Abstract
In the past 6 years, we treated 129 patients who had acute promyelocytic leukemia (APL) with a new arsenic agent, oral tetra-arsenic tetra-sulfide (As4S4). Nineteen of the patients had newly diagnosed APL, 7 had first relapse, and 103 had hematologic complete remission (HCR). HCR was achieved in all patients with newly diagnosed APL and in all those with hematologic relapse. Of 16 patients with newly diagnosed disease and available cytogenetic and molecular analyses, 14 had cytogenetic and molecular complete remission (CR). Cytogenetic and molecular CR was also obtained in 5 of the 7 patients with hematologic relapse. In the HCR group, 35 of 44 patients positive for PML-RARα at baseline became negative. In the newly diagnosed group, estimated disease-free survival (DFS) rates for 1 and 3 years were 86.1% and 76.6%, respectively, with a median follow-up time of 13.5 months (range, 2-40 months). In the HCR group, DFS rates for 1 and 6 years were 96.7% and 87.4%, respectively, with a median follow-up of 23 months (range, 2-71 months). Treatment with As4S4 was well tolerated, with only moderate side effects, including asymptomatic prolongation of corrected QT interval, transient elevation in liver enzyme levels, rash, and mild gastrointestinal discomfort; neither myelosuppression nor appreciable long-term side effects occurred. Degeneration or apoptosis of APL promyelocytes was observed during As4S4therapy. Pharmacokinetic studies showed that the agent was absorbed rapidly. Most urinary arsenic excretion occurred within the first 24 hours. Both blood and urinary arsenic levels declined after discontinuation of As4S4. Our results show, for the first time, that As4S4 treatment alone is highly effective and safe in both remission induction and maintenance therapy in patients with APL, regardless of disease stage.
Introduction
Acute promyelocytic leukemia (APL), first recognized as a distinctive clinical entity in the 1950s,1comprises about 10% of cases of acute myelocytic leukemias in adults.2,3 Combination treatment with all-trans-retinoic acid (ATRA) and chemotherapy has dramatically improved outcome and survival in patients with APL.4-9 Despite this improvement, relapse occurs within 4 to 5 years in approximately 30% of patients given such therapy10-12 and represents a major obstacle to cure. Investigators in China and the United States have reported that daily intravenous infusions of arsenic trioxide (ATO) induce complete remission (CR) in more than 80% of patients with relapsed and refractory APL, with an acceptable toxicity profile given the seriousness of the disease.13-16 The main toxic effects of ATO are fluid retention, hyperleukocytosis, gastrointestinal discomfort (nausea, vomiting, and diarrhea), fatigue, hyperglycemia, and neuropathy. Prolongation of the corrected QT interval (QTc), occasional liver damage, and sudden death have also been observed.17-22 In general, drug-related toxicity decreases during consolidation and maintenance courses of ATO compared with induction courses in cases of relapse. A drawback of ATO is that it must be administered intravenously daily in a 1- to 4-hour infusion. Thus, an oral agent with similar effectiveness and fewer side effects would provide not only cost and quality-of-life benefits but also easy access to consolidation and maintenance therapy.
Realgar, the ore composed mainly of tetra-arsenic tetra-sulfide (As4S4), has been used as a traditional medicine in China for more than 1500 years. In addition to As4S4, realgar contains up to about 10% of other ingredients, such as different types of arsenite, arsenic trioxide, and other minerals.23 Rather than being used alone to treat various diseases, realgar was commonly administered with several Chinese herbs.24 25 No previous study of the use of As4S4 as a single agent to treat disease has been reported. In the past 6 years, we administered an oral preparation of highly purified crystalline As4S4 to patients with different stages of APL. This pilot study found that oral As4S4 used alone is highly effective and safe for both remission induction and maintenance in all stages of APL.
Patients, materials, and methods
Patients
A total of 129 patients with APL were entered into this study between December 1994 and December 2000. Informed consent to participate was obtained from all patients. The diagnosis of APL was based on morphologic characteristics of the French-American-British classification AML-M3,26 cytogenetic or fluorescence in situ hybridization (FISH) analysis of t(15;17), and reverse transcription–polymerase chain reaction (RT-PCR) analysis for PML-RARα transcript. All patients with a confirmed diagnosis of APL and evidence of t(15;17) were eligible for enrollment regardless of disease stage, except for those who were unlikely to survive induction therapy because of coma or severe disseminated intravascular coagulation (DIC), those with an infection not controlled by antimicrobial therapy, those with an uncontrolled concurrent disease, and women who were pregnant or lactating.
Initially, only patients with molecular or cytogenetic evidence of persistent residual leukemia from previous ATRA treatment and chemotherapy were entered into the As4S4treatment protocol. However, after observing preliminary evidence of safety and efficacy in this poor-prognosis group, we broadened the entry criteria to include patients at all disease stages: newly diagnosed, frank hematologic relapse, and hematologic complete remission (HCR) with or without molecular CR. Patients with HCR were previously treated in our institution or were referred, chiefly for either stem cell transplantation or postremission treatment.
All 129 patients enrolled were 14 years of age or older. Nineteen had newly diagnosed untreated APL, 7 had frank hematologic relapse, and 103 had HCR. Characteristics of the patients at diagnosis are shown in Table 1.
Monitoring
Routine examination of the patients was done as described previously.27 Assessments included pretreatment and posttreatment chest x-ray studies, electrocardiography, and if necessary, echocardiography and other indicated studies. Complete blood counts, coagulation studies, serum chemistry analyses, urinalyses, blood smear examinations, and bone marrow (BM) aspiration or biopsy were done on the patient's first visit and at close periodic intervals thereafter. HCR was defined as a white blood cell (WBC) count of at least 3 × 109/L and a platelet count of at least 100 × 109/L in peripheral blood and less than 5% blasts and abnormal promyelocytes in BM smears.
Cytogenetic analyses, FISH, and RT-PCR for PML-RARα were done on BM aspirates from all patients with newly diagnosed disease or relapse, as well as most patients with HCR. Follow-up studies were performed at close intervals until cytogenetic CR was achieved or when relapse was suspected. Thereafter, cytogenetic analyses were done at regular intervals.
Cytogenetic analysis
BM cells were cultured overnight at 37°C in McCoy S5A medium supplemented with 20% fetal-calf serum for a metaphase chromosome preparation with routine G banding. Karyotypes were evaluated according to the International System for Human Cytogenetic Nomenclature.28 At least 20 metaphases per sample were analyzed.
FISH analysis
Flow cytometric analysis
Direct immune staining for 3-color phenotyping was done on BM aspirates. The antibodies used for these studies were CD45–peridinin chlorophyll protein, CD2–fluorescein isothiocyanate conjugated (FITC), and CD19-phycoerythrin (PE) or CD15-FITC and CD13-PE, CD33-FITC and CD11b-PE, HLA-DR-FITC and CD34-PE, CD38-FITC and CD117-PE, and isotype controls (Becton Coulter, Miami, FL). Samples were analyzed on a fluorescence-activated cell-sorter scanner flow cytometer, and data were analyzed by using Cell Quest software (Becton Dickinson, Mountain View, CA). At least 10 000 events were counted for each tube.
RT-PCR
Heparin-treated BM aspirates were tested by RT-PCR for PML/RARα transcript as described by Biondi et al.31 NB4 cells served as positive controls. The sensitivity of this test is 0.5 ng RNA.
Preparation of As4S4
As4S4 was prepared from mined natural realgar under the supervision of the first author and a collaborating pharmacist. The molecular structure of As4S4has been interpreted by Chia-Si Lu for more than 50 years.32
At the beginning of the study, we used moderately purified As4S4 that was mixed with an equal amount (wt/wt) of ground Seman platycladi as an excipient. To avoid any possibility that trace amounts of ATO present in the initial preparation or other arsenites in “processed-to-be-refined” realgar were responsible for the observed effects, we began to use crystallized realgar with high-purity As4S4 as starting material beginning in January 1998. The purity of our As4S4 preparation was confirmed by repeated x-ray diffraction investigations (done in collaboration with the Physical Geography Institute, Chinese Academy of Sciences). The results were compatible with pure As4S4 standards and excluded the presence of ATO and other arsenic compounds (Lei Wen and D.-P.L., unpublished data, July 2001). Highly purified As4S4 was also mixed with an equal amount of ground Seman platycladi and put into capsules containing 250 mg As4S4.
The high-purity As4S4 was given to 124 patients. However, some of the patients started preliminary therapy with the moderately purified As4S4 and switched to the highly purified As4S4 when it became available. Seventeen of the 19 patients with newly diagnosed APL and 6 of the 7 patients with hematologic relapse received only the high-purity As4S4.
As4S4 dosage and administration
As4S4 was orally administered for remission induction in patients with newly diagnosed APL or APL relapse at a dosage of 50 mg/kg of body weight per day, divided into 4 doses (approximately 750 mg 4 times daily), until HCR was documented. For patients with HCR, the same daily dose was given on a treatment schedule of 2 weeks on and 2 weeks off in the first year. Thereafter, the treatment break was increased to one month within 4 years. Therapy was discontinued in the fifth year.
The As4S4 dosage in this study was selected on the basis of results of a dose-escalation trial and studies in animals. Realgar ore has long been used in Chinese traditional medicine and regarded as a Materia medica with mild toxicity. A small study in volunteer patients showed that escalation to a single dose of 60 mg/kg As4S4 was well tolerated. In addition, pharmacokinetic and toxicity studies in mice showed that our highly purified As4S4 was safe when administered orally: even at a dose of 1 g/kg, it was well tolerated and did not cause death (Lei Wen and D.-P.L., unpublished data, July 2001). Concomitantly, studies in our laboratory found that As4S4 was effective against the NB4 cell line (an APL cell line) in culture and NB4-induced ascites in mice. The current As4S4 dosage in our clinical trial was escalated gradually from less than one hundredth of the well-tolerated dosage in animals. More important, we monitored blood arsenic levels in several patients in the trial, as well as in volunteers (see below), and regarded those levels as safe. Therefore, we proceeded to treat patients who had residual APL cells.
Determination of arsenic levels
Quantitative determination of arsenic levels in samples from patients was done in our laboratory with an atomic absorption spectrophotometer (Analyst 100; Perkin Elmer, Wellesley, MA). The arsenic standard was prepared from diarsenic trioxide with an arsenic concentration of 100 μg/mL by using material obtained from the China National Center of Materials Standardization. Urinary arsenic analysis was done according to the method described by Norin and Vahter33 by using a specific standard sample (GBW09103; 360 ± 40 μg/L arsenic). Venous blood specimens collected from patients before, during, and after treatment were anticoagulated with EDTA. Cerebrospinal fluid and 24-hour urine collections were also obtained from patients at several intervals. All these specimens were kept at 4°C for a few days or at −20°C until analysis, and all were analyzed within a week.
To determine arsenic content in hair, hairs were plucked from the occipital area, washed with detergent and deionized running water, and dried. For arsenic analysis, 2 mL anticoagulated blood or 0.2 g of the hair specimen was put into a 5-mL acid mixture (nitric acid, hyperchloric acid, and sulfuric acid [10:3:2]) and left overnight in a capped flask. The next day, samples were heated from 100°C in a step-wise fashion to 250°C and assayed in duplicate, along with controls including a standard sample and a clear specimen. Cerebrospinal fluid and urine were used directly for arsenic analysis (without digestion).
Clinical pharmacokinetic studies
Clinical pharmacokinetic studies were done in 7 volunteers with APL and HCR by using 60 mg/kg oral As4S4 in a single dose. Before this experiment, a phase I clinical trial of gradual dose escalation was carried out to ensure safety of the agent. Blood samples were collected before administration of As4S4 and at 0.5, 1, 2, 4, 6, 8, 12, 24, 36, 48, and 72 hours afterward. Urine samples were obtained as follows: a 24-hour urine collection before drug administration and collection of one sample at intervals of 0 to 4, 4 to 8, 8 to 12, 12 to 24, 24 to 48, 48 to 72, and 72 to 96 hours afterward. Arsenic quantitation in these samples was done as described above. Pharmacokinetic variables assessed included maximal blood concentration (Cmax), peak time (Tpeak), area under the concentration-time curve (AUC0-infinity), and elimination half-life (t1/2). AUC0-infinity was calculated by using the sum-of-the-trapezoids method.
Results
Patients with newly diagnosed APL
Treatment efficacy.
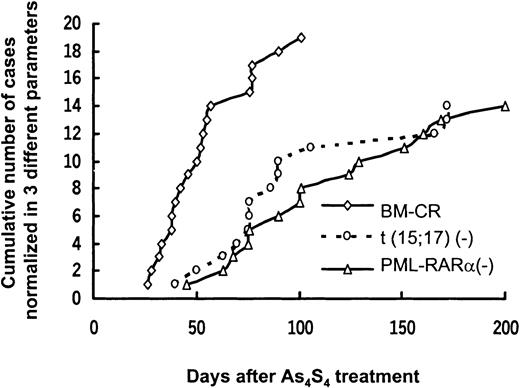
Nineteen patients with newly diagnosed APL received As4S4 alone as their initial therapy, and CR was achieved in all of them. Clinical characteristics of these patients and the time to achieve HCR and cytogenetic and molecular CR are shown in Table 2 and Figure1.
Sequential normalization of morphologic features, t(15;17), and PML/RARα after As4S4 treatment.
Each line represents a variable.
Sequential normalization of morphologic features, t(15;17), and PML/RARα after As4S4 treatment.
Each line represents a variable.
Early responses to As4S4 therapy included rapid resolution of bleeding symptoms within 7 days and a progressive rise in platelet levels to normal values. The median time to a normal platelet count (100 × 109/L) was 31 days (range, 19-47 days). Blood hemoglobin levels recovered more slowly. No patient had exacerbation of DIC. Patients had BM CR in a median time of 50 days (range, 26 to 101 days), with a median total dose of 110.2 g (range, 37.5 to 312.5 g). In 14 patients, normalization of BM blasts and promyelocytes occurred within 2 months; in the other 5, this took somewhat longer. However, the rapid improvements in clinical condition and the decreases in the number of BM promyelocytes and blasts that occurred in all patients during the first month of treatment warranted continued therapy with As4S4 alone. The remaining promyelocytes had unusual morphologic features, including (1) a reduction in dense granules in the cytoplasm (degranulation); (2) more acidophilic cytoplasm, with a changed spectrum of color from lightly basophilic to lightly acidophilic; and (3) continued presence of discernible nucleoli but development of irregularity of the nuclear contour and more coarseness in the chromatin strands than in those of APL promyelocytes in untreated patients. The morphologic findings suggested that the leukemic promyelocytes underwent degeneration rather than differentiation (Figure2).
Morphologic changes in APL cells in BM after As4S4treatment.
(A) Promyelocytes from a patient with APL (patient 8) had dense azurophilic cytoplasmic granules and ectoplasm with Wright stain before treatment. The nuclei had fine chromatin strands and nucleoli. (B) After 18 days of As4S4treatment, cells from the same patient had degranulated cytoplasm. The nuclei had an irregular contour and coarse chromatin strands. (C) Other degenerating cells in the same smear as that in Figure 2B. Original magnification, × 1000.
Morphologic changes in APL cells in BM after As4S4treatment.
(A) Promyelocytes from a patient with APL (patient 8) had dense azurophilic cytoplasmic granules and ectoplasm with Wright stain before treatment. The nuclei had fine chromatin strands and nucleoli. (B) After 18 days of As4S4treatment, cells from the same patient had degranulated cytoplasm. The nuclei had an irregular contour and coarse chromatin strands. (C) Other degenerating cells in the same smear as that in Figure 2B. Original magnification, × 1000.
After As4S4 treatment, 16 of the 19 patients with newly diagnosed APL were evaluable for disappearance of t(15;17) and loss of the fusion transcript on RT-PCR analysis. Patient 19 could not be assessed because it was too early in the treatment regimen, and patients 14 and 18 did not have an available RT-PCR analysis. Among the 16 patients studied, t(15;17) disappeared in 15 (93.7%) within a median time of 81 days (range, 40-172 days) and was detected in one (patient 11). The PML-RARα fusion transcript became undetectable by RT-PCR in 14 patients (87.5%) within a median time of 101 days (range, 45-200 days) and remained detectable in 2 patients (6 and 11). Seven patients became negative for PML-RARα on RT-PCR analysis in 100 days, 3 in 100 to 150 days, and 4 in 150 to 200 days (Figure 1). More time was required for patients to become negative on RT-PCR analysis than for cytogenetic normalization.
DFS.
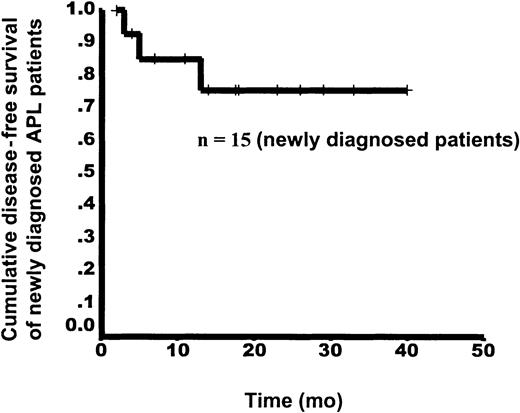
After CR, all patients received only As4S4 on the protocol schedule for maintenance. Follow-up data were available for 15 patients with newly diagnosed disease. Two patients (13 and 18) were lost to follow-up and 2 others (6 and 12) were excluded from the trial because of protocol violation and nonadherence to the suggested dosage during maintenance therapy. In the 15 patients with follow-up data, 3 relapses occurred. Patient 3 again became positive on RT-PCR analysis and had relapse in the 13th month after achievement of HCR. Patient 8 had relapse in the sixth month. This patient was found to have complex karyotypes at relapse and underwent BM transplantation after another CR obtained with chemotherapy. Patient 11, who had positive results on t(15;17) and RT-PCR assessments after HCR, had relapse in the third month. Thus, with a median follow-up time of 13.5 months (range, 2-40 months), the estimated DFS rates at 1 and 3 years were 86.2% and 76.6%, respectively (Figure3).
Kaplan-Meier curve for DFS from the time of HCR in 15 patients with newly diagnosed APL.
Kaplan-Meier curve for DFS from the time of HCR in 15 patients with newly diagnosed APL.
Patients with relapsed APL
The enrolled patients with relapse had previously received ATRA or chemotherapy. Two were treated with ATRA and 5 with ATRA and chemotherapy before As4S4 treatment began. Their clinical characteristics and time to achieve CR are shown in Table 3. After As4S4 treatment, all 7 patients with relapse had HCR, in a median time of 47.5 days (range, 32-80 days, similar to that in patients with newly diagnosed APL) and with a median total dose of 50.5 g (range, 15.7-418.7 g). Cytogenetic and molecular CRs were achieved in 5 patients. For the other 2 patients, t(15;17) and RT-PCR results were not available by the cut-off date for the statistical analysis. During follow-up, one patient with relapsed APL (R3) had another relapse, in the 10th month.
Patients with HCR
Maintenance efficacy.
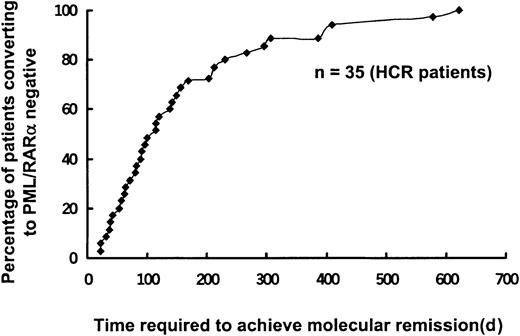
Of the 103 patients with HCR with or without molecular or cytogenetic residual leukemia, 84 had previously been treated with both ATRA and chemotherapy and 19 patients were previously given ATRA alone. As4S4 therapy was administered in these patients in accordance with the treatment protocol. No myelosuppression occurred. Before As4S4 treatment, RT-PCR analysis to detect PML-RARα messenger RNA (mRNA) yielded positive results in 44 patients and negative results in 56; 3 patients were not tested for a variety of reasons. After As4S4therapy, 35 of the 44 patients (79.5%) with positive results on RT-PCR analysis became negative on RT-PCR analysis in a median time of 114 days (range, 22-622 days; Figure 4).
Time to achieve molecular remission in patients who were positive for PML/RARα and had HCR.
Among the patients with HCR treated for residual leukemia and maintenance, 44 patients positive for PML-RARα transcript at baseline had serial follow-up assessments, and 35 of them (79.5%) had become negative for PML-RARα mRNA after As4S4treatment in a median time of 114 days (range, 22-622 days).
Time to achieve molecular remission in patients who were positive for PML/RARα and had HCR.
Among the patients with HCR treated for residual leukemia and maintenance, 44 patients positive for PML-RARα transcript at baseline had serial follow-up assessments, and 35 of them (79.5%) had become negative for PML-RARα mRNA after As4S4treatment in a median time of 114 days (range, 22-622 days).
DFS.
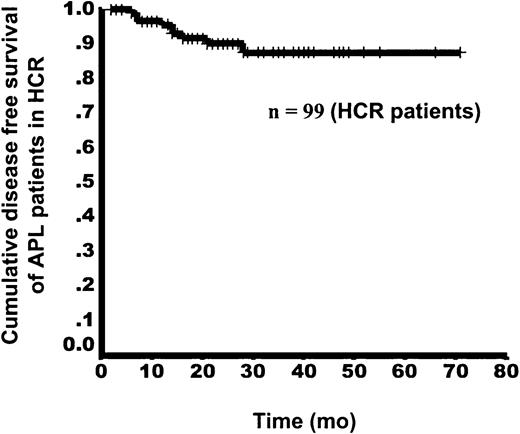
During the entire 6-year study, 99 patients in the HCR group were available for statistical analysis, and 4 were lost to follow-up. In the entire series, there were 9 relapses, including 1 extramedullary relapse and 8 hematologic relapses. At initiation of As4S4 therapy in these 9 patients, RT-PCR analysis yielded positive results in 4, negative results in 4, and was not available in one. The extramedullary relapse occurred in the central nervous system in the 28th month after As4S4 therapy began. The 8 patients with hematologic relapse had a median relapse interval of 13.5 months (range, 6-22 months). Of these 8 patients, 3 had a second HCR with administration of an increased dosage of As4S4and 5 had no response to As4S4 and underwent chemotherapy. Two of the 5 patients with no response died. With a median observation time of 23.0 months (range, 2-71 months) in this group, the estimated DFS rates at 1 and 6 years were 96.7% and 87.4%, respectively (Figure5).
Kaplan-Meier curve for DFS in APL patients with HCR, from initiation of As4S4 treatment.
Kaplan-Meier curve for DFS in APL patients with HCR, from initiation of As4S4 treatment.
Flow cytometric studies in patients with overt APL
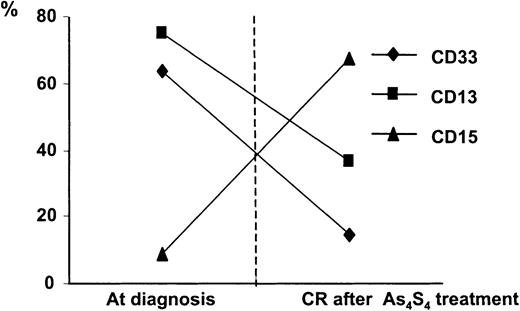
Flow cytometric evaluations for CD33, CD13, and CD15 were done on BM samples from 13 patients, 9 with newly diagnosed APL and 4 with relapse. CD33+ and CD13+ cells, which are typically associated with APL, were observed before As4S4 therapy and began to decrease after treatment, gradually reaching the normal range. Concomitantly, there was increased expression of CD15, an antigen associated with mature granulocytes34 (Figure6).
Surface-antigen analyses of BM before and after As4S4 treatment.
Expression of surface antigens of CD33, CD13, and CD15 in BM mononuclear cells was analyzed by flow cytometry before and after As4S4 treatment. Data are mean ± values from 13 patients, 9 with newly diagnosed disease and 4 with relapse. Before treatment, most cells expressed only CD33 and CD13, typical of APL. After HCR was achieved, most myeloid-lineage cells expressed CD15, the antigen usually found on mature cells.
Surface-antigen analyses of BM before and after As4S4 treatment.
Expression of surface antigens of CD33, CD13, and CD15 in BM mononuclear cells was analyzed by flow cytometry before and after As4S4 treatment. Data are mean ± values from 13 patients, 9 with newly diagnosed disease and 4 with relapse. Before treatment, most cells expressed only CD33 and CD13, typical of APL. After HCR was achieved, most myeloid-lineage cells expressed CD15, the antigen usually found on mature cells.
Adverse effects
Among 124 patients evaluated for toxic effects of high-purity As4S4, most had no severe adverse events. The main side effects observed are listed in Table4. The effect most often observed was asymptomatic QTc prolongation, which occurred in 33 of 100 patients (33%) with an available electrocardiogram and ranged from 0.440 to 0.513 seconds (median, 0.455 seconds). QTc prolongation occurred at different accumulative dosages, with the median being 76.5 g (range, 3.0-1127.5 g). However, all patients with prolonged QTc were asymptomatic and As4S4 treatment could be administered as scheduled. No patient had ventricular premature beats or ventricular tachycardia (including torsive ventricular tachycardia).
Another side effect was a transient elevation in liver enzyme levels, which occurred in 13 of 124 patients (10.5%). Among these 13 patients, neither hepatitis B virus (HBV) nor hepatitis C virus (HCV) infection was detected in 8, one had HBV DNA, and 4 had HCV RNA, with or without small increases in liver enzyme levels before As4S4 therapy. In the 8 patients without HBV or HCV infection, the elevation in serum alanine aminotransferase (ALT) and serum aspartate aminotransferase (AST) occurred on the 7th day to the 13th month after As4S4 therapy began. The peak ALT level ranged from 67 to 466 U/L (median, 214 U/L; normal range, 0 to 40 U/L) and the peak AST level from 51 to 453 U/L (median, 212 U/L; normal range, 0 to 40 U/L). The ALT and AST elevations lasted for 10 to 60 days. All 8 patients had recovery of normal values after conservative treatment, and they continued to receive As4S4 therapy after hepatic recovery. The 5 patients with HBV or HCV infection had ALT and AST elevations for longer periods. Three of those 5 patients had a small increase in ALT and AST before As4S4 therapy. In 2 patients, temporary discontinuation of As4S4 therapy was necessary.
Mild nausea without vomiting or diarrhea occurred in 4 patients (3.2%). Skin itching or puffiness of the eyelids also occurred in 4 patients (3.2%). No patient had ankle edema or peripheral neuropathy.
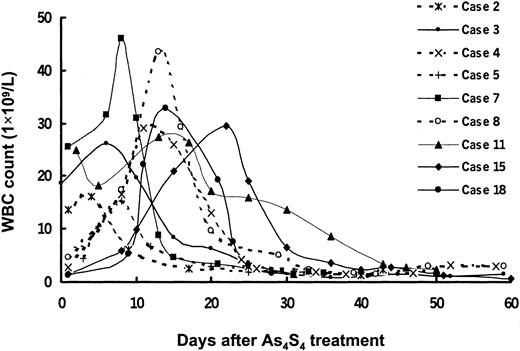
Neither myelosuppression nor retinoic acid syndrome was observed. As4S4 was given safely to patients with severe neutropenia. No patient in the HCR group had leukocytosis. However, mild and transient leukocytosis occurred in some patients with newly diagnosed APL during remission induction; these patients had an increased WBC count (> 10 × 109/L) before As4S4 treatment (4 patients) or during As4S4 therapy (5 patients). A gradual increase in peripheral WBC count developed, with peak values ranging from 15.1 to 45.9 × 109/L on the 4th to 22nd day after As4S4 was given (Figure7). In 4 patients, the elevations in WBC were easily controlled by a few doses of hydroxyurea. Patient 7 received a low dosage of harringtonine (2 mg/day for 3 days). In 4 other patients (dotted lines in Figure 7), the high WBC resolved spontaneously, without administration of cytotoxic drugs.
Dynamic changes in peripheral WBC counts during As4S4 treatment in 9 patients with newly diagnosed APL.
Four patients had baseline WBC counts above 10 × 109/L, and 5 had such counts during As4S4 treatment. The peak values in WBC counts ranged from 15.1 to 45.9 × 109/L on the 4th to 22nd day after administration of As4S4. In 4 patients, the WBC counts decreased after a few doses of hydroxyurea. Patient 7 received a low dosage of harringtonine (2 mg/day) for 3 days. In 4 other patients (dotted lines), the high WBC counts resolved spontaneously, without administration of cytotoxic drugs.
Dynamic changes in peripheral WBC counts during As4S4 treatment in 9 patients with newly diagnosed APL.
Four patients had baseline WBC counts above 10 × 109/L, and 5 had such counts during As4S4 treatment. The peak values in WBC counts ranged from 15.1 to 45.9 × 109/L on the 4th to 22nd day after administration of As4S4. In 4 patients, the WBC counts decreased after a few doses of hydroxyurea. Patient 7 received a low dosage of harringtonine (2 mg/day) for 3 days. In 4 other patients (dotted lines), the high WBC counts resolved spontaneously, without administration of cytotoxic drugs.
One patient had a mild and non–life-threatening pericardial effusion. This patient had the same problem when previously treated with ATRA or chemotherapy. No severe persistent toxicity was observed during the 6 years of this study.
Clinical pharmacokinetic study
Pharmacokinetic variables were analyzed in 7 volunteers with APL and HCR by using a single dose of As4S4. The single dose of up to 60 mg/kg As4S4 was well tolerated. Arsenic could be detected in the blood 30 minutes after oral administration of As4S4. The Tpeakwas 3.4 ± 1.4 hours and the Cmax was 24.9 ± 8.0 μg/L. There was a wide interpatient variation in AUC0-infinity (899.01 ± 705.64 μg/hour per liter) and t1/2 (30.1 ± 11.1 hours).
Mean ± SD levels of urinary arsenic excretion were 323.5 ± 271.8 μg at 0 to 4 hours, 392.9 ± 295.0 μg at 4 to 8 hours, 245.1 ± 157.7 μg at 8 to 12 hours, 582.9 ± 312.6 μg at 12 to 24 hours, 904.7 ± 475.8 μg at 24 to 48 hours, 709.4 ± 332.2 μg at 48 to 72 hours, and 355.9 ± 226.2 μg at 72 to 96 hours. To characterize arsenic excretion, we calculated percentages of daily urinary arsenic excretion of the total arsenic excreted in the first 96 hours; mean percentages were 45.5% ± 12.9% for 0 to 24 hours, 26.1% ± 8.4% for 24 to 48 hours, 20.9% ± 9.2% for 48 to 72 hours, and 7.8% ± 4.4% for 72 to 96 hours (Figure8).
Urinary arsenic excretion in the first 96 hours after one dose of As4S4.
Data are percentages of daily urinary arsenic excretion of the total arsenic excreted in the first 96 hours.
Urinary arsenic excretion in the first 96 hours after one dose of As4S4.
Data are percentages of daily urinary arsenic excretion of the total arsenic excreted in the first 96 hours.
Measurement of arsenic levels
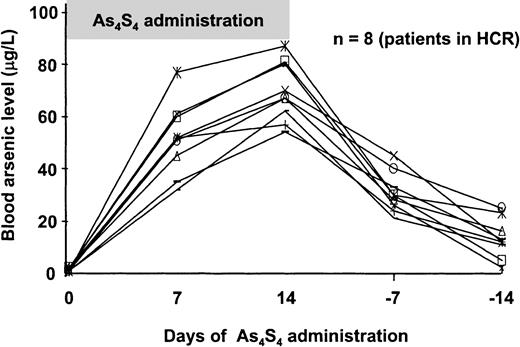
For maintenance therapy and for treatment of patients with residual disease and HCR, As4S4 was given for 2 weeks followed by a break of 2 weeks during the first year of treatment. Blood arsenic levels were measured in 8 patients in the HCR group on the 7th and 14th day after As4S4began, as well as on the 7th and 14th day after therapy was discontinued (Figure 9). Mean ± SD levels were 51.9 ± 13.6 μg/L on the 7th day and 64.9 ±11.4 μg/L on the 14th day. Blood arsenic levels decreased to 26.1 ± 6.4 μg/L and 13.1 ± 8.0 μg/L on the 7th and 14th day, respectively after discontinuation of therapy.
Blood arsenic concentration in patients with APL treated with As4S4.
During the 2-weeks-on and 2-weeks-off treatment schedule, blood arsenic levels increased during the 2 weeks of continued administration of oral As4S4 and gradually returned to normal levels after discontinuation. Blood samples were obtained for arsenic analysis every week. Blood arsenic concentrations are shown in micrograms per liter on the y-axis; the x-axis shows the days of As4S4 administration. The −7 and −14 indicate discontinuation of treatment with As4S4 for 7 and 14 days, respectively.
Blood arsenic concentration in patients with APL treated with As4S4.
During the 2-weeks-on and 2-weeks-off treatment schedule, blood arsenic levels increased during the 2 weeks of continued administration of oral As4S4 and gradually returned to normal levels after discontinuation. Blood samples were obtained for arsenic analysis every week. Blood arsenic concentrations are shown in micrograms per liter on the y-axis; the x-axis shows the days of As4S4 administration. The −7 and −14 indicate discontinuation of treatment with As4S4 for 7 and 14 days, respectively.
In 5 patients with newly diagnosed APL, plasma, red cell, and white cell arsenic levels were also measured. Baseline values were below 2 μg/L. On the fourth day of As4S4 treatment, the mean level of arsenic in red cells rose to 22.65 ± 2.65 μg/L and the plasma arsenic level was 8.6 ± 6.9 μg/L. Both arsenic levels increased gradually, to 90.9 ± 4 μg/L in red cells and 20 to 31 μg/L in plasma on the 40th day of continuous treatment and plateau levels thereafter. The red cell arsenic level was 2.1 ± 3.6 times higher than the plasma level. During treatment, the mean urinary arsenic level on the eighth day was 1733.6 ± 1365.9 μg/L (44 samples), and the average daily urinary excretion was 3.93 ± 2.44 mg/day. The urinary excretion level fell to 0.33 ± 0.3 mg/day 8 days after therapy was discontinued. The arsenic level in cerebrospinal fluid in 9 patients on the 10th day of treatment was 5.6 to 14.6 μg/L, a level similar to that in plasma. The mean arsenic level in hair (13 patients) was 3.7 ± 1.5 μg/g in the 4th month and accumulated to 13.5 ± 7.8 μg/g in the 18th month.
Discussion
ATO has been reported to induce clinical and molecular remission in a high proportion of patients with APL.11,16 Because ATO can cause severe liver damage if given orally, the agent must be administrated intravenously. However, high doses of intravenous ATO may be associated with serious acute toxicity.21 22 Therefore, administration of ATO requires close monitoring. On the other hand, we found in this series of patients with APL that oral As4S4 is effective and easy to administer, so that quality of life is improved, especially in patients in whom long-term therapy is required.
We conducted a 6-year study of treatment of APL with an oral preparation of As4S4. The therapeutic effect of As4S4 is well established. In our study, As4S4 used alone not only induced HCR in all patients with newly diagnosed and relapsed APL but also produced cytogenetic and molecular CR in 87.5% of patients with newly diagnosed disease and in most evaluable patients with hematologic relapse. In treatment for residual leukemia, 79.5% of patients in this series who had PML-RARα mRNA transcript at baseline became negative for PML-RARα mRNA after As4S4 treatment. Used as a single agent, As4S4 was associated with long-term DFS rates, including 86.1% and 76.6% at 1 and 3 years in the patients with newly diagnosed disease and 96.7% and 87.4% at 1 and 6 years in the HCR group. Without a randomized clinical trial, it is not possible to determine the relative efficacy of As4S4 and ATO. However, both agents appear to induce a high proportion of durable remissions in patients with APL refractory to ATRA and chemotherapy.
Realgar, a mined ore, contains about 90% As4S4.35 Realgar has been used in Chinese traditional medicine for more than 1500 years. Employed either externally or orally, realgar was administered to treat a variety of diseases, including syphilis, “malignant” sores, malaria and several other parasitic infections, and was regarded as having little toxicity.23 24 It was also claimed to be effective against certain “malignant” diseases in which persistent fevers and large intra-abdominal masses occurred. In provinces south of the Yangtze River, realgar was regularly used at home during the Duan-Wu festival. Realgar in wine, in which some of the agent was dissolved and some suspended to make “realgar wine,” was used as a preventive against “malign influences.” A suspension of realgar was sprinkled on the ground to keep out insects and snakes. These historical uses demonstrate the relatively nontoxic nature of realgar compared with ATO administered orally.
It is recorded in ancient Chinese Materia medica books that excavated realgar is toxic or mildly toxic.23,24 Before this agent is made available on the traditional Chinese pharmacy market, it must be “refined” in accordance with methods used in traditional Chinese medicine.23 Unrefined realgar contains appreciable amounts of ATO, as well as calcium and magnesium arsenites, which are toxic.23,24 Use of realgar as mined can produce toxic effects.36 Realgar differs greatly in the purity of its As4S4 contents. Mineral crystal with a very high proportion of As4S4 was rarely used in Chinese traditional medicine. Although realgar was used commonly, it was employed with several herbal drugs containing hundreds of components. Neither As4S4 nor other arsenic sulfides, such as diarsenic trisulfide (As2S3), were previously described as being effective against any malignant disease when used alone.
During treatment of APL in our series, As4S4was well tolerated, with moderate side effects. As4S4 caused QTc prolongation in 33% of patients, but neither symptoms nor serious cardiac events occurred. The incidence and severity of QTc prolongation appear to have been lower than with ATO. In ATO treatment, a marked percentage of cases of QTc prolongation and even torsades de pointes were reported.19 21 The mechanism of QTc lengthening with As4S4 is unknown and must be elucidated.
With respect to hepatotoxicity, As4S4 treatment led to transient injury. Patients with elevations in liver enzyme levels after As4S4 treatment had recovery within 10 to 60 days after conservative treatment. This effect was not related to accumulative doses of As4S4. Patients with concomitant with HBV or HCV infection were vulnerable to hepatic injury. Importantly, neither retinoic acid syndrome nor myelosuppression occurred during the treatment course. Only a mild, transient leukocytosis was detected in patients with newly diagnosed disease during remission induction. The WBC counts returned to normal levels spontaneously or after a few small doses of cytotoxic drugs. It is important to note that ATRA and even ATO were found to induce a level of hyperleukocytosis that was more severe and was responsible for early deaths during remission induction; this was especially true of ATRA.16 37
Orpiment has been used as a Chinese Materia medica for nearly as long as realgar.23,24 As2S3, a major ingredient of orpiment, could also be used either externally or orally. The Chinese name for orpiment is literally translated as “female yellow,” whereas that for realgar is “male yellow.” In the current study, we treated one patient with newly diagnosed APL with synthetic, chemically pure As2S3. The patient had HCR and molecular CR after monotherapy with As2S3.38 Therefore, this form of arsenic sulfide may also be an effective therapy for APL.
The morphologic appearance of APL promyelocytes after As4S4 treatment suggested degeneration and apoptosis rather than differentiation. As4S4and As2S3 have induced subdiploid cells, DNA ladder formation on agarose-gel electrophoresis, and enhancement of caspase-3 activity in the NB4 cell line. Arsenic sulfides also arrested NB4 cell growth in the G2/M phase.39Relocalization of PML protein in patients' APL cells was detected readily in cells from BM aspirates from patients treated with arsenic sulfides before the morphologic change. The decrease in promyelocytes and blast cells in BM obtained after As4S4 therapy in patients with APL was accompanied by a reduction in t(15;17) cells. Clinical improvement and platelet recovery usually preceded the decrease in promyelocytes.
Our pharmacokinetic studies revealed that rapid absorption of arsenic occurred after oral administration of As4S4. A single large dose of arsenic sulfide in 7 patients with APL produced similar and tolerable peak blood arsenic levels without appreciable side effects. Interpatient variations in arsenic absorption, metabolism, and clearance were observed. However, when As4S4 was administered daily in divided doses, blood levels were not only tolerable but fell within a certain range. Both blood and urinary arsenic levels declined after As4S4 was discontinued. Because 70% of absorbed arsenic is excreted in urine by means of the kidneys,40 41 we deduce that 5.61 ± 3.4 mg arsenic was absorbed from the intestinal tract each day by each patient in our series. On the other hand, because of the renal excretion of arsenic, the dosage should be adjusted for diminished renal function. Arsenic binds keratoprotein and can be removed through hair and nail growth and cutaneous surfaces. In this study, continuous use of oral As4S4 was associated with increased elimination of arsenic in hair. Together with the renal excretion of arsenic, this at least partly accounts for the flattening out or lessening of increases in arsenic levels. Additional studies of the metabolism and clearance of arsenic in humans requires further investigation.
Our findings lead us to conclude that oral As4S4 is highly effective in inducing CR both in patients with newly diagnosed APL and in those with APL relapse. Moreover, it is highly effective in treating residual APL and for CR maintenance. Blood and BM cell morphologic characteristics, t(15;17), and PML-RARα results on RT-PCR analysis became normal in patients with APL after As4S4 treatment. Oral arsenic can be used safely for induction or long-term treatment in most patients with APL. The highly purified arsenic used at the dosage described here produced no symptoms, signs, or laboratory evidence of adverse effects in most patients. No myelosuppression was observed, in contrast to results with other cytotoxic antineoplastic agents. An honorable gift from nature, As4S4 must be added to the list of effective therapeutic agents for the cure of APL.
We thank Drs Xiao-Jun Huang, Xi-Jing Lu, and Wei Han for valuable assistance in the clinic; Hui-Lin Shi, Xio-Ying Qin, Ya-Zhen Qin, Guo-Xuan Li, Bo Hong, and Feng-Rong Wang for helpful laboratory work; and Drs Peggy Pei-Hua Lu and Yan-Fang Liu for assistance in preparation of the manuscript.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Dao-Pei Lu, Peking University Institute of Hematology, Peking University People's Hospital, 11 Xizhimen South St, Beijing, 100044, China; e-mail: lscm@beic.gov.cn.