Abstract
Making a diagnosis of deep vein thrombosis (DVT) requires both clinical assessment and objective testing because the clinical features are nonspecific and investigations can be either falsely positive or negative. The initial step in the diagnostic process is to stratify patients into high-, intermediate-, or low-risk categories using a validated clinical model. When the clinical probability is intermediate or high and the venous ultrasound result is positive, acute symptomatic DVT is confirmed. Similarly, when the probability is low and the ultrasound result is normal, DVT is ruled out. A low clinical probability combined with a negative D-dimer result can also be used to rule out DVT, thereby obviating the need for ultrasonography. In contrast, when the clinical assessment is discordant with the results of objective testing, serial venous ultrasonography or venography is required to confirm or refute a diagnosis of DVT. Once a patient is diagnosed with an acute DVT, low-molecular-weight heparin (LMWH) is the agent of choice for initial therapy and oral anticoagulant therapy is the standard for long-term secondary prophylaxis. Therapy should continue for at least 3 months; the decision to continue treatment beyond 3 months is made by weighing the risks of recurrent thrombosis and anticoagulant-related bleeding, and is influenced by patient preference. Screening for associated thrombophilia is not indicated routinely, but should be performed in selected patients whose clinical features suggest an underlying hypercoagulable state. Several new anticoagulants with theoretical advantages over existing agents are undergoing evaluation in phase 3 studies in patients with venous thromboembolism.
Introduction
Deep vein thrombosis (DVT) affects approximately 0.1% of persons per year. The incidence is much lower in the young and higher in the elderly. Although many patients develop DVT in the presence of risk factors, such as malignancy and immobility, DVT can also occur without obvious provocation (idiopathic DVT). Some of the patients with idiopathic DVT have an inherited or acquired thrombophilia, whereas the remainder have no identifiable biochemical or genetic abnormality. Although the management of DVT is often straightforward, problems leading to morbidity and mortality can result from misdiagnosis, treatment failure, and anticoagulant-related bleeding.
This article will provide an overview of our management of adult patients with symptomatic, lower limb DVT, with an emphasis on diagnosis, treatment, and thrombophilia screening. A brief discussion of some of the new anticoagulants under clinical development, as well as other treatment modalities that still require further research, is included.
Diagnosis of first episode of DVT
Patients with DVT may have minimal or atypical symptoms and clinical features that are generally considered diagnostic of DVT can be found in nonthrombotic disorders. Only about 25% of patients who present with compatible symptoms have DVT confirmed on objective testing. Because the clinical diagnosis is insensitive and nonspecific, confirmation with objective investigations is essential. In addition, even though treatment with anticoagulant therapy is highly effective, its unnecessary use should be avoided because it can cause serious bleeding. Despite the limitations of clinical diagnosis, the first step in evaluating a patient with suspected DVT is still a history and physical examination because the clinical presentation influences the diagnostic process.1
Clinical assessment
A proper clinical assessment includes a careful evaluation of the patient's signs, symptoms, and risk factors for venous thrombosis. Patients with symptomatic DVT can present with pain, swelling, tenderness along the distribution of the deep leg veins, erythema, or cyanosis. These features are caused by venous obstruction or perivascular inflammation, but they can also be found in patients with superficial thrombophlebitis, cellulitis, ruptured Baker cyst, and other musculoskeletal conditions. Therefore, an important objective of the clinical evaluation is to determine whether the presenting features are more likely to be caused by one of these alternative diagnoses. If an alternative diagnosis is considered more likely, or if the patient has no known risk factors for venous thrombosis, the likelihood of DVT is reduced. Conversely, if an alternative diagnosis is unlikely, or if the patient has one or more known risk factors for thrombosis, the likelihood of DVT is increased. Important risk factors for venous thrombosis include malignancy, recent major surgery or trauma, recent hospitalization, prolonged immobilization, pregnancy and the puerperium, use of hormonal agents, and known thrombophilia. Obesity, smoking, and long distance flights are weaker risk factors.
The use of a clinical model to standardize the clinical assessment is recommended. The first clinical model designed to assess the pretest probability (clinical likelihood) of DVT was developed and validated by Wells and colleagues in outpatients who present with suspected DVT.2 Based on the clinical presentation, patients are stratified into low-, intermediate-, or high-probability categories for having DVT. Outpatients with classical findings of DVT and at least one risk factor have an 85% probability of having DVT, whereas those with atypical features and no identifiable risk factors have only about a 5% probability of having thrombosis.2 Table1 shows a simplified version of the original model that is currently used in our clinic.3 The most problematic item in this 9-point clinical model is the identification of an alternative diagnosis because it is subjective. Nevertheless, the model has been applied successfully to different patient populations, including patients in the hospital and patients who present to the emergency department.4,5 Recently, Wells has further streamlined the diagnostic process by stratifying patients into 2 risk categories: “DVT unlikely” if the clinical score is 1 or less, and “DVT likely” if the score is more than 1.6 Other investigators have adapted the 9-point model but removed the alternative diagnosis item. Junior medical staff were able to use this modified model without difficulty to triage patients presenting to the emergency department with suspected DVT.7
Initial objective testing: venous ultrasonography and D-dimer testing
The most useful objective tests for diagnosing DVT are venous ultrasonography and D-dimer testing. In combination with clinical assessment, these investigations have markedly reduced the need for contrast venography, the reference standard for diagnosing DVT. Rigorously performed cohort studies have shown that diagnostic strategies incorporating clinical pretest probability, ultrasonography, and D-dimer testing are safe and reliable in managing patients with suspected DVT.
Venous ultrasonography.
Venous ultrasonography is our first objective test of choice in patients with high or moderate pretest probabilities. Noncompressibility of the common femoral vein or popliteal vein or both is diagnostic for proximal DVT. Compression B-mode ultrasonography with or without color duplex imaging has a sensitivity of 95% and a specificity of 96% for diagnosing symptomatic, proximal DVT, and a sensitivity and specificity of 60% to 70% for isolated calf vein thrombosis.8 Therefore, when the clinical likelihood and ultrasound evaluations of the proximal venous system are concordant, the accuracy and predictive values of the positive and negative combinations approach 100%.9 Accordingly, when the pretest probability is intermediate or high and the ultrasound result is positive, DVT is confirmed, whereas when the clinical suspicion is low and the ultrasound result is negative, it is safe to exclude a diagnosis of DVT and withhold anticoagulant therapy. In contrast, further objective testing is required when other combinations occur because the posttest probability of DVT ranges between 14% and 63%.2 3 The major limitations of ultrasonography are its reduced accuracy in diagnosing distal thrombosis and its limited availability outside of regular clinic or hospital radiology department hours.
D-dimer testing.
We use D-dimer testing as the first objective test in patients with low pretest probability. D-dimer assays were developed about 2 decades ago to measure this degradation product of cross-linked fibrin. Since then, many different assays have been evaluated for their accuracy and utility in diagnosing DVT. In general, a positive D-dimer result is not useful because the test lacks specificity. D-dimer levels are elevated not only in the setting of acute thrombosis, but also in other conditions such as pregnancy, infection, and malignancy. In contrast, a negative result using a sensitive D-dimer test is useful for excluding acute DVT. Unfortunately, commercially available D-dimer assays vary in their sensitivity and specificity and, therefore, the performance of one assay cannot be extrapolated to another.10 Currently, the most reliable and extensively evaluated tests are 2 rapid enzyme-linked immunosorbent assays (ELISAs; Instant-IA D-dimer, Stago, Asnières, France and VIDAS DD, bioMérieux, Marcy-l'Etoile, France) and a rapid whole blood assay (SimpliRED D-dimer, Agen Biomedical, Brisbane, Australia). The sensitivity of the rapid ELISAs is over 95% and that of the SimpliRED D-dimer assay is approximately 85%.
Results of earlier cohort studies suggested that DVT can be excluded in outpatients who have a low pretest probability on standardized clinical assessment and a negative D-dimer result.11,12 The validity of this proposed approach is now supported by management studies that indicate an initial ultrasound examination is not necessary in patients with a low pretest probability and a negative D-dimer result because less than 2% of these patients will develop symptomatic DVT over the next 3 months.7,13 Using this approach, an initial ultrasound can be avoided in 23% to 40% of patients who present with a suspected first episode of DVT.7,13 Management studies have also shown that ultrasonography can be combined with D-dimer testing to reduce, by about 60%, the number of patients who have to undergo serial ultrasonography.14,15 Thus, independent of the pretest probability, if the initial ultrasound result is normal and the D-dimer result is negative, further testing with serial ultrasonography is unnecessary and anticoagulant therapy can be withheld safely.14-16 Therefore, D-dimer testing can greatly reduce the number of ultrasound examinations required to investigate patients who present with a first episode of suspected DVT.
Follow-up testing: serial ultrasonography or venography
When further testing is indicated because there is disagreement among the clinical assessment or ultrasound or D-dimer result, serial venous ultrasonography or venography should be performed. If the clinical probability is moderate or high but the ultrasound is negative, further testing is indicated to detect a calf vein thrombus. Isolated calf vein thrombosis occurs in 15% to 20% of patients with symptomatic confirmed thrombi and only about 20% of calf thrombi that are undetected at initial presentation will extend proximally within 1 to 2 weeks of initial presentation.17 Serial testing is a safe approach because it detects calf vein thrombi that extend into the popliteal vein and because calf vein thrombi that remain isolated to the calf during the period of testing do not produce serious complications. Serial testing involves bringing the patient back for another ultrasound examination in 5 to 7 days, or sooner if the symptoms worsen or are severe and do not abate. Based on a systematic review of management studies using serial ultrasonography, the ultrasound will convert from a negative to a positive result in about 1% to 2% of the patients during the period of testing, and the risk of a patient dying from pulmonary embolism while awaiting serial testing is 0.06% (95% CI, 0.00%-0.32%).9 Although serial ultrasound testing is safe and appropriate in the majority of patients, we perform venography in patients who cannot return for serial ultrasonography or have severe symptoms and high clinical probability. Venography can also be considered in patients who have poor cardiorespiratory reserve. In addition, in patients with unexplained swelling of the entire leg but a negative ultrasound examination, it is important to consider the possibility of an isolated iliac vein thrombus because the iliac veins are not routinely visualized with lower limb ultrasonography. Although isolated iliac vein thrombosis is infrequent, it can occur in pregnancy and in patients who have extensive pelvic malignancy or have undergone recent pelvic surgery.
In the uncommon situation in which the clinical probability is low but the ultrasound result is positive, we re-evaluate the history and review the ultrasound with the radiologist. Not infrequently, we find that the imaging was technically difficult, or that the abnormality is more suggestive of old (eg, thickening of the vessel wall and well-developed collateral venous channels) rather than recent thrombosis. If the ultrasound result is inconclusive, venography is indicated to confirm or refute the diagnosis of DVT. An intraluminal filling defect on venography is considered as evidence of new or recent thrombosis. If the diagnosis is still inconclusive, it is reasonable to treat patients with proximal venous abnormalities with anticoagulant therapy and follow patients with abnormalities in distal veins with serial ultrasonography.
Pregnant women with suspected DVT
The diagnosis of venous thrombosis in pregnancy can be challenging because (1) unilateral left leg swelling can be caused by compression of the left iliac vein by the gravid uterus, (2) leg swelling can be caused by isolated common iliac vein thrombosis that may not be detectable by compression ultrasonography, and (3) venographic examination of pelvic veins is problematic because it exposes the fetus to irradiation. Accepting these caveats, ultrasonography is the initial test of choice in all patients and the use of venography is limited to the rare patient with suspected isolated iliac vein thrombosis when the vein cannot be identified by ultrasonography. Although venography exposes the fetus to irradiation, the risk of a fatal pulmonary embolism from a missed iliac thrombus likely outweighs the risk of radiation exposure to the fetus. Examination of the external and common iliac veins is technically feasible in the first 2 trimesters and can sometimes be done even in the third trimester with appropriate positioning. As in nonpregnant women, patients who have a negative initial ultrasound should be followed up with serial testing.
An approach to diagnosing DVT: an algorithm
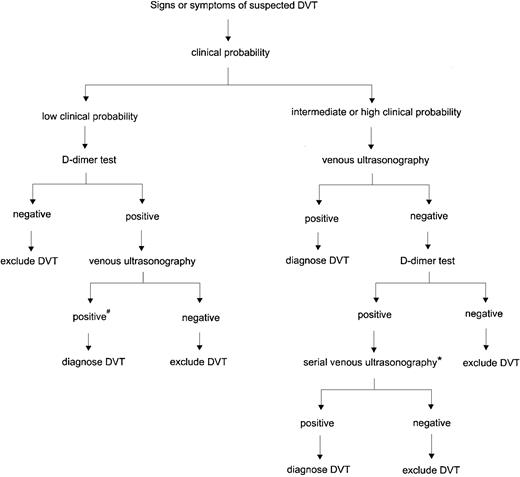
Based on the balance of evidence, we recommend a streamlined diagnostic strategy that combines clinical assessment using a standardized model, rapid ELISA or SimpliRED D-dimer testing, and venous ultrasonography (Figure 1). In patients with a low pretest probability, D-dimer testing should be the first investigation. If the D-dimer result is negative, further testing with ultrasonography is not necessary and DVT can be excluded; if the D-dimer result is positive, venous ultrasonography should be performed. For all patients who have an intermediate or high pretest probability, the first investigation should be a venous ultrasound. If the ultrasound result is negative, D-dimer testing is helpful in selecting patients for further evaluation. Follow-up testing is not required if the D-dimer test is negative, whereas serial ultrasonography or venography is indicated if the D-dimer result is positive. This strategy simplifies the diagnostic process and reduces the cost by decreasing the number of patients who require both D-dimer testing and ultrasound examinations. As for all algorithms, there is room for the clinician to exercise clinical judgment. For example, serial ultrasonography should be performed earlier than 5 to 7 days if the patient has severe or worsening symptoms, and venography should be considered in a patient with a high clinical probability, a normal ultrasound, and severe calf symptoms. Furthermore, if confirmatory tests cannot be performed in a timely manner and the clinical suspicion is high, empiric anticoagulant therapy should be started before objective testing if there are no contraindications.
Algorithm for diagnosing DVT using clinical assessment, venous ultrasonography, and D-dimer testing.
#Re-evaluate history and review ultrasound for features suggestive of old rather than new thrombosis. If ultrasound findings are inconclusive, venography should be considered. *In patients with a high clinical probability or who cannot return for serial ultrasonography, venography is recommended. Venography can also be considered in patients with cardiorespiratory compromise.
Algorithm for diagnosing DVT using clinical assessment, venous ultrasonography, and D-dimer testing.
#Re-evaluate history and review ultrasound for features suggestive of old rather than new thrombosis. If ultrasound findings are inconclusive, venography should be considered. *In patients with a high clinical probability or who cannot return for serial ultrasonography, venography is recommended. Venography can also be considered in patients with cardiorespiratory compromise.
Diagnosis of recurrent DVT
Our approach to the diagnosis of suspected recurrent DVT is similar to that used in patients with suspected first episode of DVT. We routinely perform clinical assessment, ultrasonography, and D-dimer testing in all patients who present with suspected recurrent DVT. However, establishing a diagnosis of recurrent DVT is more difficult because we lack a validated clinical model, and residual organized thrombus can complicate the interpretation of compression ultrasonography or venography.
Two important determinants influence pretest probability of recurrent DVT. These are the history of postphlebitic syndrome (PPS) and the current use of anticoagulant therapy. In patients with established PPS, it may be difficult to distinguish between an acute exacerbation of chronic symptoms and an episode of recurrent DVT. In patients already receiving anticoagulant therapy, the likelihood of recurrence is reduced if the international normalized ratio (INR) is in the therapeutic range, although patients with advanced malignancy or antiphospholipid antibody syndrome are at increased risk for recurrence despite having a therapeutic INR value.18 19
Although a new noncompressible segment on compression ultrasonography is diagnostic of recurrent thrombosis, an earlier test result is needed to make this determination. Compression ultrasonography remains abnormal in up to 50% of patients 1 year after the initial diagnosis, therefore, a single abnormal ultrasound, especially when there is no previous result available for comparison, does not necessarily confirm recurrent DVT. An increase of more than 4 mm in the compressed diameter of a previously involved venous segment has been reported to provide strong evidence of recurrent thrombosis, but this observation requires confirmation.20 In contrast, an intraluminal filling defect on venography is diagnostic for DVT and a previous examination is not required for comparison. However, venography is technically demanding, is not readily available, and is impractical for repeated use.
We also use the SimpliRED D-dimer assay to guide management of patients with suspected recurrence. Although D-dimer testing has not been formally evaluated in this setting, there is no reason why a negative D-dimer result should not be as reliable for excluding a diagnosis of recurrent DVT as it is for first episode of venous thrombosis.
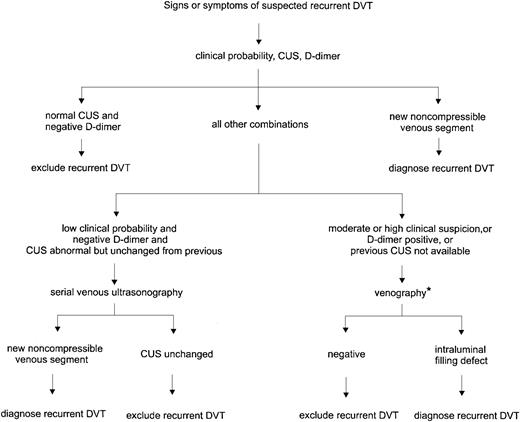
Based on the above considerations, we confirm a diagnosis of recurrent DVT if there is a new noncompressible segment on ultrasonography. Alternatively, we rule out recurrence if the patient has a normal ultrasound and negative D-dimer result. In patients who have a high clinical suspicion or other combinations of ultrasound and D-dimer results, venography or serial ultrasonography is required. Figure2 outlines the general strategy we use in diagnosing recurrent DVT. This management scheme is practical and allows us to make a clinical decision in most patients.
Diagnosis of recurrent DVT.
CUS indicates compression ultrasonography. *If venography is contraindicated, difficult to interpret, or nondiagnostic, serial ultrasonography is recommended to follow changes.
Diagnosis of recurrent DVT.
CUS indicates compression ultrasonography. *If venography is contraindicated, difficult to interpret, or nondiagnostic, serial ultrasonography is recommended to follow changes.
Treatment of acute DVT
Two main advances in the treatment of DVT have been made in the last decade. The first is the introduction of low-molecular-weight heparin (LMWH) as a replacement for unfractionated heparin (UFH) and the second is an improved ability to identify patients who are likely to benefit from a longer duration of anticoagulant therapy. Large meta-analyses have shown that unmonitored, weight-adjusted subcutaneous LMWH is safer and likely more effective than UFH administered by continuous infusion guided by the activated partial thromboplastin time (aPTT).21 22 As a result, subcutaneous LMWH is replacing intravenous UFH for the initial therapy of acute venous thromboembolism (VTE). Coumarins are highly effective for long-term therapy, but they require laboratory monitoring and are problematic in some patients. Several new anticoagulants with more convenient and potentially safer profiles are now undergoing clinical evaluation in randomized controlled trials.
Initial anticoagulant therapy
In the majority of patients with confirmed DVT, LMWH is the anticoagulant of choice for initial therapy. The predictable pharmacokinetic properties of the LMWHs allow this class of compounds to be given as weight-adjusted subcutaneous injections without the need for laboratory monitoring.23 Depending on the LMWH agent used, a dose of 100 antifactor Xa U/kg twice daily or 150 to 200 anti-Xa U/kg daily is given subcutaneously. Although laboratory monitoring is not usually required for patients receiving LMWHs, we recommend checking the 4-hour anti-Xa level in patients who have advanced renal disease, are morbidly obese, or are pregnant, because there are theoretical reasons why they might respond differently to weight-adjusted doses of LMWHs. If necessary, the LMWH dose should be adjusted to a target anti-Xa level of 0.6 to 1.0 IU/mL at 4 hours after an injection of a twice-daily regimen, and 1.0 to 2.0 IU/mL for once-daily administration.23 In addition to their convenient dosing administration, LMWHs are more cost effective than UFH because of the elimination of laboratory monitoring in most patients results in a reduction in the length of hospitalization.24 Another advantage of LMWHs over UFH is a lower risk of heparin-induced thrombocytopenia.25
At our hospitals, about 80% of outpatients with newly diagnosed DVT are treated entirely at home. We encourage out-of-hospital treatment and teach all suitable patients or family members to administer subcutaneous injections, or arrange for home care support in those who are visually impaired or physically unable to inject themselves. Outpatient treatment requires an organized service with dedicated nurses to provide patient support and education and an appropriately trained physician available 24 hours a day through an on-call service to handle patient concerns.
Despite the advantages of LMWH, we often use UFH in patients with extensive iliofemoral DVT with circulatory compromise and those who are hemodynamically unstable from associated major pulmonary embolism because these groups were excluded in clinical trials that compared LMWHs with UFH. In addition, patients who have inadequate insurance coverage and who are limited financially may need to be treated with intravenous or subcutaneous UFH. The usual intravenous regimen for UFH is a loading dose of 5000 U followed by a continuous infusion of at least 30 000 U every 24 hours. The dose of UFH is adjusted according to the aPTT by following a validated standard nomogram to maintain a therapeutic heparin level.23 Alternatively, a weight-adjusted loading dose and nomogram can be used.26If subcutaneous UFH is used, it is given at a starting dose of 17 500 U twice daily and the dose is adjusted to achieve a therapeutic aPTT at 6 hours after an injection. The aPTT target range should correspond to a heparin level of 0.4 to 0.7 IU/mL by anti-Xa assay.23 In patients requiring large doses of UFH (> 35 000 U/24 h), the heparin level should be monitored by anti-Xa assay or protamine sulfate titration. LMWH or UFH should be administered for a minimum of 5 days in patients with uncomplicated thrombosis and for 7 days or longer in patients who have extensive disease (eg, iliofemoral DVT or massive pulmonary embolism). Oral anticoagulant therapy can be started on the first day of treatment and LMWH/UFH should not be stopped until the INR has been at least 2.0 for 2 consecutive days. A platelet count can be done on days 5 to 7 to check for heparin-induced thrombocytopenia if the patient is receiving UFH.
Long-term anticoagulant therapy
After an initial course of LMWH or UFH, continuing anticoagulant therapy with coumarin derivatives is required to prevent recurrence. Warfarin is the most common agent used in North America. We start with an average maintenance warfarin dose of 5 mg on the first and second days with the expectation that the INR will be in the range of 2.0 to 3.0 in 4 or 5 days. We use smaller doses (2-4 mg) in the elderly, in patients who have a low body weight, or in those with compromised nutrition. The use of a loading dose is discouraged because it may be associated with a transient period of excessive anticoagulation without a corresponding antithrombotic effect.27 The INR is measured after the first 2 or 3 doses of warfarin, and subsequent doses are adjusted to maintain the INR within the target range. Because the therapeutic window for oral anticoagulant therapy is narrow, frequent monitoring of the INR is essential to reduce the risks of recurrent thrombosis and anticoagulant-related bleeding. Appropriate adjustments in the dose of warfarin usually require twice-weekly monitoring for the first 1 to 2 weeks, followed by weekly monitoring for the next 4 weeks, then once every 2 weeks for a month, and finally every 4 weeks if the INRs have remained in the therapeutic range on a stable warfarin dose and the patient has not experienced any adverse effects. It is unwise to leave the INR unchecked for longer than a 4-week interval even in patients who have maintained a stable warfarin dose because of the potential interactions of warfarin with food or drugs. If there are changes in the patient's medications, more frequent monitoring is needed until a stable dose response is achieved.28
Because oral anticoagulant therapy is inconvenient, LMWH is being evaluated as an alternative for long-term treatment of VTE. LMWH has a number of advantages over warfarin. First, because LMWH does not require INR monitoring, it can be used when laboratory monitoring is problematic (eg, geographic inaccessibility or difficult venous access). Second, LMWH has a more rapid onset and offset of action than warfarin, thereby rendering it more convenient to use in patients who require intercurrent procedures. Third, there is a clinical impression that LMWH is more effective than warfarin in patients with thrombosis and cancer and in those who develop recurrent thrombosis despite therapeutic warfarin therapy. Despite these advantages, however, the routine use of LMWH is not practical or economical because LMWH requires administration by subcutaneous injection and is more expensive than warfarin. Thus far, randomized controlled trials comparing LMWH with oral anticoagulant therapy have shown that the rates of recurrent thrombosis and major bleeding between the 2 treatment groups are similar.29 30 At our center, we use long-term LMWH in patients who have had a recurrence on warfarin or cannot tolerate warfarin therapy; most of these patients have advanced cancer and are receiving aggressive antineoplastic treatments.
Duration of anticoagulant therapy
The duration of anticoagulant therapy is influenced by the estimated competing risks of bleeding and recurrent thrombosis and is influenced by patient's preference. The risk of bleeding during the initial period of anticoagulation with UFH or LMWHs is 2% to 5%, whereas the estimated risk of major bleeding with oral anticoagulant therapy is about 3% annually.31 Because 20% of major bleeds are fatal, the annual case fatality rate from anticoagulant-related bleeding is about 0.6%. The risk of bleeding is increased by patient-specific factors such as age (65 years or older), comorbid illness (renal failure, diabetes, peptic ulcer disease, cerebrovascular disease, malignancy), and by the concomitant use of antiplatelet agents.32 33 Evidence also indicates that the risk of bleeding on anticoagulant therapy is reduced over time, so the long-term fatality rate is likely to be lower in patients who have tolerated months or years of anticoagulant treatment without bleeding. On the other hand, the case fatality rate from recurrent VTE is about 5%, with the rate being higher within the first 3 months of an episode of pulmonary embolism. Therefore, at an annual recurrence rate of 12%, the risk of death from recurrent thrombosis is balanced by the risk of death from anticoagulant-mediated bleeding.
In general, patients should be treated with anticoagulant therapy for a minimum of 3 months. Patients with a reversible risk factor have a low risk of recurrence after 3 months of anticoagulant therapy. In contrast, patients with idiopathic or unprovoked DVT who are treated for only 3 months have a 10% to 27% risk of recurrence in the year after anticoagulants are discontinued.34-36 Recent evidence suggests that extending therapy beyond 6 months in patients with idiopathic thrombosis does not reduce the risk of recurrent thrombosis to less than 10% in the year after discontinuing anticoagulant therapy. Continuing warfarin after this period protects the patient against future recurrence but also exposes the patient to the risk of anticoagulant-related bleeding.
Based on results of prospective studies and extrapolation from studies on the risk of recurrence after a first episode of venous thrombosis, patients can be stratified into low-, moderate-, high-, and very high-risk groups for recurrence when anticoagulants are discontinued (Table 2). Low-risk patients are those who had an important risk factor for thrombosis (eg, major surgery, pelvic or leg trauma, or major medical illness) from which they have fully recovered. Their risk of recurrence when anticoagulants are discontinued at 3 months is estimated to be 4% to 5% in the next year and somewhat less in subsequent years. These patients should be encouraged to have prophylactic anticoagulants if exposed to a high-risk state and in general should be encouraged to seek alternatives to estrogens for contraception or postmenopausal use. Moderate-risk patients are those without inherited or acquired biochemical markers for thrombophilia and who had a thromboembolic event in association with a minor risk factor such as estrogen use or long distance plane travel. Their risk of recurrent thrombosis after 6 months of anticoagulants is likely to be less than 10% in the year after stopping anticoagulants, provided that the precipitating risk factor is avoided; they should be treated with anticoagulants for 6 months. If, however, the precipitating factor cannot be avoided (eg, estrogens) they should be given the option of remaining on anticoagulants during the period of exposure. High-risk patients are those who have an unprovoked venous thromboembolic event and who either have no biochemical markers for thrombophilia or are heterozygous for factor V Leiden or the prothrombin G20210A mutation. Their risk of recurrence after 6 months of anticoagulant therapy is likely to be about 10%/y. In general, anticoagulant therapy can be stopped after 6 months in these high-risk patients; however, if the bleeding risk is low, INR monitoring is smooth and convenient, and if the patient prefers to remain on anticoagulant therapy, treatment can be continued and the treatment duration reviewed on an annual basis. Very high-risk patients are those with more than one unprovoked thromboembolic event; patients with inherited deficiencies of antithrombin, protein C, or protein S; those with antiphospholipid antibody syndrome or advanced malignancy; and those who are homozygous for factor V Leiden or double heterozygotes. The risk of recurrence after a 6-month course of anticoagulants is likely to be more than 12% annually, and in general, these patients should remain on anticoagulants indefinitely. Firm evidence for this last recommendation is not available but because the listed thrombophilic states are strong risk factors for a first episode of VTE, they are likely, also, to increase the risk of recurrent VTE.
PPS and its prevention
PPS occurs in about 20% of patients following an episode of DVT.37 The typical features are leg pain and swelling, which are exacerbated by standing and physical activity and reduced with elevation of the affected leg. In severe cases, venous ulceration can develop. PPS occurs as a result of venous hypertension, which is most commonly caused by venous valvular incompetence and less frequently by persistent venous obstruction. Not all patients with valvular incompetence develop the clinical features of PPS.38
Two approaches have been proposed to prevent and treat PPS: thrombolytic therapy to reduce the damage to venous valves and graduated compression stockings to counter venous hypertension. However, results from clinical trials have not clearly shown beneficial effects with either method.37,39 40 The development of PPS is more likely to occur after recurrent episodes of DVT; therefore, every effort should be made to reduce the likelihood of recurrent thrombosis by using an appropriate course of anticoagulant therapy for the initial episode and anticoagulant prophylaxis in subsequent high-risk situations.
At our hospitals, graduated compression stockings are not routinely prescribed in patients with acute DVT. Rather, we offer stocking therapy to patients with established PPS who have troublesome symptoms and recommend continued use only if they experience symptomatic improvement.
Treatment of DVT in pregnancy
Prior to the introduction of LMWHs, UFH was the standard treatment for DVT in pregnant women in North America. Warfarin is generally avoided because of the risk of warfarin embryopathy and other potential teratogenic effects. UFH has a number of limitations, including heparin-induced osteoporosis, the need for twice-daily subcutaneous injections and the necessity for aPTT monitoring. These disadvantages are virtually eliminated with LMWH.
Although there have been no randomized controlled trials comparing UFH with LMWH in pregnancy, there is no reason to expect that the advantages of LMWH in the nonpregnant population would not apply to pregnant women.41 In addition to the convenience of once-daily injection without the need for frequent laboratory monitoring, like UFH, LMWH is not teratogenic and does not pass into breast milk. We usually treat pregnant women throughout their pregnancy with LMWH and arrange for a planned induction of labor in consultation with the obstetrician. The controlled delivery date allows discontinuation of LMWH 24 hours prior to induction, thereby reducing the risk of bleeding during delivery.
Screening for thrombophilia
The indications for screening patients who present with a first episode of venous thrombosis to identify underlying inherited or acquired thrombophilia are controversial. From a practical viewpoint, screening would be indicated if the results influenced the duration of anticoagulant therapy or the need for family counseling. As discussed in the previous section, the duration of anticoagulant therapy is influenced by finding deficiencies in antithrombin, protein C, or protein S, homozygous factor V Leiden, double heterozygosity, and persistently elevated antiphospholipid antibodies or a lupus anticoagulant. Family counseling is particularly important for female carriers who are contemplating estrogen use. Based on these considerations, we think that it is reasonable to perform screening for thrombophilia in the following groups: first episode of idiopathic thrombosis at age 50 or younger; history of 2 or more episodes of recurrent thrombosis, especially if the events were unprovoked; thrombosis in an unusual site (eg, cerebral, mesenteric); positive family history with 2 or more first-degree relatives with documented venous thrombosis; women who develop thrombosis during pregnancy or in the setting of a hormonal agent; and women who have unexplained recurrent pregnancy loss. This latter group requires special consideration because anticoagulant and antiplatelet therapy may improve future pregnancy outcomes if underlying thrombophilia is documented.42
Our standard screening panel includes functional assays for antithrombin and protein C, free protein S level, activated protein C resistance assay with DNA testing for factor V Leiden if positive, molecular assay for prothrombin G20210A mutation, a phospholipid-based clotting test for lupus anticoagulant, ELISAs for antiphospholipid antibodies, and a fasting homocysteine level. We check for elevated homocysteine levels because even though hyperhomocysteinemia does not alter the duration of anticoagulant therapy, vitamin supplementation with folic acid, vitamin B6, and vitamin B12 lowers the homocysteine levels in most patients.43 Although there is no convincing evidence that correction of the homocysteine level reduces the risk of recurrent thrombosis, these vitamins are inexpensive and innocuous, and it is reasonable to recommend their use if hyperhomocysteinemia is confirmed. Insufficient data are available to support routine performance of other tests, such as coagulation factor assays and plasminogen levels.
New antithrombotic agents
Several new antithrombotic agents that target single steps in the coagulation cascade are under development. Three compounds—the heparinoid danaparoid and 2 direct inhibitors of thrombin, hirudin and argatroban—have been approved for the treatment of heparin-induced thrombocytopenia. Two compounds—synthetic pentasaccharide and ximelagatran—are being evaluated in phase 3 studies in patients with VTE. Both agents are given without coagulation monitoring and have the potential to replace existing anticoagulants. Synthetic pentasaccharide is administered as a once-daily subcutaneous injection and is being compared with UFH for initial treatment of DVT and pulmonary embolism. This new agent has the advantage of a longer half-life than LMWH and is unlikely to produce heparin-induced thrombocytopenia. A newer form of synthetic pentasaccharide with a longer half-life that allows once-weekly subcutaneous injection is also being evaluated for the out-of-hospital longer-term treatment of patients with VTE. Large randomized controlled trials have shown that pentasaccharide is superior to enoxaparin in thromboprophylaxis after major orthopedic surgery.44,45 Ximelagatran is administered orally and is being compared against standard anticoagulants for thromboprophylaxis in orthopedic surgery, atrial fibrillation, as well as initial and long-term treatment of VTE.46 47
Thrombolytic therapy for DVT
The role of thrombolysis in DVT treatment remains unclear. Based on venographic studies, thrombolytic agents can produce rapid lysis of venous thromboemboli and restore venous flow. Consequently thrombolytic therapy has the potential to provide prompt symptomatic relief and reduce the risk of the PPS.48,49 Despite documented improvements on radiologic imaging, however, appropriate studies have not been performed to demonstrate improvements over standard anticoagulant therapy alone using clinically relevant outcomes. Thrombolytic therapy increases the risk of major bleeding about 3-fold over that observed with UFH alone, and the observed rate of intracranial hemorrhage is approximately 2%.50 Further, there is no agreement on whether systemic or catheter-directed thrombolysis is the preferred method of delivery. A recent randomized controlled trial comparing UFH alone with 4 regimens of systemic or regional thrombolysis showed greater venographic improvement at 12 months with systemic thrombolytic therapy, but at a cost of substantially higher rates of major bleeding and pulmonary embolism compared with UFH.51 Therefore, even if thrombolysis is effective in reducing the risk of recurrent thrombosis or PPS, the cost, bleeding risk, and the technical expertise required for this aggressive therapy are major obstacles to its routine use. We limit thrombolytic therapy to select, younger patients with massive iliofemoral vein thrombosis who have limb-threatening circulatory compromise.
Vena caval interruption
The accepted indications for placement of an inferior vena caval filter are active bleeding, risk of serious bleeding that precludes the use of anticoagulant therapy, and failure of therapeutic anticoagulant therapy. The use of filters remains controversial in other clinical situations, for example, for preventing embolization of “free-floating” thrombi in iliofemoral disease and as the first-line treatment (alone) in patients with central nervous system malignancy and acute DVT.52
To date, only one randomized controlled trial has evaluated the use of vena caval filters in patients with proximal DVT, all of whom also received anticoagulant therapy.53 There was an initial reduction in the incidence of pulmonary embolism in the filter group, but this advantage was lost with longer follow-up. In addition, patients with a filter had a higher risk of recurrent DVT and there was no difference in the overall mortality after 2 years. Similar results are reported by a population-based analysis in more than 3600 patients in whom a filter was inserted for DVT.54
At our hospitals, we insert filters in patients with a newly diagnosed proximal DVT or pulmonary embolism who have to undergo urgent surgery, who have severe thrombocytopenia, or have active and potentially life-threatening bleeding. In all cases, anticoagulant therapy is restarted when normal hemostasis is achieved. We do not routinely insert filters in patients with reversible, controllable bleeding or brain tumors. In the former setting, we reduce the dose or withhold anticoagulant therapy temporarily and reintroduce anticoagulants when hemostasis is re-established. In patients with brain tumors or cerebral metastases, we prescribe standard anticoagulant therapy with frequent INR monitoring unless there is a documented history of symptomatic intracranial bleed. The warfarin dosing in the patients with cerebral malignancy can be difficult to manage when they are given moderate or high doses of dexamethasone because high-dose steroid therapy can cause unpredictable fluctuations in their INR values.55 When warfarin dosing is problematic, we treat the patient with LMWH injections rather than insert a filter.
A.Y.Y.L. is a recipient of a New Investigator Award from the Canadian Institutes of Health Research/R and D Research Program.
References
Author notes
Jack Hirsh, Henderson Research Centre, 711 Concession St, Hamilton, Ontario, Canada L8V 1C3; e-mail:jhirsh@thrombosis.hhscr.org.