Mixed warm and cold autoimmune hemolytic anemia (AHIA) is characterized by the presence in the serum of both an IgG warm autoantibody and a cold-active IgM antibody with wide thermal amplitude.1,2 The disease presents with severe hemolysis, responds to steroids, but usually runs a chronic course with intermittent exacerbations.1,2 Rituximab is a monoclonal antibody against CD20 antigen, which has been introduced recently for the treatment of some autoimmune disorders related to a B-cell clone.3-5 We report here the use of rituximab to treat for the first time a patient with mixed warm and cold AIHA who relapsed after tapering off corticosteroids.
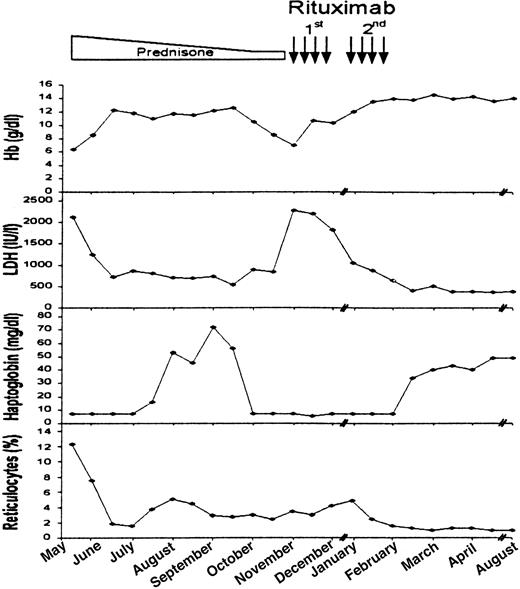
A 68-year-old white woman was referred to the hospital because of weakness and dyspnea. At admission, laboratory investigations showed severe normochromic, normocytic anemia (hemoglobin level of 6.4 g/dL and hematocrit of 16.5%), with a reticulocyte count of 12.2%. The white blood cell count (WBC) was 4500/μL, with neutrophils, 81%; lymphocytes, 12%; and monocytes, 3%. The platelet count was 273 000/μL. Biochemical evidence of hemolysis was supported by bilirubin level of 2.5 mg/dL, lactate dehydrogenase (LDH) of 2140 IU/L, and haptoglobin below 7 mg/dL (Figure 1). The direct antiglobulin test was positive for polyvalent serum (++-), complement (C3d) (++-), and IgG (++-). Cold agglutinins of IgM type have a titer of 1:1024. Serologies for human immunodeficiency virus, hepatitis B and C viruses, and Mycoplasma pneumoniae were negative. Rheumatoid factor and antinuclear antibodies were undetectable. Total body computed tomography showed neither adenomegaly nor splenomegaly. Bone marrow biopsy examination revealed erythroid hyperplasia and scant lymphocytic infiltrates (< 5% of total cellularity). Prednisone therapy was started at a dose of 1 mg/kg intravenously, daily. Hemoglobin level rose to 11 g/dL, concomitantly with the improvement of hemolytic signs. A reduction of positivity of both direct and indirect antiglobulin tests (polyvalent serum +−−; C3d +−−; IgG+−−), as well as a reduction of cold agglutinin titers (1:128), was observed 8 weeks after corticosteroid therapy. Three months later, corticosteroids were tapered to a maintenance dose of 25 mg daily. Hemolysis subsided with the fall of hemoglobin to 7 g/dL. The direct antiglobulin test recurred positive for polyvalent serum (+++), complement (+++), and IgG (+++), while cold agglutinin titers again became strongly positive (1:256). Immunophenotyping of bone marrow cells showed that 10% of all the cells were CD20 and CD19 positive. Rituximab was started at the dose of 375 mg/mq once weekly, for a total of 4 doses (Figure 1). Hemoglobin value reached 13.5 g/dL just before the third dose, although biochemical signs of hemolysis remained substantially unaltered. One month after the first cycle of therapy, a second course of rituximab was administered, at the same dosage schedule. At the end of therapy, the hemolytic signs disappeared, the direct and indirect antiglobulin tests became negative, and cold agglutinin titers fell to 1:32 (Figure 1). Immunophenotyping of bone marrow cells showed the absence of CD20 and CD19 B cells. The patient did not experience side effects during rituximab treatment. Actually, 7 months after the last administration of rituximab, the patient is in complete remission and out of therapy.
Clinical course of the patient and response to treatment.
Changes in hemoglobin, LDH, haptoglobin levels, and reticulocyte count after initial rituximab therapy. Rectangle represents treatment with corticosteroids. Black arrows represent single rituximab infusions.
Clinical course of the patient and response to treatment.
Changes in hemoglobin, LDH, haptoglobin levels, and reticulocyte count after initial rituximab therapy. Rectangle represents treatment with corticosteroids. Black arrows represent single rituximab infusions.
This is the first reported case of mixed warm and cold AIHA not associated with an overt malignant lymphoproliferative disease responding to rituximab therapy. The notable finding is represented by the complete recovery of the disease, which has been obtained by 2 courses of rituximab. To our knowledge, rituximab, often in association with corticosteroids and/or chemotherapy, has so far shown efficacy in only a few cases of cold agglutinin disease.6-11 A single course of rituximab also has been used in combination with corticosteroids for the treatment of one case of idiopathic warm hemolytic anemia, leading only to a partial improvement of the hemoglobin level and hemolytic signs.12 Although further studies are warranted on larger series of patients with longer follow-up, rituximab may represent a decisive therapeutic option in the treatment of mixed warm and cold AIHA. The possibility of a second course of rituximab also should be considered in partial responders.
Supported by a research grant from Associazione Italiana per la Ricerca sul Cancro (AIRC, Milan, Italy) to M.L.