Abstract
Fas (CD95) is a death receptor involved in apoptosis induction on engagement by Fas ligand (CD95L). Although CD95L-mediated apoptosis has been proposed as a pathogenic mechanism in a wide range of diseases, including graft-versus-host disease, systemic CD95 engagement in mice by agonistic CD95-specific antibodies or by soluble multimeric CD95L (smCD95L), though lethal, has been reported to cause apoptosis only in a limited range of cell types, that is, hepatocytes, hepatic sinusoidal endothelial cells, and lymphocytes. Another member of the tumor necrosis factor (TNF)/CD95L family, TNF-α, induces disseminated vascular endothelial cell apoptosis, which precedes apoptosis of other cell types and lethal multiorgan failure. Here we show that systemic CD95 engagement in vivo by agonistic CD95-specific antibody or smCD95L causes rapid, extensive, and disseminated endothelial cell apoptosis throughout the body, by a mechanism that does not depend on TNF-α. Disseminated endothelial cell apoptosis was also the first detectable lesion in a murine model of acute tissue damage induced by systemic transfer of allogeneic lymphocytes and did not occur when allogeneic lymphocytes were from CD95L-defective mice. Both vascular and additional tissue lesions induced by agonistic CD95-specific antibody, smCD95L, or allogeneic lymphocytes were prevented by treatment with an inhibitor of caspase-8, the upstream caspase coupled to CD95 death signaling. Vascular lesions are likely to play an important role in the pathogenesis of allogeneic immune responses and of other diseases involving circulating CD95L-expressing cells or smCD95L, and the prevention of CD95-mediated death signaling in endothelial cells may have therapeutic implications in these diseases.
Introduction
The Fas (CD95) protein is a cell surface receptor belonging to the tumor necrosis factor (TNF) receptor family that transduces death signaling on engagement by multimeric Fas ligand (CD95L), either in its membrane-bound form or in its soluble form resulting from cleavage by a putative metalloproteinase.1,2 Apoptosis induced by inappropriate or excessive expression of CD95L has been proposed as an important pathogenic mechanism in several diseases, involving various organs, tissues, and cell types, and including acute hepatitis,1,3,4 acute graft-versus-host disease (aGVHD),5-7 organ-specific autoimmune diseases,8,9 allergic10 and toxic11 cutaneous diseases, systemic tissue damage caused by lymphomas and leukemias,12 and tumor progression.13 On the other hand, systemic CD95 engagement induced in the mouse by agonistic CD95-specific antibodies or by soluble multimeric CD95L (smCD95L), though lethal, has been reported to cause tissue damage in a limited range of organs, that is, the liver and the lymphoid organs, through apoptosis induction in only 3 cell types, specifically, hepatocytes,3,14-16 hepatic sinusoidal endothelial cells,14,17 and lymphocytes.16 18
A possible explanation for this discrepancy may be that several diseases in which a role for CD95L-induced apoptosis has been proposed in fact require additional facilitating or effector mechanisms that are not induced in these murine models of systemic CD95 engagement. Alternately, these murine models may involve apoptosis induction in additional cell types and tissues that have not yet been identified. The latter possibility could be consistent with 2 previous reports of sinusoidal endothelial cell death in the hemorrhagic liver of mice injected with agonistic CD95-specific antibodies.14,17Although this may be an indirect consequence of the complete liver destruction resulting from the massive hepatocyte apoptosis caused by CD95 engagement,3 14-16 an alternate possibility is that hepatic endothelial cell death represents only a particular example of a general in vivo sensitivity of endothelial cells to CD95-mediated death.
Endothelial cells express CD95, but in vitro studies of their response to CD95 engagement have led to discordant reports of either spontaneous resistance19-23 or sensitivity24,25 to CD95-mediated death signaling, which may depend on the origin of the endothelial cells used or on the culture conditions. Also, on some particular in vitro treatments,21-23 initially resistant endothelial cells can be rendered sensitive to CD95-mediated death. In vivo, endothelial cells have so far been reported to undergo CD95-mediated death in only 2 organs: the liver, in response to agonistic CD95-specific antibody injection,14,17 as mentioned above, and the eye, in which endothelial cell apoptosis induced by local CD95L expression is involved in the physiologic control of subretinal blood vessel growth after injury.26However, endothelial cells from at least some arteries in some animal species, such as rabbit ear and rat carotid arteries, constitutively express both CD95 and CD95L,27,28 and rat carotid artery endothelial cells have been recently reported to survive adenovirus vector–mediated CD95L overexpression.28 Thus, whether in vivo sensitivity to CD95-mediated death signaling is restricted to endothelial cells from particular blood vessels in a few tissues or is a general characteristic of endothelial cells from most blood vessels throughout the body remains so far unknown.
Tumor necrosis factor-α, another member of the TNF/CD95L family, has been reported to induce disseminated endothelial cell apoptosis29 that precedes induction of apoptosis in additional cell types and lethal multiorgan failure. Here we show that CD95L shares with TNF-α the capacity to cause disseminated endothelial cell apoptosis and vascular lesions, through a mechanism that does not depend on TNF-α expression. We also identify disseminated endothelial cell apoptosis as an early and major lesion in a murine model of acute tissue damage induced by systemic transfer of allogeneic lymphocytes into immunodeficient mice. Our findings indicate that the capacity of the allogeneic lymphocytes to express functional CD95L is required for the induction of both these vascular lesions and additional tissue lesions in the recipient mice. Vascular damage may play an important and previously unrecognized role in the pathogenesis of allogeneic immune responses and of other diseases involving inappropriate or excessive CD95L expression, by causing further tissue damage through the induction of hypoxia and vascular leakage of proinflammatory cells.
Materials and methods
Mice
Six- to 7-week-old wild-type (wt) C57BL/6 (H-2b) and BALB/c (H-2d) mice were obtained from Iffa Credo (L'Arbresle, France); CD95-defective lpr/C57BL/6, CD95L-defective gld/C57BL/6, and SCID/C57BL/6 mice from the Jackson Laboratory (Bar Harbor, ME); TNF-α−/−/lymphotoxin (Lt)-α−/−/C57BL/6 mice from CNRS (Orléans, France); and SCID/BALB/c mice from Harlan (Oxen, United Kingdom). Severe combined immunodeficient (SCID) mice were housed in sterilized cages with filter-cap, and all mice allowed to rest for 1 week before the experiments were conducted. All the mouse studies reported here have been approved by the Animal Housing and Experiment Board of the French government.
Injection of CD95-specific antibody and soluble CD95 ligand
Mice were injected intravenously, under brief ether anesthesia, with antibodies or other reagents suspended in 200 μL sterile isotonic saline solution. Purified hamster monoclonal antibody (mAb) against mouse CD95 (JO2 clone, IgG isotype, containing < 0.01 ng lipopolysaccharide [LPS]/μg antibody) and purified control hamster monoclonal IgG were purchased from Pharmingen (San Diego, CA); soluble recombinant human CD95L (with a FLAG sequence) and the anti-FLAG antibody were from Alexis (San Diego, CA); the caspase-8 inhibitor z-IETD-fmk was from Calbiochem (La Jolla, CA); and bacterial LPS was from Sigma (St Louis, MO). When not killed, mice were monitored for up to 12 hours after injection, and surviving mice daily for 1 week.
Injection of allogeneic lymphocytes
Lymphocytes were isolated from the spleen of killed mice by density gradient centrifugation with Lympholyte M (TEBU, Le Perray en Yvelines, France), after hypotonic lysis of red blood cells. Following washing in RPMI supplemented with 10% fetal calf serum (FCS), 2 mM l-glutamine, and 1 mM pyruvate (Gibco BRL, Rockville, MD), and macrophage and monocyte depletion by incubation at 37°C in 5% CO2 for 2 hours, cell suspensions were analyzed with a FACScan (Becton Dickinson, Mountain View, CA), using phycoerythrin-conjugated mAb against mouse Thy-1.2, Cy-Chrome–conjugated mAb against mouse B220, fluorescein isothiocyanate–conjugated mAb against mouse natural killer cells, and isotype-matched control immunoglobulin (Pharmingen). Donor splenic lymphocyte preparations with more than 95% viability (trypan blue exclusion), and containing around 40% T lymphocytes, were resuspended in phosphate-buffered saline, and 500 μL of the suspension (containing either 2.5 × 107 or 108 cells) was immediately injected intraperitoneally in each SCID recipient mouse. Allogeneic lymphocyte transfers were from C57BL/6 mice into Balb/c SCID recipients, and syngeneic lymphocyte transfers were either from Balb/c into Balb/c SCID or from C57BL/6 (in particular forlpr or gld mice) into C57BL/6 SCID. When not killed, recipient mice were monitored daily and scored for clinical manifestations of systemic allogeneic responses, such as weight loss of more than 20%, associated with posture modifications (hunching), and activity loss.5-7
Histology
Killed mice were immediately dissected, and specimens from skin (ear), liver, spleen, kidneys, different levels of digestive tract, pancreas, heart, lungs, and brain were immediately cut into 3 parts: one was snap-frozen, another was fixed in 2% glutaraldehyde in cacodylate buffer and further processed for electron microscopy, and the third part was fixed in 10% formalin and further processed for paraffin embedding. Analysis of apoptotic cells, vascular damage, and hemorrhages was performed on 3-μm–thick paraffin sections stained with hematoxylin and eosin. Apoptosis was confirmed by in situ detection of fragmented DNA, using the terminal deoxytransferase-catalyzed DNA nick end labeling (TUNEL) assay,29 30 on deparaffinized 3–μm–thick sections, treated with proteinase K (20 μg/mL) for 15 minutes at room temperature. Ultrastructural analysis of apoptotic cells was performed on a JEOL 100B electron microscope.
Quantification of vascular lesions
The percentages of damaged blood vessels were assessed independently by 2 pathologists who were blinded to the treatment, on 3-μm–thick hematoxylin and eosin–stained sections of each explored organ, at magnification of ×400, on at least 3 different microscopic fields, to obtain a minimum count of 50 blood vessel sections per organ. All blood vessels in which endothelial cells were either apoptotic or lacking were counted as damaged. For each organ, in each experimental condition, counts were performed on tissue sections from 4 to 9 mice, and percentages of damaged vessels were the mean of the counts performed by the 2 pathologists. Statistical significance was assessed using the Wilcoxon test. A log-linear regression model for Poisson data with random effect, performed using the pathologist as the unit of analysis, demonstrated no significant pathologist effect.
Results
Agonistic CD95-specific antibody and smCD95L induce disseminated endothelial cell apoptosis and vascular damage
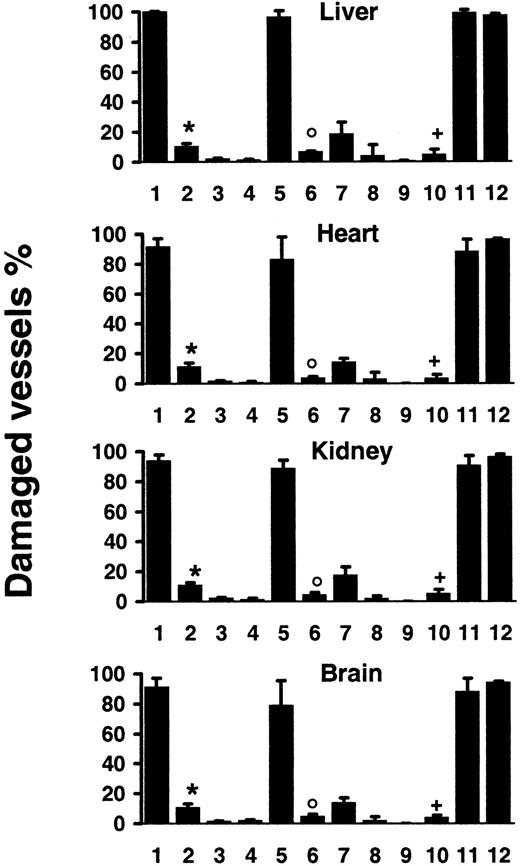
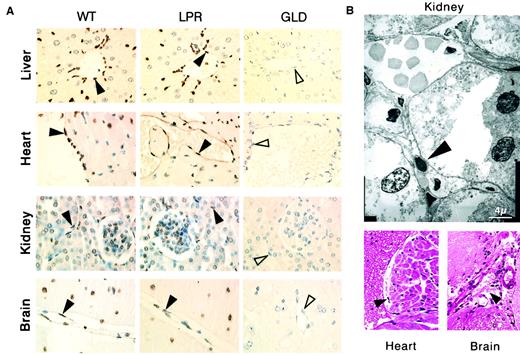
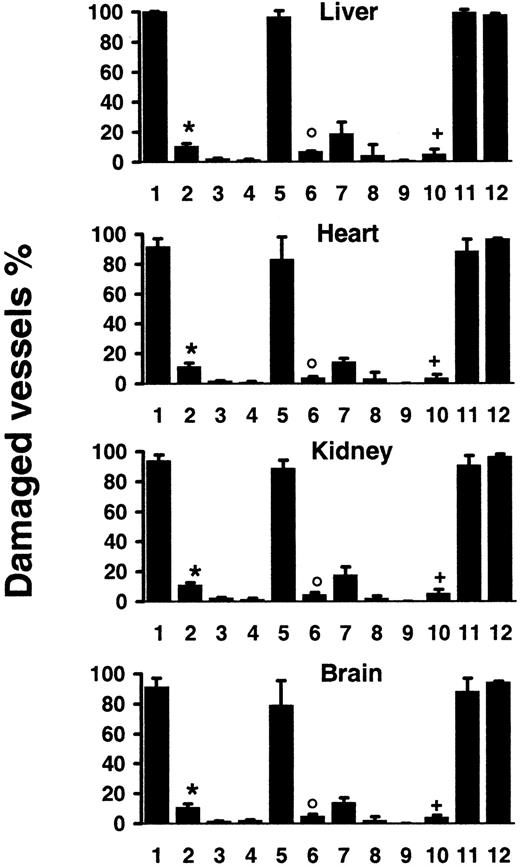
Mice injected with the agonistic JO2 CD95-specific mAb (0.5 μg/g or 10 μg for a 20-g mouse) died within 2.5 to 5 hours,3,14,15 and tissue section analysis of mice killed 2 hours after injection showed, as previously described, hepatocyte apoptosis with hemorrhages3,14,15,17 and vascular endothelial cell apoptosis in the liver14 17 (Figure1A). In addition, we identified extensive, disseminated vascular endothelial cell apoptosis (Figure 1A) in all other organs explored, including kidneys, heart, brain (Figures1 and 2), as well as lungs, intestine, pancreas, and skin (data not shown), associated with localized hemorrhage suffusions in several organs, including the brain (Figure1B). Quantification of vascular lesions in the liver, kidneys, heart, and brain indicated that more than 90% of blood vessels were damaged (Figure 2), with either apoptotic endothelial cells or no detectable remaining endothelial cell lining. JO2 also caused apoptosis of endocardial cells (Figure 1B), of additional cell types in the heart and brain cells (Figure 1A), and of intestine gland epithelial cells and basal skin keratinocytes (data not shown). Similar lesions were induced by JO2 in both males and females from 2 different mouse strains, BALB/c and C57BL/6, as well as in immunodeficient SCID and recombination-activating gene-defective (RAG) mice (data not shown).
Systemic CD95 engagement induces disseminated vascular endothelial cell death.
(A) The TUNEL assay shows apoptosis (brown staining) of vascular endothelial cells (filled arrowheads) and additional cell types in tissue sections from liver, heart, kidneys, and brain of wild-type (WT) mice killed 2 hours after injection of the JO2 CD95-specific antibody, whereas no apoptotic lesions were detected in CD95-defectivelpr (LPR) mice killed 6 hours after JO2 injection (the unstained endothelial cells are indicated by open arrowheads). (B) Endocardial lesions (heart) and subarachnoidal hemorrhage suffusions (brain), as well as typical electron microscopy features of endothelial cell apoptosis (kidney) were observed in wt mice killed 2 hours after JO2 injection (filled arrowheads).
Systemic CD95 engagement induces disseminated vascular endothelial cell death.
(A) The TUNEL assay shows apoptosis (brown staining) of vascular endothelial cells (filled arrowheads) and additional cell types in tissue sections from liver, heart, kidneys, and brain of wild-type (WT) mice killed 2 hours after injection of the JO2 CD95-specific antibody, whereas no apoptotic lesions were detected in CD95-defectivelpr (LPR) mice killed 6 hours after JO2 injection (the unstained endothelial cells are indicated by open arrowheads). (B) Endocardial lesions (heart) and subarachnoidal hemorrhage suffusions (brain), as well as typical electron microscopy features of endothelial cell apoptosis (kidney) were observed in wt mice killed 2 hours after JO2 injection (filled arrowheads).
Quantification of vascular lesions induced by systemic CD95 engagement.
Wt mice were injected with JO2 (1) (n = 9), JO2 plus caspase-8 inhibitor (2) (n = 4), control immunoglobulin (3) (n = 4), LPS (> 150-fold excess of LPS content in JO2) (4) (n = 4), smCD95L (5) (n = 4), smCD95L plus caspase-8 inhibitor (6) (n = 4), sCD95L (7) (n = 5), the anti-FLAG antibody used to multimerize the sCD95L (8) (n = 5), or remained untreated (9) (n = 5); JO2 was injected in lpr mice (10) (n = 7), in gld mice (11) (n = 7), or in TNF-α−/−–Ltα−/−mice (12) (n = 4). Percentages are mean ± SD of 2 independent counts on tissue sections from liver, heart, kidneys, and brain of(n) different mice, as described in “Materials and methods.” *P < .007 compared to 1; °P < .03 compared to 5;+P < .002 compared to 1, 11, and 12 (Wilcoxon test).
Quantification of vascular lesions induced by systemic CD95 engagement.
Wt mice were injected with JO2 (1) (n = 9), JO2 plus caspase-8 inhibitor (2) (n = 4), control immunoglobulin (3) (n = 4), LPS (> 150-fold excess of LPS content in JO2) (4) (n = 4), smCD95L (5) (n = 4), smCD95L plus caspase-8 inhibitor (6) (n = 4), sCD95L (7) (n = 5), the anti-FLAG antibody used to multimerize the sCD95L (8) (n = 5), or remained untreated (9) (n = 5); JO2 was injected in lpr mice (10) (n = 7), in gld mice (11) (n = 7), or in TNF-α−/−–Ltα−/−mice (12) (n = 4). Percentages are mean ± SD of 2 independent counts on tissue sections from liver, heart, kidneys, and brain of(n) different mice, as described in “Materials and methods.” *P < .007 compared to 1; °P < .03 compared to 5;+P < .002 compared to 1, 11, and 12 (Wilcoxon test).
The specific involvement of CD95 engagement in the hepatocyte apoptosis induced by JO2 in vivo has been previously validated by the use of 2 series of control experiments.3 The first one is the injection of control monoclonal immunoglobulin of the same species and isotype as JO2, which induces no hepatocyte lesion. The second one is the injection of the JO2 antibody in lpr and gldmice, that are, respectively, defective in CD95 and CD95L; JO2 induces similar hepatocyte apoptosis in wt mice and in gld mice, while causing no hepatocyte lesion in the CD95-defective lprmice. To assess the specific involvement of CD95 engagement in the disseminated vascular lesions induced in vivo by JO2, we used the same approaches. No vascular lesions were observed in any organ after injection in C57BL/6 wt mice of 10 μg control monoclonal immunoglobulin of the same species and isotype as JO2 (Figure 2) and JO2 induced no detectable cell death in C57BL/6 lpr mice, defective in CD951 (Figures 1A and 2). In contrast, C57BL/6gld mice, defective in CD95L but not in CD95,1showed the same extent of lesions as wt mice (Figure 2). To rule out global vital function failure induced by CD95 engagement as a possible cause for vascular damage, we injected wt mice with a nonlethal dose (5 μg for a 20-g mouse) of JO2. In mice killed 6 hours after injection, although vascular lesions were less extensive than in mice injected with 10 μg JO2, we observed disseminated endothelial cell apoptosis in all the organs mentioned above (data not shown). To assess whether endothelial cells represent early targets of CD95 engagement, we killed wt mice 30 minutes or 1 hour after JO2 (10 μg) injection. Whereas at 1 hour all the lesions mentioned above were already induced, at 30 minutes the only cell types undergoing extensive apoptosis were hepatocytes and endothelial cells throughout the body (data not shown).
We then investigated whether CD95L, the physiologic ligand of the CD95 receptor,1,2 may also cause vascular lesions. The capacity of soluble CD95L (sCD95L) to trigger apoptosis appears to depend on its multimerization degree,16,31 which determines the CD95 aggregation degree it induces. Injection of recombinant soluble multimeric CD95L (smCD95L) (5 μg) was lethal in 3 to 5 hours, as described,16 and in mice killed 2 hours after injection, in addition to the previously reported massive hepatocyte apoptosis,16 we observed the same disseminated vascular damage (Figure 2) as after JO2 injection. Injection of the anti-FLAG antibody (10 μg) used to multimerize the sCD95L induced no vascular damage (Figure 2). Injection of sCD95L in the absence of anti-FLAG antibody caused almost no vascular lesions (Figure 2), indicating that CD95L multimerization was required to induce extensive endothelial cell death in vivo.
CD95-mediated endothelial cell death does not depend on TNF-α expression and is prevented by an inhibitor of caspase-8
Tumor necrosis factor-α causes endothelial cell apoptosis in several organs,29 and Lt-α, which can engage TNF-α receptors, may share this property. High doses of LPS (> 50 μg) also trigger endothelial cell apoptosis through a mechanism that strictly depends on TNF-α secretion.29 To rule out the possibilities that vascular damage may result from intermediate steps of CD95-mediated TNF-α (or Lt-α) secretion, or from LPS contamination of JO2 (10 μg) or smCD95L (5 μg), we performed the following experiments. First, because 10 μg JO2 contained less than 0.1 ng LPS, we injected wt mice with 15 ng (> 150-fold excess) LPS, and observed no vascular lesions (Figure 2). Second, we injected either JO2, smCD95L, or high doses of LPS (90 μg) in either wt,lpr (CD95 defective), or TNF-α−/−–Ltα−/− mice. CD95 engagement induced similar extensive vascular damage in wt and TNF-α−/−–Ltα−/− mice (Figure 2), whereas high-dose LPS induced vascular lesions in wt and lprmice, but no lesions in TNF-α−/−–Ltα−/− mice (data not shown).
CD95 engagement triggers caspase activation, and injection of lethal doses of JO2 together with either the broad caspase inhibitor peptide z-VAD or with the z-IETD peptide that selectively inhibits caspase-8, the most proximal caspase activated by CD95 engagement,1,2 prevents the induction of both hepatocyte apoptosis and hepatic hemorrhage and allows mice to survive.15,17 32 We injected wt mice with JO2 (10 μg) or smCD95L (5 μg) and z-IETD (500 μg). Treatment with z-IETD allowed survival of all treated mice, and we observed a very significant prevention of vascular damage in mice killed 6 hours later (Figure 2) or 24 hours later (data not shown), indicating that the inhibition of caspase-8 activation allowed the survival of endothelial cells.
A murine model of systemic allogeneic response induces CD95L- and caspase-8–dependent disseminated endothelial cell death
A pathogenic role for CD95L expressed by effector allogeneic lymphocytes has been proposed in the induction of acute tissue damage causes by aGVHD.5-7 Because human aGVHD, the major complication of allogeneic bone marrow grafting, is caused by mature T lymphocytes of donor origin, most murine models involve the transfer of allogeneic mature splenic lymphocytes.5-7 Several of these models raise complex problems of interpretation, due either to recipient irradiation (which induces several tissular lesions), or to the presence of mature lymphocytes of recipient origin (in the parent to F1 model). To avoid these problems, we used the simplest possible model of systemic allogeneic immune response: injection of mature splenic lymphocytes from immunocompetent mice into major histocompatibility complex I– and II–mismatched SCID recipients.
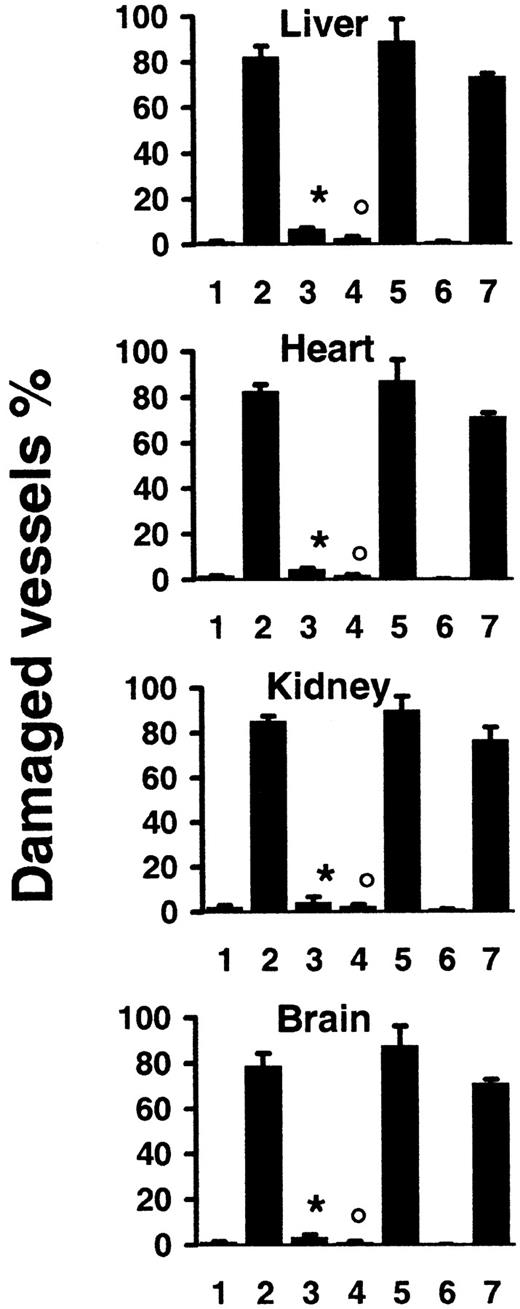
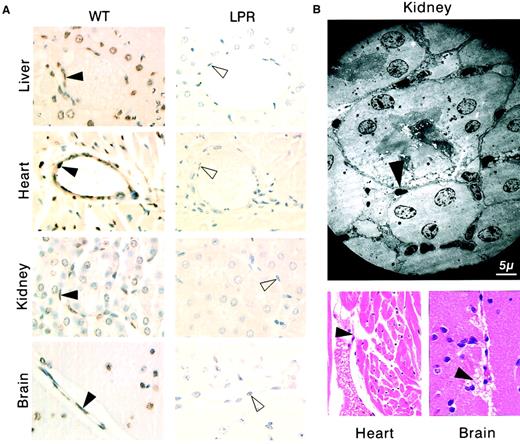
In recipient mice killed 2 days after transfer of 108allogeneic lymphocytes, we observed tissular lesions typical of aGVHD,5-7 that is, apoptosis of hepatocytes (Figure3A), liver biliary duct cells, intestinal gland epithelial cells, and basal skin keratinocytes (data not shown). In addition, we identified extensive disseminated endothelial cell apoptosis (Figure 3A) in all organs explored, including liver, kidneys, heart, brain (Figures 3 and 4), as well as lungs, intestine, pancreas, and skin (data not shown), with localized hemorrhage suffusions in several organs, including the brain (Figure 3B). Quantification of vascular lesions in the liver, kidneys, heart, and brain indicated that more than 75% of blood vessels were damaged (Figure 4), with either apoptotic endothelial cells or no detectable remaining endothelial cell lining. Apoptosis also occurred in endocardial cells (Figure 3B), and in additional cell types in the heart and brain (Figure 3A). Thus, the scope of tissue lesions in this model of acute allogeneic response recapitulated that induced by systemic injection of the agonistic CD95-specific JO2 antibody or of smCD95L. Allogeneic lymphocyte infiltration in tissues 2 days after transfer was minimal or lacking (Figure 3A,B), suggesting that if cell death was induced by CD95L, it might involve the ligand in its soluble form, a possibility consistent with the previous report of increased serum levels of sCD95L during aGVHD in humans.33No lesions were induced in recipients of 108 syngeneic lymphocytes (Figure 4). In recipients of allogeneic lymphocytes killed 1 day after transfer, the only lesion we detected was disseminated vascular damage, indicating that endothelial cells were the earliest targets of the allogeneic response in this model (data not shown).
Systemic allogeneic immune responses induce disseminated vascular endothelial cell death.
(A) The TUNEL assay shows apoptosis (brown staining) of vascular endothelial cells (filled arrowheads) and additional cell types in tissue sections from liver, heart, kidney, and brain of immunodeficient SCID recipient mice killed 2 days after transfer of 108lymphocytes from allogeneic wild type (WT) mice or from allogeneic CD95-defective lpr (LPR) mice, whereas no apoptotic lesions were detected in SCID recipient mice 2 days after transfer of 108 lymphocytes from allogeneic CD95L-defectivegld (GLD) mice (the unstained endothelial cells are indicated by open arrowheads). (B) Endocardial lesions (heart) and subarachnoidal hemorrhage suffusions (brain), as well as typical electron microscopy features of endothelial cell apoptosis (kidney) were observed in SCID recipients killed 2 days after transfer of 108 lymphocytes from allogeneic wt mice (filled arrowheads).
Systemic allogeneic immune responses induce disseminated vascular endothelial cell death.
(A) The TUNEL assay shows apoptosis (brown staining) of vascular endothelial cells (filled arrowheads) and additional cell types in tissue sections from liver, heart, kidney, and brain of immunodeficient SCID recipient mice killed 2 days after transfer of 108lymphocytes from allogeneic wild type (WT) mice or from allogeneic CD95-defective lpr (LPR) mice, whereas no apoptotic lesions were detected in SCID recipient mice 2 days after transfer of 108 lymphocytes from allogeneic CD95L-defectivegld (GLD) mice (the unstained endothelial cells are indicated by open arrowheads). (B) Endocardial lesions (heart) and subarachnoidal hemorrhage suffusions (brain), as well as typical electron microscopy features of endothelial cell apoptosis (kidney) were observed in SCID recipients killed 2 days after transfer of 108 lymphocytes from allogeneic wt mice (filled arrowheads).
Quantification of vascular lesions induced by systemic allogeneic immune responses.
SCID recipient mice were killed 2 days after transfer of 108 lymphocytes from syngeneic wt mice (1) (n = 4), or from allogeneic wt mice in the absence (2) (n = 9) or presence of recipient treatment with caspase-8 inhibitor (3) (n = 4), or after transfer of 108 lymphocytes from allogeneicgld mice (4) (n = 4), or from allogeneic lprmice (5) (n = 4); SCID recipients were killed 14 days after transfer of 2.5 × 107 lymphocytes from syngeneic (6) (n = 4) or allogeneic wt mice (7) (n = 5). Percentages are mean ± SD of 2 independent counts on tissue sections from liver, heart, kidneys, and brain of (n) different mice, as described in “Materials and methods.” *P < .007 compared to 2; °P < .007 compared to 2 and 5 (Wilcoxon test).
Quantification of vascular lesions induced by systemic allogeneic immune responses.
SCID recipient mice were killed 2 days after transfer of 108 lymphocytes from syngeneic wt mice (1) (n = 4), or from allogeneic wt mice in the absence (2) (n = 9) or presence of recipient treatment with caspase-8 inhibitor (3) (n = 4), or after transfer of 108 lymphocytes from allogeneicgld mice (4) (n = 4), or from allogeneic lprmice (5) (n = 4); SCID recipients were killed 14 days after transfer of 2.5 × 107 lymphocytes from syngeneic (6) (n = 4) or allogeneic wt mice (7) (n = 5). Percentages are mean ± SD of 2 independent counts on tissue sections from liver, heart, kidneys, and brain of (n) different mice, as described in “Materials and methods.” *P < .007 compared to 2; °P < .007 compared to 2 and 5 (Wilcoxon test).
Previous studies, using several different murine models, have suggested various degrees of contribution of CD95L, TNF-α, or perforine/granzyme, expressed or released by allogeneic lymphocytes, in the pathogenesis of aGVHD.5-7,34 We investigated the potential contribution of CD95L to endothelial cell apoptosis induction in our murine model of systemic allogeneic response by transferring 108 allogeneic (or syngeneic) lymphocytes from either wt, CD95L-defective gld, or CD95-defective lpr mice, all from the same C57BL/6 background. CD95L-defective gldmice and CD95-defective lpr mice have similar lymphocyte anomalies and develop similar time-dependent lymphoproliferative disorders.1 Syngeneic transfers induced no detectable lesion (data not shown). Two days after allogeneic transfer, no lesions were detected in recipients of gld cells, whereas multiorgan lesions, including extensive vascular damage, were similar in recipients of lpr and of wt cells (Figures 3A and 4).
We then treated recipient mice with the caspase-8 inhibitor peptide z-IETD (500 μg) 2 hours before (and 1 day after) transfer of 108 allogeneic wt lymphocytes. In mice killed 2 days after transfer, we detected only very few vascular lesions (Figure 4) and other tissue damage (data not shown), indicating that inhibition of caspase-8 activation allowed the survival of endothelial cells in the recipients of the allogeneic lymphocytes.
To investigate a more progressive model of acute tissue damage induced by allogeneic lymphocytes, we used transfers of lower numbers (2.5 × 107) of splenocytes. Characteristic clinical features of aGVHD, including major body weight loss (> 20%,P < .04) occurred within 2 weeks in all recipients of allogeneic wt cells, but not in recipients of syngeneic cells. In allogeneic wt cell recipients killed 14 days after transfer, we observed disseminated vascular lesions (Figure 4) associated with lymphocyte infiltration and with tissue lesions typical of aGVHD in the liver, intestine, and skin (data not shown). Finally, in recipients of allogeneic lymphocytes from gld mice killed 14 days after transfer, although perivascular lymphocyte infiltration was greater than in recipients of allogeneic cells from wt mice, we detected only rare vascular and other tissular lesions; in contrast, vascular and tissular lesions were as extensive in the recipients of allogeneic lymphocytes from lpr mice as in the recipients of allogeneic lymphocytes from wt mice (data not shown). Together, these results strongly suggested that the induction of disseminated endothelial cell death by allogeneic lymphocytes depended on their capacity to express functional CD95L.
Discussion
Here, we identified disseminated endothelial cell apoptosis and vascular loss of integrity as an early and major pathologic consequence of systemic CD95 engagement in vivo, whether induced by an agonistic CD95-specific antibody or by smCD95L. TNF-α has been previously reported to induce disseminated endothelial cell apoptosis,29 which precedes apoptosis induction in other cell types and lethal multiorgan failure. Because we observed that systemic CD95 engagement causes the same vascular damage in TNF-α−/− mice as in wt mice, our findings indicate that CD95L shares with TNF-α the capacity to induce vascular lesions, but through a mechanism that does not depend on TNF-α.
Endothelial cells express CD95, but have been shown to undergo CD95-mediated death in vivo so far in 2 organs only: the liver, in response to agonistic CD95-specific antibodies,14,17 and the eye, in which endothelial cell apoptosis induced by local CD95L expression appears to be involved in the physiologic control of subretinal angiogenesis after injury.26 Our findings indicate that these are only 2 particular examples of a broad in vivo sensitivity of endothelial cells to CD95-mediated death. It should be noted, however, that endothelial cells from some arteries in some tissues and animal species, such as the rabbit ear and rat carotid arteries, have been shown to constitutively express both CD95 and CD95L,27,28 and very recently, rat carotid artery endothelial cells have been reported to survive adenovirus vector–mediated CD95L overexpression in vivo.28 Thus, though it is possible that the activation and proinflammatory signals induced by adenovirus vector gene expression have played a role in the repression of CD95-mediated death, these findings indicate that endothelial cells from at least some blood vessels may be resistant to CD95 death signaling induced by local CD95L expression.
In several cell types, sensitivity or resistance to CD95L- or TNF-α–mediated death signaling can be regulated by additional signals present in the environment, and, in some instances, CD95L or TNF-α can even induce cell differentiation or proliferation.35,36 In endothelial cells, systemic TNF-α expression causes disseminated apoptosis,29 but local expression of TNF-α can induce proliferation,37 leading to neoangiogenesis. Interestingly, agonistic CD95-specific antibodies have been reported to induce local inflammation and neoangiogenesis when implanted subcutaneously in matrigel, in the presence of heparin.38 Whether local expression of CD95L in similar conditions may also exert such proangiogenic effects is an important question that remains to be investigated. Indeed, because several tumors express CD95L,13 and because tumor neovascularization is essential for cancer growth and spread,39 the local response of endothelial cells to CD95L—proliferation or death—may be of major importance in determining cancer progression. Although our findings do not allow us to infer the particular sensitivity of any given blood vessel to a local expression of CD95L, they clearly indicate that systemic CD95 engagement results in disseminated vascular damage. A possibility that we cannot exclude at this stage is that endothelial cells from several or even most blood vessels might be spontaneously resistant to CD95 death signaling, but that systemic CD95 engagement induces a rapid release of yet unidentified circulating mediators that render these endothelial cells sensitive to CD95-mediated death.
Systemic engagement of CD95 can be induced either by circulating smCD95L or by circulating CD95L-expressing cells. In this study, we identified disseminated endothelial cell apoptosis as the earliest lesion occurring in a murine model of systemic allogeneic immune response, and our findings indicated that vascular damages, as well as the subsequent additional tissular damage, were not induced in the recipient mice when the allogeneic lymphocytes were from CD95L-defective donor mice. Thus, although it is possible that cytokines, including TNF-α, released by the allogeneic lymphocytes may participate in endothelial cell death, our study indicates that CD95L, either in its membrane-bound cell surface expressed form, or in its soluble/released form, is required for the induction of these vascular lesions.
Our findings also raise the more general question of the potential outcome of interactions between CD95L expressed by activated circulating T lymphocytes and CD95 expressed by endothelial cells during nonallogeneic immune responses, including those directed against infectious pathogens or tumors and those involved in allergic and autoimmune diseases. It is possible that moderate immune responses, leading to T-cell recruitment into localized tissue targets may either cause focal endothelial cell death or proliferation, depending on the nature of the cytokines produced at the site of tissue inflammation. However, it is tempting to speculate that intense and systemic immune responses, leading to the prolonged recirculation of large numbers of CD95L-expressing T lymphocytes or to the release of high quantities of smCD95L may lead to disseminated endothelial cell death similar to that induced by the systemic allogeneic responses that we explored.
Another important question is the potential role that the induction of early vascular damage may play in the development of further tissular lesions. On completion of our study, it was reported that endothelial cell apoptosis induced in the gastrointestinal (GI) tract by radiation is the primary lesion responsible, in a murine model, for the subsequent induction of GI stem cell death and lethal GI syndrome.40 Thus, it is possible that the early induction of endothelial cell apoptosis by allogeneic lymphocytes that we observed may also play a role in the subsequent tissular damage caused by the allogeneic immune response. Intravenous injection of basic fibroblast growth factor has been shown to prevent endothelial cell apoptosis and subsequent tissue damage and lethality induced by either TNF-α29 or GI radiation.40 Our preliminary results (data not shown) suggest that treatment with basic fibroblast growth factor fails to prevent CD95-mediated endothelial cell death. However, we found that treatment with an inhibitor of caspase-8 prevents endothelial cell apoptosis induced by either CD95-specific antibody, smCD95L, or allogeneic lymphocytes. In vitro, CD95 engagement has been reported to induce cell death either through a caspase-dependent pathway that requires caspase-8 activation,1,2 or, at least in some cell types in the presence of caspase inhibitors, through a caspase-independent pathway that may involve the generation of oxygen radicals41 or the recruitment of the kinase receptor interacting protein.42In vivo, however, CD95-mediated hepatocyte death induction depends on caspase activation, and treatment with either broad caspase inhibitors15,17 or selective caspase-8 inhibitors,32 prevents hepatic failure and hepatic hemorrhage, and allows animal survival. Our findings indicate that this is also the case for endothelial cell apoptosis and that treatment with a caspase-8 inhibitor prevents the induction of both vascular lesions and other tissular damage by CD95 engagement in vivo.
CD95L expression has been proposed to play an important role not only in aGVHD,5-7 but also in several other immune-mediated diseases affecting various organs, tissues, and cell types including acute hepatitis,1,4 organ-specific autoimmune diseases,8,9 and allergic10 and toxic11 cutaneous diseases. Our findings suggest that vascular loss of integrity induced by circulating CD95L-expressing cells or by smCD95L may represent an important pathogenic event in these diseases, by causing tissue damage through the induction of hypoxia and vascular leakage of proinflammatory cells. Our findings also suggest that the development of strategies aimed at preventing CD95-mediated death signaling in endothelial cells might have therapeutic implications for the management of acute allogeneic immune responses, such as those leading to aGVHD, and of other diseases resulting from inappropriate or excessive CD95L expression.
We thank N. Crossi, L. Garry, L. Legres, F. Bouhidel, and F. Petit for technical assistance.
Supported by Institut National de la Santé et de la Recherche Médicale (INSERM), Université Paris 7, Assistance Publique-Hôpitaux de Paris (AP-HP), grants from Etablissement Français des Greffes, Agence Nationale de recherche sur le Sida (ANRS), Ensemble Contre le Sida (ECS), and Université Paris 7 Valorisation (to J.C.A.); and an ECS postdoctoral fellowship (to K.A.).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Jean Claude Ameisen, INSERM EMI-U 9922, CHU Bichat, 46 rue Henri Huchard, 75877 Paris cedex 18, France; e-mail:ants@club-internet.fr.