs are more resistant to HCMV infection than immature ones, indicating that mature DCs may acquire mechanisms that inhibit viral entry and/or replication. This increased resistance of mature DCs to infection can also be observed with some other viruses, for example, with vaccinia, HSV-1, and HIV,3,4 48but its nature is not known.
In summary, our study demonstrates the presence of an important viral evasion strategy by HCMV; namely, the impairment of the maturation and function of professional APCs. Interference with the stimulation of naı̈ve and memory T cells specific for HCMV antigens is likely to aid HCMV to escape T-cell recognition, whereas impaired DC function against other pathogens may contribute to HCMV-associated immunosuppression.
We thank C. Sinzger for the TB40E strain of HCMV and for stimulating discussions; N. Rice for the NF-κB polyclonal antibodies; G. Wilkinson and M. Rowe for helpful comments on the manuscript; and our donors for their support.
Supported by a University of Wales College of Medicine PhD scholarship to M.M., by the Leukaemia Research Fund to A.M.M., and by program grant 048665 from the Wellcome Trust to Z.T.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Zsuzsanna Tabi, Research Oncology Unit, Velindre Hospital, Whitchurch, Cardiff CF14 2TL, United Kingdom; e-mail:tabiz@cf.ac.uk.