Abstract
Myasthenia gravis (MG) is the leading paraneoplastic manifestation of thymomas and is probably related to the capacity of thymomas to mature and export potentially autoreactive T cells. Why some thymomas are MG associated (MG+) and others are not (MG−) has been unclear. We addressed this question by comparing the percentages of intratumorous naive mature CD45RA+ thymocytes in 9 MG(+) and in 13 MG(−) thymomas by fluorescence-activated cell sorting analysis. Our results show that intratumorous naive CD4 T cells were present in all MG(+) thymomas and in one MG(−) thymoma with the development of MG only 2 months after surgery. By contrast, the percentage of naive CD4+ T cells was significantly reduced in all 13 MG(−) thymomas (P < .0001). Alterations in intratumorous thymopoiesis were reflected by corresponding alterations of naive T-cell subset composition in the blood, in that only MG(−) patients had significantly decreased levels (P = .02) of naive CD4+ T cells compared with age- and sex-matched control persons. We conclude that paraneoplastic MG is highly associated with the efficiency of thymomas to produce and export naive CD4+T cells. The acquisition of the CD45RA+ phenotype on CD4+ T cells during terminal intratumorous thymopoiesis is associated with the presence of MG in most thymoma patients.
Introduction
Myasthenia gravis (MG) is an autoimmune disease mediated by anti–acetylcholine receptor autoantibodies that cause muscle weakness by impairing neuromuscular transmission.1Of note, autoantibody production in MG is a CD4+T-cell–dependent process.2-5
The frequent but incomplete association of thymomas (ie, thymic epithelial tumors) with paraneoplastic MG and the generally unpredictable clinical course of MG after thymectomy are issues with relevance for basic immunobiology and for patient management.2 The current World Health Organization (WHO) classification of thymomas6 recognizes 5 thymoma subtypes that can be associated with MG: type A (or medullary), type AB (or mixed), type B1 (or organoid), type B2 (or cortical), and type B3 (or well-differentiated thymic carcinoma). Among these, AB, B2, and B3 thymomas are by far the most frequent subtypes and at the same time show the highest association with MG.6 7
In recent years, it has been recognized that MG-associated thymomas share critical features, namely (1) intratumorous enrichment for acetylcholine receptor reactive T cells,4 (2) intratumorous thymopoiesis with the generation of mature and potentially autoreactive T cells from immature precursors,5,6,8 and (3) export of mature T cells to the peripheral compartment of the immune system.9,10 The importance of export of potentially autoreactive T cells from thymomas for MG development has also been supported by the observation that a minority of initially MG(−) thymoma patients begin to acquire MG months or years after thymoma resection.11,12 In addition, histologic thymoma subtypes with minimal or absent thymopoiesis—WHO type A (medullary) and type C thymomas (thymic carcinomas)—have a much lower or almost absent MG risk compared with WHO type AB (mixed) or type B (cortical) thymomas that exhibit prominent intratumorous thymopoiesis.6 7
However, when the histologic thymoma subtype is taken into account, no systematic difference between MG(+) and MG(−) thymomas with respect to intratumorous thymopoiesis has been reported so far. In both MG(+) and MG(−) thymomas, there is impaired maturation almost exclusively of the CD4 lineage, whereas development of the CD8 lineage is better preserved.13,14 Furthermore, all known thymocyte maturation stages, in terms of CD3, CD69, CD4, and CD8 expression, can be detected in virtually all MG(+) and MG(−) thymomas.12-15 So far, the only significant differences between MG(+) and MG(−) thymoma patients with respect to the distribution of T-cell subsets have been recognized in the blood. Specifically, thymoma patients have been reported to exhibit a reduced CD4-CD8 ratio among naive CD45RA+ T cells in comparison with controls due to an increased export of CD8+ naive T cells from MG(−) and MG(+) thymomas.9 Because the reduction of the CD4-CD8 ratio was more pronounced in MG(−) patients, it was hypothesized that the export of CD4+ T cells might be better maintained among MG(+) thymomas. This hypothesis was directly proven by a recent study10 applying quantitative assessment of so-called T-cell receptor rearrangement excision circles (TRECS) to determine the contribution of the thymus and supposedly the thymoma to the peripheral T-cell pool.16 Buckley et al10clearly showed that the numbers of naive CD4+ and CD8+ T cells were abnormally increased in the blood of MG(+) thymoma patients, whereas an increase only of naive CD8+ T cells was detected in the blood of 3 MG(−) thymoma patients.10 Both studies9,10 suggested a pivotal role of thymoma-derived CD4+ T cells for the pathogenesis of paraneoplastic MG. However, because both investigations were based on blood T cells, they did not resolve the underlying pathophysiology at the level of intratumorous thymopoiesis or exclude the alternative possibility that exported naive T cells may disappear more quickly from the peripheral T-cell pool of MG(−) thymoma patients by either peripheral deletion17 or conversion into the CD45RO+ (TREClow) memory pool.16
To address these questions, we first analyzed the intratumorous generation of the mature naive CD45RA+ thymocyte subsets13 in thymomas with and without associated MG, and we next analyzed the circulating naive (CD45RA+) T-cell subsets by flow cytometry. Determination of the naive T-cell phenotype is difficult and has led to the use of complex techniques, such as polychromatic flow cytometry18 or molecular biologic analysis of TRECS.16 From these studies, however, it is also known that the contamination of the naive T-cell pool with memory cells is relatively small.16,18,19 Moreover, analysis of naive T cells by measurement of TRECS may also be confounded by mechanisms such as peripheral homeostatic proliferation.19Thus, the assessment of thymic output by measurement of CD45RA can still be regarded as an orientating, pragmatic method for use in routine procedures.
Our results clearly indicate that the presence of paraneoplastic MG is highly correlated with the efficiency of thymomas to produce and export mature naive CD4+ T cells. The identification of this T-cell subset in thymomas may help to identify patients who are MG(−) at the time of surgery but are at risk for MG after thymectomy.
Patients, materials, and methods
Patients
Approval was obtained by the biological and medical faculties of the University of Würzburg for these studies. Informed consent was provided according to the Declaration of Helsinki. Clinical data of the patients and control subjects in this study are summarized in Table1. Blood samples were taken at the time of thymectomy. Neither MG(+) nor MG(−) patients showed leukocytosis or lymphocytosis. WBC counts in MG(+) patients were 1000 to 11 600/μL (mean, 6620 μL), and lymphocyte counts were 857 to 3630/μL (mean, 2060 μL). WBC counts in MG(−) patients were 2300 to 9200/μL (mean, 6670 μL), and lymphocyte counts were 1260 to 3590/μL (mean, 2145). Only patients without steroid or immunosuppression treatment before surgery were included in this study.
Clinical data of patients
| Patient . | Case number . | Sex . | Age, y . | WHO thymoma subtype . | MG . | Follow-up, mo . |
|---|---|---|---|---|---|---|
| 1 | 94/24 864 | F | 75 | AB | Yes | 60 |
| 2 | 98/5 633 | M | 36 | AB | Yes | 30 |
| 3 | 99/13 575 | F | 39 | B2 | Yes | 21 |
| 4 | 95/1 064 | M | 61 | B2 | Yes | 5 |
| 5 | 00/25 907 | F | 58 | B2 | Yes | 13 |
| 6 | 00/22 717 | F | 74 | B2 | Yes | 10 |
| 7 | 00/20 223 | F | 50 | B2 of B3 | Yes | 10 |
| 8 | 99/21 994 | M | 65 | B2 | Yes | 20 |
| 9 | 99/3 983 | F | 70 | B2 | Yes | 18 |
| 10 | 95/15 091 | M | 41 | AB | No | 48 |
| 11 | 00/21 586 | M | 67 | AB | No | 10 |
| 12 | 95/12 211 | F | 62 | AB | No | 48 |
| 13 | 00/1 495 | F | 53 | AB | No | 19 |
| 14 | 01/4 054 | M | 50 | AB | No | 5 |
| 15 | 01/261 | F | 66 | AB | No | 6 |
| 16 | 93/23 169 | M | 39 | B2 | No | 70 |
| 17 | 00/3 045 | M | 51 | B2 | No | 12 |
| 18 | 97/6 335 | F | 83 | B2 | No | 22 |
| 19 | 01/5 447 | M | 58 | B2 | No | 1 |
| 20 | 98/5 186 | F | 69 | B2 + B3 | No | 5 |
| 21 | 01/6 600 | F | 70 | B2 + B3 | No | 4 |
| 22 | 97/20 308 | F | 68 | B2 + B3 | No | 26 |
| Patient . | Case number . | Sex . | Age, y . | WHO thymoma subtype . | MG . | Follow-up, mo . |
|---|---|---|---|---|---|---|
| 1 | 94/24 864 | F | 75 | AB | Yes | 60 |
| 2 | 98/5 633 | M | 36 | AB | Yes | 30 |
| 3 | 99/13 575 | F | 39 | B2 | Yes | 21 |
| 4 | 95/1 064 | M | 61 | B2 | Yes | 5 |
| 5 | 00/25 907 | F | 58 | B2 | Yes | 13 |
| 6 | 00/22 717 | F | 74 | B2 | Yes | 10 |
| 7 | 00/20 223 | F | 50 | B2 of B3 | Yes | 10 |
| 8 | 99/21 994 | M | 65 | B2 | Yes | 20 |
| 9 | 99/3 983 | F | 70 | B2 | Yes | 18 |
| 10 | 95/15 091 | M | 41 | AB | No | 48 |
| 11 | 00/21 586 | M | 67 | AB | No | 10 |
| 12 | 95/12 211 | F | 62 | AB | No | 48 |
| 13 | 00/1 495 | F | 53 | AB | No | 19 |
| 14 | 01/4 054 | M | 50 | AB | No | 5 |
| 15 | 01/261 | F | 66 | AB | No | 6 |
| 16 | 93/23 169 | M | 39 | B2 | No | 70 |
| 17 | 00/3 045 | M | 51 | B2 | No | 12 |
| 18 | 97/6 335 | F | 83 | B2 | No | 22 |
| 19 | 01/5 447 | M | 58 | B2 | No | 1 |
| 20 | 98/5 186 | F | 69 | B2 + B3 | No | 5 |
| 21 | 01/6 600 | F | 70 | B2 + B3 | No | 4 |
| 22 | 97/20 308 | F | 68 | B2 + B3 | No | 26 |
The diagnosis of MG was based on clinical features, decrement testing on 3 Hz serial stimulation, and detection of anti-AChR antibodies as described previously.13 All MG(+) patients were autoantibody positive. Among the MG(−) patients, data on anti-AChR titers were available only in 3 patients. Interestingly, one of these 3 MG(−) patients (patient 17) showed a high anti-AChR titer (9.8 nM) at the time of surgery, without the subsequent development of postsurgery MG (follow-up for 12 months).
Tumors and cell preparation
Thymomas were classified according to the recent WHO classification.6 For the thymic compartment, 13 nonneoplastic thymuses with inflammatory changes (thymic lymphofollicular hyperplasia [TFH]) were used as controls (henceforth designated as thymus controls). For the peripheral compartment, peripheral blood mononuclear cells (PBMCs) from 33 healthy sex- and age-matched donors were used as controls (henceforth referred to as blood controls). Before staining for fluorescence-activated cell sorter (FACS) analysis (see below), thymocytes and PBMCs from all samples were isolated by Ficoll-Hypaque density-gradient centrifugation as described in detail previously.13
Nomenclature of naive thymocyte subsets and T-cell subsets
In the thymus, T cells expressing either CD4 or CD8 together with CD3, CD69, and CD45 RA were designated as mature naive CD4 or CD8 T cells, respectively. In the peripheral blood, the term naive CD4 or CD8 T cells refers to T cells expressing CD4 or CD8 together with CD3 and CD45RA, but not CD69, which is lost on emigration from the thymus.20
Flow cytometry and cell separation procedures
Sampling and data analyses of thymocytes and PBMCs were performed on a FACScan flow cytometer with Lysis II software (Becton Dickinson, Heidelberg, Germany) as described.13 The CD3+ T-cell subset was gated for all analyses.
Cells (2 × 105) were stained for 3-color FACS analysis with a panel of surface antigen–directed monoclonal antibodies, as described previously.13 The panel of antibodies included anti-CD3 (phycoerythrin [PE]-labeled), anti-CD4 (fluorescein isothiocyanate [FITC]–labeled), and anti-CD8 (PE-labeled) (DAKO, Hamburg, Germany); anti-CD69 (either FITC- or PE-labeled) (Becton Dickinson); anti-CD45RO and anti-CD45RA (either FITC- or PE-labeled) (Dianova, Hamburg, Germany); and anti-CD3 (Tricolor-labeled) and the isotype control IgG2a (FITC-labeled) (Medac, Hamburg, Germany). Other isotype controls and anti-CD3 (FITC-labeled) were purchased from Sigma (Deisenhofen, Germany).
To further characterize a phenotypically unusual CD4+CD8−CD3high cell population detected in 3 MG(−) patients, CD8+ cells were removed with anti-CD8–coated cell recovery columns from the thymoma-derived thymocyte suspensions of the 3 patients and 3 control thymoma patients following the protocol of the manufacturer (Camon, Wiesbaden, Germany). The CD8-thymocyte suspension was then stained as described above for CD3, CD4, and a third marker of interest (CD1a, CD25, CD45RA, CD45RO, or CD69).
Confocal laser scanning microscopy
Immunofluorescence double staining of formalin-fixed, paraffin-embedded tissues was performed in 4 patients with combination of CD45RA and CD4 or CD8. Four-micrometer sections were dewaxed overnight in xylene. Indirect immunohistochemical staining was performed in the following order at room temperature: (1) blocking with 5% donkey serum (Dianova) for 20 minutes; (2) primary monoclonal antibody against CD4 (1:5) or CD8 (DAKO) (1:10 for 1 hour); (3) biotinylated goat anti–mouse IgG (Biogenex supersensitive; 1 hour); (4) Cy3-conjugated streptavidin (Jackson ImmunoResearch, West Grove, PA; 1 hour); (5) anti-CD45RA, directly conjugated with FITC (DAKO, 1 hour); (6) rabbit anti-FITC antibody (Molecular Probes, Eugene, OR; 1 hour); and (7) Cy2-conjugated donkey anti–rabbit antibody (Dianova; 1 hour). The latter 2 steps were necessary to reach adequate signal strength. For each fluorochrome labeling, negative and positive control sections were included. Confocal fluorescence images were obtained using a Leica TCS SP2 (Leica Microsystems, Heidelberg, Germany) system. Images were taken using a ×40 1.25 NA objective. Color photomicrographs were taken from the single fluorescences and from electronic overlays.
Statistical analyses
All statistical analyses were performed using χ2analysis and Mann-Whitney U test (P < .05, unless otherwise indicated) provided by the SPSS software (version 2000; SPSS, Chicago, IL). The Φ coefficient was used to determine correlations between different parameters (low, 0.01-0.40; moderate, 0.41-0.70; high, 0.71-1.00). In all column graphs shown, the statistical mean ± SEM are depicted.
Results
MG(−), but not MG(+), thymomas contain a highly reduced percentage of mature naive CD4 T cells
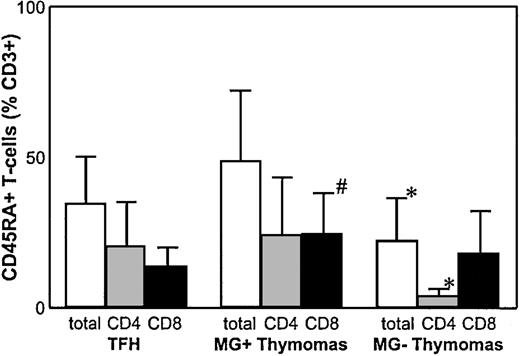
Analyzing thymocytes from 22 thymomas for CD3, CD4, CD8, and CD69 expression, we confirmed reports12-14 on impaired, but largely maintained, generation of all maturational stages of the CD4 and the CD8 lineages without apparent differences between MG(+) and MG(−) thymomas (data not shown). In MG(+) thymomas, the percentage of mature naive CD8+ T cells was statistically significantly (P = .01) increased compared with nonneoplastic thymus controls, whereas the percentage of total CD3+CD45RA+ cells and of mature naive CD4+ T cells was not significantly altered, resulting in a highly decreased CD4:CD8 ratio. In MG(−) thymomas, by stark contrast, the percentage of CD45RA+ thymocytes among the CD3+ subpopulation was highly significantly reduced compared with MG(+) thymomas (49.2% ± 23.1 vs 20.1 ± 15.2;P = .003) and thymus controls (P = .03). Further analysis of the naive CD4+ and CD8+subsets revealed that the percentage of the CD4+ subset was highly significantly reduced in all MG(−) thymomas analyzed compared with MG(+) thymomas and thymus controls (4.3 ± 2.0 in MG(−) thymomas vs 24.4 ± 18.9 in MG(+) thymomas; P < .0001; Table 2; Figure1). Thus, on χ2analysis, the presence of MG was significantly linked to high levels of mature naive CD4+ T cells (P < .01). However, our finding of 9.8% naive CD4+ T cells in the thymoma of the MG(−) patient 17, compared with the virtually identical percentages in MG(+) patients 1 and 2 (10.1% and 10.0%, respectively), show that a minor overlap between the 2 groups exists. This overlap extended to the CD8+ subset because several patients (eg, patients 9, 10, 12, 17) had marked reduction of mature naive CD8+ cells among MG(+) and MG(−) thymomas. In addition, this latter finding suggests for the first time that maturation not only of the CD4+ but also of the CD8+ lineage might be affected in a subset of thymomas (Table 2; Figure 2).
FACS analysis of CD45RA subset composition in thymomas of MG(+) and MG(−) patients, gated on CD3+ T cells
| Patient . | Case number . | WHO thymoma subtype . | MG . | CD4-CD8 ratio . | CD45RA+cells, % CD3 . | % CD45RA+ CD3+ . | |
|---|---|---|---|---|---|---|---|
| CD4 . | CD8 . | ||||||
| 1 | 94/24 864 | AB | Yes | 0.24 | 26.0 | 10.6 | 15.4 |
| 2 | 98/5 633 | AB | Yes | 1.8 | 32.6 | 10.0 | 22.6 |
| 3 | 99/13 575 | B2 | Yes | 1.2 | 52.0 | 26.7 | 25.3 |
| 4 | 95/1 064 | B2 | Yes | 1.2 | 52.1 | 24.4 | 27.7 |
| 5 | 00/25 907 | B2 | Yes | 0.58 | 41.7 | 27.1 | 14.6 |
| 6 | 00/22 717 | B2 | Yes | 0.57 | 48.8 | 12.1 | 36.8 |
| 7 | 00/20 223 | B2 of B3 | Yes | 0.79 | 93.7 | 71.4 | 22.3 |
| 8 | 99/21 994 | B2 | Yes | 3.0 | 74.5 | 22.7 | 51.7 |
| 9 | 99/3 983 | B2 | Yes | 4.2 | 21.7 | 14.6 | 7.1 |
| Average, patients 1-9 | 1.51 | 49.2 ± 23.1 | 24.4 ± 18.9 | 24.8 ± 13.2 | |||
| 10 | 95/15 091 | AB | No | 0.5 | 6.8 | 2.5 | 4.5 |
| 11 | 00/21 586 | AB | No | 0.3 | 46.9 | 5.6 | 41.4 |
| 12 | 95/12 211 | AB | No | 0.16 | 8.9 | 2.9 | 6.0 |
| 13 | 00/1 495 | AB | No | 0.11 | 12.7 | 2.7 | 10.0 |
| 14 | 01/4 054 | AB | No | 0.18 | 18.9 | 4.2 | 14.7 |
| 15 | 01/261 | AB | No | 0.26 | 24.0 | 3.7 | 20.7 |
| 16 | 93/23 169 | B2 | No | 0.45 | 16.1 | 4.5 | 11.6 |
| 17 | 00/3 045 | B2 | No | 1.8 | 17.6 | 9.8 | 7.8 |
| 18 | 97/6 635 | B2 | No | 0.06 | 46.9 | 2.4 | 44.5 |
| 19 | 01/5 447 | B2 | No | 0.26 | 27.0 | 5.2 | 22.1 |
| 20 | 98/5 186 | B2 + B3 | No | 0.12 | 13.2 | 3.1 | 10.1 |
| 21 | 01/6 600 | B2 + B3 | No | 0.25 | 14.1 | 4.3 | 9.9 |
| 22 | 97/20 308 | B2 + B3 | No | 0.16 | 41.4 | 5.1 | 36.3 |
| Average, patients 10-22 | 0.35 | 20.1 ± 15.2 | 4.3 ± 2.0 | 18.4 ± 13.7 | |||
| 23-35 thymus controls | TFH | Yes | 1.0-3.3 | 17.6-55.6 | 8.1-32.8 | 9.5-22.2 | |
| Average, patients 23-35 thymus controls | 2.5 | 33.7 ± 12.7 | 19.1 ± 10.6 | 12.9 ± 3.9 | |||
| Patient . | Case number . | WHO thymoma subtype . | MG . | CD4-CD8 ratio . | CD45RA+cells, % CD3 . | % CD45RA+ CD3+ . | |
|---|---|---|---|---|---|---|---|
| CD4 . | CD8 . | ||||||
| 1 | 94/24 864 | AB | Yes | 0.24 | 26.0 | 10.6 | 15.4 |
| 2 | 98/5 633 | AB | Yes | 1.8 | 32.6 | 10.0 | 22.6 |
| 3 | 99/13 575 | B2 | Yes | 1.2 | 52.0 | 26.7 | 25.3 |
| 4 | 95/1 064 | B2 | Yes | 1.2 | 52.1 | 24.4 | 27.7 |
| 5 | 00/25 907 | B2 | Yes | 0.58 | 41.7 | 27.1 | 14.6 |
| 6 | 00/22 717 | B2 | Yes | 0.57 | 48.8 | 12.1 | 36.8 |
| 7 | 00/20 223 | B2 of B3 | Yes | 0.79 | 93.7 | 71.4 | 22.3 |
| 8 | 99/21 994 | B2 | Yes | 3.0 | 74.5 | 22.7 | 51.7 |
| 9 | 99/3 983 | B2 | Yes | 4.2 | 21.7 | 14.6 | 7.1 |
| Average, patients 1-9 | 1.51 | 49.2 ± 23.1 | 24.4 ± 18.9 | 24.8 ± 13.2 | |||
| 10 | 95/15 091 | AB | No | 0.5 | 6.8 | 2.5 | 4.5 |
| 11 | 00/21 586 | AB | No | 0.3 | 46.9 | 5.6 | 41.4 |
| 12 | 95/12 211 | AB | No | 0.16 | 8.9 | 2.9 | 6.0 |
| 13 | 00/1 495 | AB | No | 0.11 | 12.7 | 2.7 | 10.0 |
| 14 | 01/4 054 | AB | No | 0.18 | 18.9 | 4.2 | 14.7 |
| 15 | 01/261 | AB | No | 0.26 | 24.0 | 3.7 | 20.7 |
| 16 | 93/23 169 | B2 | No | 0.45 | 16.1 | 4.5 | 11.6 |
| 17 | 00/3 045 | B2 | No | 1.8 | 17.6 | 9.8 | 7.8 |
| 18 | 97/6 635 | B2 | No | 0.06 | 46.9 | 2.4 | 44.5 |
| 19 | 01/5 447 | B2 | No | 0.26 | 27.0 | 5.2 | 22.1 |
| 20 | 98/5 186 | B2 + B3 | No | 0.12 | 13.2 | 3.1 | 10.1 |
| 21 | 01/6 600 | B2 + B3 | No | 0.25 | 14.1 | 4.3 | 9.9 |
| 22 | 97/20 308 | B2 + B3 | No | 0.16 | 41.4 | 5.1 | 36.3 |
| Average, patients 10-22 | 0.35 | 20.1 ± 15.2 | 4.3 ± 2.0 | 18.4 ± 13.7 | |||
| 23-35 thymus controls | TFH | Yes | 1.0-3.3 | 17.6-55.6 | 8.1-32.8 | 9.5-22.2 | |
| Average, patients 23-35 thymus controls | 2.5 | 33.7 ± 12.7 | 19.1 ± 10.6 | 12.9 ± 3.9 | |||
Percentage of mature naive CD4++ T cells is highly reduced in MG(−) thymomas.
FACS analysis of naive thymocyte subsets shows a highly significant reduction of mature naive CD4+ T cells in MG(−) thymomas compared with MG(+) thymoma patients and nonneoplastic thymus controls and a significantly increased percentage of mature naive CD8+ T cells in MG(+) thymomas. *Statistically significant alteration in comparison with MG(+) thymomas and thymus controls. #Statistically significant alteration compared with thymus controls.
Percentage of mature naive CD4++ T cells is highly reduced in MG(−) thymomas.
FACS analysis of naive thymocyte subsets shows a highly significant reduction of mature naive CD4+ T cells in MG(−) thymomas compared with MG(+) thymoma patients and nonneoplastic thymus controls and a significantly increased percentage of mature naive CD8+ T cells in MG(+) thymomas. *Statistically significant alteration in comparison with MG(+) thymomas and thymus controls. #Statistically significant alteration compared with thymus controls.
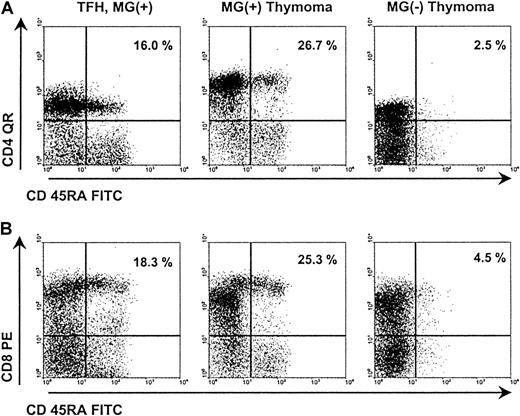
Representative results of FACS analysis of MG(+) and MG(−) thymomas.
FACS analysis of naive CD4+ and CD8+ subsets in a nonneoplastic thymus control (patient 24), a WHO type B2 thymoma with MG (patient 3), and a WHO type AB thymoma without MG (patient 10). Both subsets are highly reduced in the MG(−) thymoma compared with the control thymus and the MG(+) thymoma.
Representative results of FACS analysis of MG(+) and MG(−) thymomas.
FACS analysis of naive CD4+ and CD8+ subsets in a nonneoplastic thymus control (patient 24), a WHO type B2 thymoma with MG (patient 3), and a WHO type AB thymoma without MG (patient 10). Both subsets are highly reduced in the MG(−) thymoma compared with the control thymus and the MG(+) thymoma.
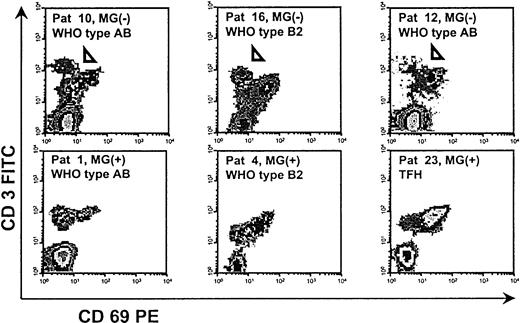
Some MG(−), but not MG(+), thymomas or control thymuses contain a peculiar thymocyte population with a CD4+CD8−CD3high phenotype
Three of 13 MG(−) thymomas (2 WHO type AB and 1 type B2) but none of the MG(+) thymomas contained a peculiar population of CD3high thymocytes that were CD4+ and CD8−. This population has hitherto not been described in the human thymus and was also absent from all nonneoplastic thymuses studied (Figure 3). The cells were small by forward and side scatter (not shown). To further characterize this population, we removed CD8+ thymocytes from thymoma-derived thymocyte suspensions of the 3 patients through anti-CD8 cell depletion columns and then stained for a marker of interest in addition to CD3 and CD4. The CD3high T cells were negative for CD69 (Figure3), nonactivated, and negative (CD25−) for all other surface molecules tested, in particular CD1a, and for CD45RA and CD45RO (not shown).
Detection of a peculiar, presumably apoptotic, CD4+CD3highCD69− thymocyte population in 3 MG(−) thymomas (arrowheads).
FACS analysis after depletion of CD8+ cells. All other applied surface markers, including CD1a, CD25, CD45RA, and CD45RO, were negative (not shown).
Detection of a peculiar, presumably apoptotic, CD4+CD3highCD69− thymocyte population in 3 MG(−) thymomas (arrowheads).
FACS analysis after depletion of CD8+ cells. All other applied surface markers, including CD1a, CD25, CD45RA, and CD45RO, were negative (not shown).
CD45RA+CD4+ T cells are reduced in the blood of thymoma patients without MG
Having shown that MG status was significantly associated with distinct abnormalities of intratumorous thymopoiesis (Figures 1 and 2), we next asked whether the observed differences in thymopoiesis might be mirrored by respective abnormalities in the peripheral blood. To this end, we compared the percentages of CD4+ and CD8+ T cells among the naive CD45RA+ subset in the blood of thymoma patients and of MG(+) patients with nonneoplastic thymuses to age- and sex-matched blood controls (Table3; Figure4). This strategy has been shown to be a valid method to indirectly assess T-cell export from the thymus and presumably from thymomas.9 21 Compared with blood controls, MG(+) and MG(−) thymoma patients showed a statistically significant increase of the naive CD8+ subset (P = .009 and P = .002, respectively) and a decreased CD4-CD8 ratio (P = .01 andP = .006, respectively). Both alterations were slightly more prominent in MG(−) than in MG(+) patients. By contrast, the percentage of naive CD4+ T cells was significantly reduced only in MG(−) (P = .02), but not in MG(+), patients (P = .34) (Figure 4). Together, these data clearly indicate that virtually all thymomas contribute to the naive peripheral T-cell pool by export of mature naive T cells. This conclusion was further supported by a moderate positive correlation between percentages of naive CD4+ T cells in the thymoma and the peripheral blood of MG(+) patients (r = 0.59). However, presence of MG appears to be critically associated only with the export of mature naive CD4+, but not CD8+, T cells from the thymoma into the peripheral compartment.
Summary of FACS analyses of naive T-cell subsets
| Patients . | Diagnosis . | MG . | CD4-CD8 ratio (range) . | CD45RA+ cells, % CD3 . | CD3+CD45RA+ . | |
|---|---|---|---|---|---|---|
| CD4+ . | CD8+ . | |||||
| 1-9 | Thymoma | Yes | 2.6 (0.7-11.0)3-150 | 63.2 ± 17.4 | 31.0 ± 10.8 | 32.2 ± 16.73-150 |
| Control | No | 4.9 (2.0-8.3) | 57.1 ± 16.6 | 39.4 ± 16.1 | 14.5 ± 9.1 | |
| 10-22 | Thymoma | No | 2.6 (0.43-12.6)3-150 | 61.9 ± 25.4 | 25.4 ± 10.33-150 | 36.5 ± 18.93-150 |
| Control | No | 4.6 (1.9-9) | 51.2 ± 17.3 | 37.0 ± 15.7 | 14.0 ± 6.8 | |
| 23-35 | TFH | Yes | 2.3 (0.6-6.0)3-150 | 64.2 ± 15.6 | 37.3 ± 18.8 | 26.9 ± 10.2 |
| Control | No | 2.3 (1.0-4.3) | 76.0 ± 9.6 | 41.1 ± 10.6 | 34.9 ± 11.4 | |
| Patients . | Diagnosis . | MG . | CD4-CD8 ratio (range) . | CD45RA+ cells, % CD3 . | CD3+CD45RA+ . | |
|---|---|---|---|---|---|---|
| CD4+ . | CD8+ . | |||||
| 1-9 | Thymoma | Yes | 2.6 (0.7-11.0)3-150 | 63.2 ± 17.4 | 31.0 ± 10.8 | 32.2 ± 16.73-150 |
| Control | No | 4.9 (2.0-8.3) | 57.1 ± 16.6 | 39.4 ± 16.1 | 14.5 ± 9.1 | |
| 10-22 | Thymoma | No | 2.6 (0.43-12.6)3-150 | 61.9 ± 25.4 | 25.4 ± 10.33-150 | 36.5 ± 18.93-150 |
| Control | No | 4.6 (1.9-9) | 51.2 ± 17.3 | 37.0 ± 15.7 | 14.0 ± 6.8 | |
| 23-35 | TFH | Yes | 2.3 (0.6-6.0)3-150 | 64.2 ± 15.6 | 37.3 ± 18.8 | 26.9 ± 10.2 |
| Control | No | 2.3 (1.0-4.3) | 76.0 ± 9.6 | 41.1 ± 10.6 | 34.9 ± 11.4 | |
Data based on the peripheral blood of MG(+) and MG(−) thymoma patients and MG(+) patients with nonneoplastic thymuses (TFH) compared with the respective sex- and age-matched control cohort. For detailed graphical overview of data, see Figure 4.
Statistically significant alteration.
Naive CD4+ are significantly decreased in the peripheral blood of MG(−) thymoma patients.
FACS analysis of naive T-cell subsets in the peripheral blood of 9 MG(+) and 12 MG(−) thymoma patients and of 13 MG(+) patients with nonneoplastic thymuses compared to age- and sex-matched healthy control subjects (missing data for MG(−) patient 14). Statistically significant increase of naive CD8+ T cells in MG(+) and MG(−) thymoma patients, with a statistically significant decrease of naive CD4+ T cells only in MG(−) thymoma patients. There was no statistically significant alteration in the blood of MG(+) patients with nonneoplastic thymuses, though there was a trend toward reduction of the naive CD8+ subset (grey lines, regression lines for values of control subjects).
Naive CD4+ are significantly decreased in the peripheral blood of MG(−) thymoma patients.
FACS analysis of naive T-cell subsets in the peripheral blood of 9 MG(+) and 12 MG(−) thymoma patients and of 13 MG(+) patients with nonneoplastic thymuses compared to age- and sex-matched healthy control subjects (missing data for MG(−) patient 14). Statistically significant increase of naive CD8+ T cells in MG(+) and MG(−) thymoma patients, with a statistically significant decrease of naive CD4+ T cells only in MG(−) thymoma patients. There was no statistically significant alteration in the blood of MG(+) patients with nonneoplastic thymuses, though there was a trend toward reduction of the naive CD8+ subset (grey lines, regression lines for values of control subjects).
The percentage of CD45RA+ T cells was not increased in the peripheral blood of patients with TFH compared with healthy age- and sex-matched control persons (P = .17), and there were no statistically significant alterations with respect to the percentage of naive CD4+ and CD8+ T cells (P = .74 and P = .06, respectively). However, there was a trend toward a reduction of peripheral naive CD8+ T cells in patients with TFH (Figure 4), and the percentages of intrathymic and peripheral naive CD8+ T cells showed a moderate statistical correlation (r = 0.43).
Clinical course after thymectomy
The clinical course after thymectomy was followed for up to 66 months in all 22 thymoma patients. In patients with MG at the time of surgery, thymectomy in combination with immunosuppression generally resulted in amelioration of myasthenic symptoms (9 of 9 patients), but complete cure without the requirement for medication was not achieved in any patient (follow-up 5-60 months). None of the patients without MG at the time of thymectomy acquired postsurgery MG (follow-up 1 to 70 months), though this is a well-known but rare complication in primarily MG(−) thymomas22-25 and though one MG(−) patient (patient 17) had high anti-AChR antibody titers.
Detection of intratumorous CD45RA+CD4+thymocytes in a thymoma patient with postsurgery MG
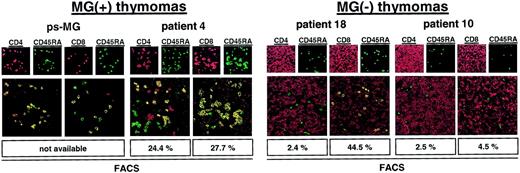
Among the thymoma patients studied here by flow cytometry, none acquired MG after thymoma resection (postsurgery MG [ps-MG]). In addition, among MG(−) thymomas, only one patient (patient 17) maintained production of mature naive CD4+ thymocytes. The latter finding is surprising because the occurrence of ps-MG has been taken as circumstantial evidence that thymomas might start to generate and export mature autoreactive T cells long before MG develops.5 7 To address this question, we checked our files for thymoma patients with ps-MG (2 in 532 patients). Paraffin-embedded tissue was available in one of them, but frozen material was not available in either. We applied double-immunofluorescence laser scanning microscopy to analyze this material and 3 exemplary thymomas from the FACS series (patient 4, MG(+) thymoma with a high percentage of CD4+ and CD8+ mature naive thymocytes; patient 18, MG(−) thymoma with a high percentage of mature naive CD8+ cells, but strong reduction of naive CD4+ cells; patient 10, MG(−) thymoma with virtual absence of both naive T-cell subsets; Table 2). Moreover, 3 nonneoplastic thymuses were stained as controls. Results of the immunofluorescence investigations (Figure5) were highly concordant with the FACS data and showed abundant numbers of mature naive CD4+thymocytes inside nonneoplastic thymuses and MG(+) thymomas and a significantly reduced percentage of naive CD4+ thymocytes in MG(−) thymomas. In the patient with ps-MG, naive CD4+thymocytes were as frequent as in the patient with MG(+) thymoma. These preliminary findings suggest that the presence of mature naive CD4+ T cells in MG(−) thymomas is rare but might be predictive of the risk for ps-MG. Of note, immunofluorescence microscopy findings in a given section were remarkably homogeneous throughout at least 20 microscopic fields studied per patient, indicating that thymopoietic incompetence during the very late stage of thymopoiesis, as indicated by FACS (Table 2), was an evenly distributed malfunction in a given tumor. We did not detect “hot spots” along with either well-preserved or highly reduced terminal thymopoiesis. This observation suggests that incompetence for terminal thymopoiesis in MG(−) thymomas is probably related to an early genetic event in the clonal neoplastic thymic epithelial cells of thymomas.
Naive CD4+ T cells are present in a thymoma with ps MG.
Confocal laser scanning microscopy analysis of the thymomas of a patient with ps-MG and of a representative patient with development of MG before thymoma detection showing abundant naive CD4+ T cells in both tumors. By contrast, naive CD4+ T cells were almost undetectable in 2 MG(−) thymomas. Moreover, naive CD8+ T cells were virtually absent in one MG(−) thymoma (patient 10). Sections were double stained with either CD4-Cy3 (red) and CD45RA-Cy2 (green) or CD8-Cy3 (red) and CD45RA-Cy2 (green). Cells with double staining show a yellow fluorescence in electronic overlays of the red and green images. Percentages of naive T-cell subsets as determined by flow cytometry (Table 2) are given at the bottom of each panel for comparison. Images are representative of at least 20 microscopic high-power fields studied per double staining in each patient. Original magnification, × 400.
Naive CD4+ T cells are present in a thymoma with ps MG.
Confocal laser scanning microscopy analysis of the thymomas of a patient with ps-MG and of a representative patient with development of MG before thymoma detection showing abundant naive CD4+ T cells in both tumors. By contrast, naive CD4+ T cells were almost undetectable in 2 MG(−) thymomas. Moreover, naive CD8+ T cells were virtually absent in one MG(−) thymoma (patient 10). Sections were double stained with either CD4-Cy3 (red) and CD45RA-Cy2 (green) or CD8-Cy3 (red) and CD45RA-Cy2 (green). Cells with double staining show a yellow fluorescence in electronic overlays of the red and green images. Percentages of naive T-cell subsets as determined by flow cytometry (Table 2) are given at the bottom of each panel for comparison. Images are representative of at least 20 microscopic high-power fields studied per double staining in each patient. Original magnification, × 400.
Discussion
In this article, we demonstrate for the first time a significant qualitative difference in intratumorous thymocyte maturation between MG(+) and MG(−) thymomas of the most frequent histologic subtypes.6,7 In MG(−) thymomas, the percentage of mature naive CD4+ T cells20 was highly significantly reduced, whereas this subset was present in all MG(+) thymomas at percentages comparable to those of control thymuses. Normal numbers of naive CD4+ T cells were also detectable in the thymoma of a single patient in whom ps-MG developed. Thus, our findings directly link the presence of paraneoplastic MG in patients with WHO type AB and B thymomas with the intratumorous production of mature naive CD4 T cells. A further finding in a substantial subset of patients with MG(−), but also in one with MG(+), thymoma was a high reduction in the naive CD8 T-cell subset, suggesting for the first time that the spectrum of thymopoietic incompetence in thymomas may extend not only to the CD4 but also to the CD8 lineage.12-15
Although the exact mechanisms for the defective T-cell maturation in MG(−) thymomas are unknown, it may be of interest that 3 of the 13 MG(−) thymomas harbored a phenotypically distinct CD4+thymocyte population that we were unable to identify in the normal thymus or in MG(+) thymomas (Figure 3). Because the immunophenotype of this abnormal population (CD3high, CD1a−, CD45RA−, CD25−, CD69−) shares similarity with the phenotype of dying thymocytes after CD3- and CD2-triggered apoptosis,26 it is possible that the lack of terminally mature CD4+ T cells in this subset of MG(−) thymomas was caused by efficient intra-tumorous negative selection, as shown in the mouse.27 Conversely, inefficient negative selection in MG(+) thymomas explains the presence of terminally mature T cells and the enrichment for autoreactive T cells in MG(+) thymomas.11,28 In this regard, our results suggest that the transition from the CD45RA− to the CD45RA+stage is not only of pivotal significance for the acquisition of mature T-cell effector functions,20 but it may mark a checkpoint during late T-cell development analogous to the well-characterized checkpoints during early thymopoiesis.29-31 Because knowledge is limited about the mechanisms regulating terminal stages of thymopoiesis,30 31 thymomas appear to be a promising model to study the regulation of these late stages of T-cell development in humans.
The observed reduction of intratumorous mature naive CD4+ T cells in MG(−) thymomas explains former observations with proliferation assays that MG(+), but not MG(−) thymomas are enriched for autoreactive T cells9,11 because only terminally mature CD4+ thymocytes can significantly proliferate after T-cell receptor–mediated signaling.20 Finally, our findings explain the clinical observation that thymoma subtypes with minimal or absent intra-tumorous thymopoiesis7,9,12 are rarely (WHO type A) or not at all (WHO type C) associated with MG.15,32-34 However, given that even type A thymomas with minimal thymopoiesis can be associated with MG and other autoimmune diseases,7 we cannot exclude the possibility that the sensitization of mature T cells in the peripheral blood may play a key role in the pathogenesis of autoimmunity in these tumors.
In the second part of the investigation, we asked whether the observed differences in thymopoiesis between MG(+) and MG(−) thymomas were reflected by alterations of the naive T-cell pool in the peripheral blood. In line with the generally undisturbed maturation of the CD8 lineage in both MG(+) and MG(−) thymomas (Table 2), the percentage of peripheral blood naive CD8+ T cells was significantly increased in MG(+) and MG(−) patients compared with healthy age- and sex-matched control persons. By contrast, there was a statistically significant reduction of the percentage of the peripheral naive CD4+ T-cell subset only in MG(−), but not in MG(+) patients (Table 3). Although both alterations, increases in naive CD8+ T cells,9,10 and decreases in naive CD4+ T cells in MG(−) patients compared with MG(+) patients10 have been described recently in the blood of thymoma patients, ours is the first report to directly relate skewing of the peripheral pool of naive CD4+ and CD8+T-cell subsets to corresponding alterations in intratumorous thymopoiesis. A recent study using TREC analysis clearly demonstrated that CD4+and CD8+ thymocytes are exported from MG(+) thymomas in considerable quantities,10 but the percentage of naive CD45RA+ T cells in our present study and a previous study9 was increased only slightly in the peripheral blood of MG(+) and MG(−) patients. Even more puzzling was our finding that the percentage of peripheral naive CD4+ T cells in MG(−) patients was decreased and was not maintained at control levels. These findings can be explained by previous observations showing that the size of the naive T-cell pool and its composition are differentially regulated. Specifically, the absolute size of the peripheral naive T-cell pool is tightly regulated by peripheral mechanisms, irrespective of the CD4 or CD8 subset composition, as previously suggested by thymectomy studies in humans35 and by transplantation of thymic lobes in mice.36,37 By contrast, the CD4-CD8 ratio of the naive T-cell pool is mainly affected by central mechanisms (ie, thymic output), as shown for HIV patients receiving antiretroviral therapy16 and for mice that received transplants of congenic thymuses.36 37
With respect to the clinical history of MG after thymoma surgery, follow-up of the MG(+) patients included in this study showed the typical course with slow, moderate amelioration but not with complete resolution of the myasthenic symptoms over years. By contrast, none of the MG(−) patients acquired MG after surgery, even though MG(−) patient 17 had a high percentage of intra-tumorous mature naive CD4+ T cells and a high anti-AChR titer. Both alterations in this patient were indicative of a high risk for autoimmune symptoms and strongly suggested that thymectomy preceded the potential manifestation of clinical MG. Furthermore, the clinical course in this patient indicates that individual peripheral tolerance mechanisms might be important in the control of MG. To explain the observed postsurgical course of MG, we hypothesize that only thymomas that skew the peripheral naive CD4+ T-cell pool to an extent overruling peripheral tolerance mechanisms are associated with MG. Once disturbance of the naive peripheral CD4+ T-cell pool has led to autoimmunity, surgical removal of the source of the autoreactive T cells will not lead to significant clinical improvement because the peripheral compartment is not affected by this measure.35This break in peripheral tolerance apparently can occur even after thymoma removal, as suggested by our patient with ps-MG.
In summary, our findings provide new conceptual insight into the pathogenesis of paraneoplastic MG. A maintained but nontolerogenic intra-tumorous T-cell maturation appears to be a prerequisite for MG development, whereas more profound disturbances with the abrogation of intra-tumorous thymopoiesis interfere with autoimmunization. In particular, our findings highlight the pivotal role of thymoma-derived CD4+ T cells in the pathogenesis of paraneoplastic MG. A comparison of MG(+) and MG(−) thymomas may give clues to the molecular mechanisms that govern terminal T-cell maturation in humans.
We thank Andrea Homburger, Sonja Rotzoll, Elke Oswald, Sabine Roth, and Erwin Schmidt for expert technical assistance.
Supported by DFG grant FOR 303/2 (P.S., A.M.), BMBF 2000 grant IZKF 01 KS 903/C5 (V.H.), the National Health and Medical Research Council of Australia, and the Ian Potter Foundation (S.R.L.).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Philipp Ströbel, Institute of Pathology, University of Wuerzburg, Josef-Schneider-Strasse 2, 97080 Wuerzburg, Germany; e-mail: path036@mail.uni-wuerzburg.de.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal