Abstract
Because tumor-specific antigens have been identified in multiple myeloma (MM), immunotherapy might provide an additional treatment modality for the disease. Expression of CD40 ligand (CD40L) proximate to the MM cells might serve this purpose, either by increasing their capacity to present self-antigens by activation through their CD40 receptor or by the recruitment of professional antigen-presenting cells (APCs) able to take up and present tumor-associated antigens. To distinguish between these possibilities and predict whether human CD40− myeloma might respond to this approach, we examined 3 murine plasmacytoma cell lines, 2 (MPC-11 and S107) expressing the CD40 molecule and 1 (X-24) lacking such expression. Syngeneic BALB/CBYJ mice were inoculated subcutaneously with tumor cells mixed with CL7.1 fibroblasts, retrovirally transduced to express either the mCD40L or the neo gene. For all 3 plasmacytoma cell lines, coinjection with CL7.1/mCD40L significantly reduced local tumor growth compared with controls. This effect was mediated by a systemic antitumor immune response, since mice immunized with tumor and CL7.1/mCD40L were resistant to subsequent challenge with tumor, and tumor growth inhibition was abolished when CD8+or CD4+ lymphocytes were depleted. Because expression of CD40L gave equivalent protection from CD40+ and CD40− tumors and transgenic-CD40L failed to up-regulate costimulatory molecules in either tumor, the protective effects of CD40L probably resulted from recruitment/activation of professional APCs rather than from CD40 activation of plasmacytoma cells. As further support of this concept, we found that mice were also well protected if CL7.1 and CD40L were injected together with apoptotic plasmacytoma cells from these tumors. Hence, transgenic CD40L expression may produce an antimyeloma immune response against either CD40+ or CD40− tumors and may be of therapeutic value for both types of myeloma in humans.
Introduction
High-dose chemotherapy followed by autologous stem cell transplantation has improved the overall survival of multiple myeloma (MM) patients.1,2 However, almost all patients achieving complete remission ultimately experience relapse,1,2 and immunotherapy may represent a means of maintaining complete remission. Several antigens have been identified as potential targets of T- and/or B-cell immune responses in MM, including the idiotype of monoclonal immunoglobulin secreted by malignant plasma cells,3-5 the underglycosylated form of MUC-1,6 and antigens encoded by the MAGE-type genes and by the FRAME gene.7
Most clinical studies of immunotherapy for MM have targeted the specific idiotype.8-11 This specific tumor-associated antigen has been used directly as a protein8 or DNA vaccine9 or used to pulse dendritic cells in vitro.10 Although this strategy can induce an anti-idiotype immune response, little cytotoxic-effector function against myeloma cells is recruited.8-11 Whole tumor cells, rather than single antigens, may be more likely to recruit a potent and efficient cytotoxic antitumor response, because they may provide both class I and class II major histocompatibility complex (MHC)–restricted antigenic epitopes, allowing interaction between CD4+ and CD8+ T cells.12 To favor recruitment and activation of immune effector cells, tumor cells have been genetically modified to produce immunomodulatory molecules, such as granulocyte-macrophage colony-stimulating factor or interleukin-2 (IL-2).13-15 Paracrine production of these molecules results in local recruitment of professional antigen-presenting cells (APCs)16 that process tumor antigens derived from the irradiated tumor cells injected as the vaccine and help generate a systemic antitumor immune response.13-16 Other immunostimulatory molecules may be equally effective. CD40 ligand (CD40L, CD154) is a member of the tumor necrosis factor gene family; it is expressed mainly on antigen-activated CD4+ T lymphocytes and plays a crucial role initiating antigen-specific cytotoxic T-cell response.17 In addition, the interaction between CD40L and its receptor (CD40) expressed on the surface of professional APCs, induces these cells to mature, enabling them to directly recruit and activate CD8+ T lymphocytes.17-20 For this reason, transgenic expression of CD40L by tumor cells is currently being explored as an antitumor vaccine.21-23
Transgenic expression of CD40L may also directly affect tumor cells themselves. Tumor cells may fail to present antigens because they lack the costimulatory molecules (such as B7-1/CD80 and B7-2/CD86) necessary for activation of the T-cell receptor complex.24Up-regulation of these costimulatory molecules on the surface of tumor cells may therefore enhance their immunogenicity.24,25Many B-cell tumors, including follicular lymphoma, lymphoblastic leukemia, chronic lymphocytic leukemia (CLL), and some MMs, express the CD40 antigen on their surface; in lymphoma/CLL cells, the engagement of CD40L has been shown to increase CD80/B7.1 and CD86/B7.2 expression, up-regulate HLA class II molecules and cell-adhesion molecules, and enhance their antigen-presenting capability.21 26-28
Because CD40L may enhance antigen presentation both by tumor cells and by professional APCs, we investigated whether transgenic CD40L expression in a murine model of MM was protective. Because human MM may be either CD40+ or CD40−,29 we also determined whether enhanced antigen presentation by the tumor cell itself was critical for any of the protective effects mediated by CD40L or whether these benefits were mediated predominantly by its actions on professional APCs.
Materials and methods
Cell lines and cultures
In this study, 3 murine plasmacytoma cell lines were used. The MPC-11 (167-CCL) cell line, obtained from the American Type Culture Collection (Rockville, MD), was cultured in Dulbecco modified Eagle medium (DMEM) (BioWhittaker, Walkersville, MD) supplemented with 10% heat-inactivated horse serum and 4 mM l-glutamine (all from BioWhittaker). The S107 and X-24 cell lines (kindly provided by Dr Stuart Rudikoff, National Cancer Institute, Bethesda, MD), were cultured in RPMI-1640 (BioWhittaker) supplemented with 10% heat-inactivated fetal calf serum (FCS) (Summit Biotechnology, Fort Collins, CO), 2 mM l-glutamine, 25 IU/mL penicillin, 25 mg/mL streptomycin (all from BioWhittaker), and 50 μM β-mercaptoethanol (Sigma, St Louis, MO). The fibroblast cell line CL7.1 (TIB-80) was purchased from the American Type Culture Collection and cultured in DMEM supplemented with 10% heat-inactivated FCS, 2 mMl-glutamine, 25 IU/mL penicillin, and 25 mg/mL streptomycin. Cells were maintained in a humidified atmosphere containing 5% CO2 at 37°C.
Vectors and fibroblast transduction
The fibroblasts were transduced with the retroviral vector pG1amCD40L, containing the mCD40L complementary DNA (cDNA), or with the retroviral vector G1Na, containing the neomycin phosphotransferase gene (neo), as previously described.21 Briefly, transduction with supernatant collected from the producer cell line (BOSC) transfected with the plasmid pG1amCD40L was performed at 37°C in 5% CO2 in the presence of 6 μg/mL polybrene. Fibroblasts were then selected for mCD40L expression by fluorescence-activated cell sorting (FACS). We used fibroblasts stably expressing mCD40L on more than 60% of cells.21 To transduce the neo gene, fibroblasts were cultured with supernatant containing the G1Na vector and selected in the presence of G418 sulfate (GIBCO, Gaithersburg, MD) at an active concentration of 1 mg/mL.
Animal experiments
In vivo experiments were performed in female BALB/CBYJ mice aged 8 to 10 weeks (The Jackson Laboratory, Bar Harbor, ME) syngeneic with all 3 plasmacytoma cell lines and with the CL7.1 fibroblast cell line. Animals were housed under specific pathogen-free conditions and treated according to the Baylor College of Medicine (Houston, TX) animal husbandry guidelines.
In vivo tumor growth
To assess the effect of CD40L transgene expression on tumor growth, mice were inoculated with 2 × 106 MPC-11 or S107 cells mixed with 1 × 106 CL7.1/mCD40L or CL7.1/neo fibroblasts (ratio, 2:1) on day 1. Fibroblasts were γ-irradiated (1000 cGy) immediately before injection. Because unmodified MPC-11 and S107 plasmacytoma cell lines are highly aggressive in vivo,30 31 cells were sublethally γ-irradiated (3500 cGy) immediately before injection. The X-24 cell line has lower tumorigenicity compared with MPC-11 and S107 cell lines and was administered in correspondingly higher numbers (4 to 6 × 106 cells per animal) and without irradiation. The tumor cell/fibroblast mixture was washed in phosphate-buffered saline (PBS) (GIBCO BRL, Grand Island, NY), resuspended in 200 μL final volume, and injected subcutaneously (SC) into the right leg. Tumor growth was monitored 2 or 3 times a week by measuring the 2 maximum diameters of the tumor at the site of inoculation with a caliper and was reported as a mean of the 2 diameters. In accordance with the Animal Husbandry Committee Guidelines of the Animal Facility at Baylor College of Medicine, the animals were killed when the tumor size was larger than 10% of the animal's body weight or when the tumor induced distress or ulceration.
In vivo T-lymphocyte depletion
To evaluate the role of T lymphocytes in reducing tumor burden, mice were depleted of T lymphocytes by intraperitoneal (IP) inoculation of Gk1.5 (anti-CD4) and 2.43 (anti-CD8) monoclonal antibodies (mAbs) kindly provided by Dr Peter Doherty (St Jude Children's Research Hospital, Memphis, TN). The mAbs were inoculated IP with sublethally irradiated plasmacytoma cells mixed with CL7.1/mCD40L the day before the injection and then twice a week for 4 weeks. This protocol depletes more than 98% of the relevant T-cell subset in mice,21 an efficiency confirmed in these studies by FACS analysis of isolated splenocytes obtained from euthanized animals.
Tumor-challenge studies
Mice were inoculated SC with CL7.1/mCD40L fibroblasts mixed with sublethally irradiated plasmacytoma cell lines on the right leg on day 1 and in the opposite leg on day 14. At 2 weeks after the second injection, these animals were challenged on the back with the corresponding live plasmacytoma cell line. We used 5 × 105 cells per mouse for MPC-11 and S107 cell lines and 2 to 6 × 106 cells per mouse for the X-24 cell line.30 31 As a control, an equal number of live cells were injected in the backs of nonimmunized animals. Tumor growth was followed by measurement of the 2 maximum diameters of the tumor at the site of challenge, and mice were killed when the tumor size was larger than 10% of the animal's body weight or had induced distress or ulceration. To evaluate the specificity of the immune response, we also performed cross-challenge experiments, in which animals vaccinated with S107 cells mixed with CL7.1/mCD40L fibroblasts were subsequently challenged with live MPC-11 cells.
Vaccination with apoptotic/necrotic tumor cells
Apoptotic tumor cells were also used as a source of tumor-associated antigens for immunization. For all plasmacytoma cell lines, apoptotic tumor cells were obtained after exposure to UV irradiation for 60 seconds and culture overnight in serum-free conditions. Apoptotic cells obtained from a starting culture of 2 × 106 tumor cells were washed in PBS, combined with either CL7.1/mCD40L fibroblasts or CL7.1/neo fibroblasts (ratio, 2:1), resuspended in 200 μL final volume, and injected SC in the right leg on day 1. At 2 weeks later, these animals received a second, identical SC injection in the opposite leg and, on day 28, were challenged on the back with live tumor cells as described above. Tumor growth was followed by measurement of the 2 maximum diameters of the tumor at the site of challenge. Mice were killed when the tumor size was larger than 10% of the animal's body weight or had induced distress or ulceration.
Culture experiments
Irradiated CL7.1 fibroblasts expressing either themCD40L or the neo genes were resuspended in medium and seeded in 6-well plates at 2 × 105 cells per well. After overnight incubation, exponentially growing plasmacytoma cells (MPC-11, S107, or X-24) were added at different ratios in 5 mL final volume in the presence of the appropriate medium. Cells were recovered for phenotypic analysis after 24, 48, and 72 hours of culture at 37°C in 5%CO2. For the proliferation assay, CL7.1/neo and CL7.1/mCD40L fibroblasts were γ-irradiated (1000 rad) and placed in a 96-well plate (1 × 105 cells per well). MPC-11, S107, or X-24 cells, resuspended in the appropriate medium, were added to the wells at different ratios in a total volume of 200 μL. After 24, 48, and 72 hours of culture in a humidified atmosphere containing 5% CO2 at 37°C, cells were pulsed with 0.5 μCi (0.018 MBq) methyl-3H-thymidine (Amersham, Braunschweing, Germany) and cultured for additional 15 hours. The cells were harvested onto filters and dried, and thymidine uptake was measured in a β-scintillation counter (TrisCarb 2500 TR; Packard BioScience, Downers Grove, IL). Three replicates were used for each condition.
Immunophenotype and detection of apoptotic cells
Next, 50 000 tumor cells, fibroblasts, or mouse splenocytes were stained with phycoerythrin (PE)-conjugated or fluorescein isothiocyanate (FITC)–conjugated antibodies. The antimouse antibodies were as follows: CD3 (17A2), CD4 (L3T4), CD8a (Ly-2), CD80 (B7-1), CD86 (B7-2), CD154 (mCD40L, gp39), H-2Kd (anti–MHC class I), I-Ad/I-Ed (anti–MHC class II), and CD40 (clones 3/23 and HM40-3) (all from Pharmingen, San Diego, CA). Control samples labeled with an appropriate isotype-matched antibody (Pharmingen) were added to each experiment. After staining for 30 minutes at 4°C, cells were washed, fixed in paraformaldehyde 0.5%, and evaluated on a FACScan analyzer (Becton Dickinson, San Jose, CA) equipped with the filter set for triple fluorescence signals. Apoptosis was measured by cell staining with FITC–annexin-V (Pharmingen) antibody and propidium iodide, according to the manufacturer's instruction.
Reverse transcription–polymerase chain reaction amplification
Total RNA was extracted from plasmacytoma cells, mouse splenocytes, and fibroblasts by means of the Qiagen kit (Valencia, CA) according to the manufacturer's instructions. Then, 2 μg RNA were reverse transcribed to complementary DNA (cDNA) by means of the murine leukemia virus reverse transcriptase (GIBCO BRL). Polymerase chain reaction (PCR) amplification was performed in a final volume of 50 μL with the use of 2 μL cDNA, 0.25 mM deoxynucleoside 5′-triphosphate, 0.5 μM each primer, and 2 U AmpliTaq Gold Polymerase (Perkin-Elmer Cetus, Norwalk, CT) in PCR buffer. One cycle at 95°C for 6 minutes followed by 30 cycles, each consisting of 60 seconds at 94°C, 60 seconds at 56°C, and 90 seconds at 72°C, were performed in a Perkin-Elmer Cetus DNA Thermal Cycler. The primers for the murine CD40 amplification were 5′-ACGTGCAGTGACAAACAGTAC-3′ (sense) and 5′-CACAGCTTGTCCAGGGATAAC-3′ (antisense). The integrity of RNA extracted was evaluated in each experiment by amplification of the murine β-actin. The reaction products were electrophoretically separated through a 1.5% agarose gel and stained with ethidium bromide. The expected products generated by PCR were 429 base pairs (bp) and 462 bp for CD40 and β-actin, respectively. To exclude contamination, appropriate negative controls were added to each experiment.
Statistical analysis
All data are presented as mean ± 1 SD. Studentt test was used to determine the statistical significance of differences between samples, and P < .05 was accepted as indicating significance. Tumor-free survival was analyzed by Kaplan-Meier analysis, and the statistical significance of observed differences was assessed by log-rank testing.
Results
Transgenic expression of CD40L reduces the in vivo tumor growth of CD40+ plasmacytoma cell lines
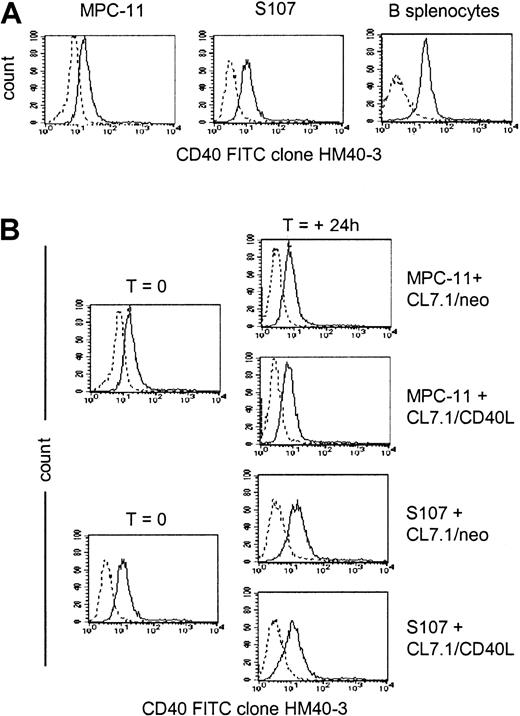
As shown in Figure 1A, the murine plasmacytoma cell lines MPC-11 and S107 expressed CD40 antigen on their surface, as detected by staining with anti-CD40 mAb (clone HM40-3). Addition of CD40L-expressing fibroblasts did not significantly modulate the surface expression of CD40 on either cell line (Figure1B), and the expression of CD40 on MPC-11 and S107 cells growing in vitro was essentially identical to the expression detected on cells growing in vivo in BALB/CBYJ mice (36 ± 14 percent of positive cells and 32 ± 11 percent of positive cells, respectively, versus 40 ± 3 percent of positive cells and 27 ± 15 percent of positive cells, respectively).
CD40 expression by MPC-11 and S107 plasmacytoma cell lines.
Data are representative of 3 independent experiments. (A) Exponentially growing MPC-11 and S107 murine plasmacytoma cells as well as normal murine splenocytes were stained with the anti-CD40–FITC (clone HM40-3)–conjugated mAb (solid lines) or with an appropriate isotype-matched antibody (dashed lines). (B) Expression of the CD40 on MPC-11 and S107 cells cocultured for 24 hours with either CL7.1/mCD40L or CL7.1/neo.
CD40 expression by MPC-11 and S107 plasmacytoma cell lines.
Data are representative of 3 independent experiments. (A) Exponentially growing MPC-11 and S107 murine plasmacytoma cells as well as normal murine splenocytes were stained with the anti-CD40–FITC (clone HM40-3)–conjugated mAb (solid lines) or with an appropriate isotype-matched antibody (dashed lines). (B) Expression of the CD40 on MPC-11 and S107 cells cocultured for 24 hours with either CL7.1/mCD40L or CL7.1/neo.
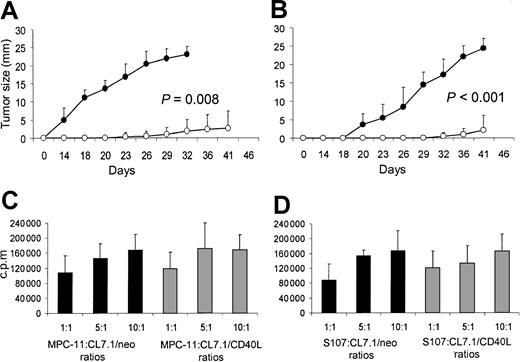
To evaluate the effects of transgenic CD40L expression on plasmacytoma cell growth in vivo, mice were inoculated SC with sublethally irradiated MPC-11 or S107 cells mixed with irradiated CL7.1/mCD40L or CL7.1/neo fibroblasts (ratio, 2:1). Tumor growth was followed at the injection site. For both MPC-11 and S107 cell lines, transgenic expression of CD40L significantly reduced tumor growth compared with control animals receiving tumor cells mixed with CL7.1/neo fibroblasts (P = .008 and P < .001, respectively) (Figure 2A-B). At 6 weeks after tumor injection, all control animals (8 of 8) receiving sublethally irradiated MPC-11 or S107 cells mixed with CL7.1/neo fibroblasts were euthanized because of massive tumor growth. In contrast, only 2 of 8 animals injected with sublethally irradiated MPC-11 or S107 cells mixed with CL7.1/mCD40L fibroblasts had measurable tumors (Table1).
Effect of transgene expression of CD40L on local tumor growth of CD40+ plasmacytoma cell lines.
Transgene expression of CD40L reduces local tumor growth of CD40+ plasmacytoma cell lines. Data represent the mean ± SD of 5 independent experiments in which tumor proliferation was evaluated after 72 hours of coculture. To evaluate the effect of CD40L on myeloma cell growth in vivo, mice were inoculated SC with sublethally irradiated MPC-11 (panel A) or S107 (panel B) cells mixed (ratio, 2:1) with CL7.1/mCD40L (white boxes) or CL7.1/neo (black boxes) fibroblasts. For both plasmacytoma cell lines, transgenic expression of CD40L significantly reduces local tumor growth compared with control animals receiving tumor cells mixed with CL7.1/neo fibroblasts. The tumor size in the 8 animals in each group is reported as mean millimeters ± SD of the 2 maximum diameters. (C) (D) MPC-11 (panel C) and S107 (panel D) tumor cells cocultured in vitro with irradiated CL7.1/neo (black bars) or CL7.1/mCD40L fibroblasts (gray bars) at different ratios and pulsed with 0.5 μCi (0.018 μBq) methyl-3H-thymidine after 24, 48, and 72 hours of culture have similar proliferative activity.
Effect of transgene expression of CD40L on local tumor growth of CD40+ plasmacytoma cell lines.
Transgene expression of CD40L reduces local tumor growth of CD40+ plasmacytoma cell lines. Data represent the mean ± SD of 5 independent experiments in which tumor proliferation was evaluated after 72 hours of coculture. To evaluate the effect of CD40L on myeloma cell growth in vivo, mice were inoculated SC with sublethally irradiated MPC-11 (panel A) or S107 (panel B) cells mixed (ratio, 2:1) with CL7.1/mCD40L (white boxes) or CL7.1/neo (black boxes) fibroblasts. For both plasmacytoma cell lines, transgenic expression of CD40L significantly reduces local tumor growth compared with control animals receiving tumor cells mixed with CL7.1/neo fibroblasts. The tumor size in the 8 animals in each group is reported as mean millimeters ± SD of the 2 maximum diameters. (C) (D) MPC-11 (panel C) and S107 (panel D) tumor cells cocultured in vitro with irradiated CL7.1/neo (black bars) or CL7.1/mCD40L fibroblasts (gray bars) at different ratios and pulsed with 0.5 μCi (0.018 μBq) methyl-3H-thymidine after 24, 48, and 72 hours of culture have similar proliferative activity.
Tumor development and survival 6 weeks after injection of CD40+ plasmacytoma cell lines and fibroblasts expressing CD40 ligand or neo gene
| Mice . | MPC-11 cell line . | S107 cell line . | ||
|---|---|---|---|---|
| neo . | mCD40L . | neo . | mCD40L . | |
| Total inoculated | 8 | 8 | 8 | 8 |
| Developed tumor after inoculation | 8 | 2 | 8 | 2 |
| Surviving 6 weeks after inoculation | 0 | 8 | 0 | 8 |
| Mice . | MPC-11 cell line . | S107 cell line . | ||
|---|---|---|---|---|
| neo . | mCD40L . | neo . | mCD40L . | |
| Total inoculated | 8 | 8 | 8 | 8 |
| Developed tumor after inoculation | 8 | 2 | 8 | 2 |
| Surviving 6 weeks after inoculation | 0 | 8 | 0 | 8 |
We next excluded the possibility that improved survival was due to the direct inhibitory effect of CD40L transgene expression on tumor cell growth, mediated through tumor CD40 stimulation. MPC-11 or S107 cells were cocultured in vitro with CL7.1/mCD40L fibroblasts or CL7.1/neo fibroblasts at different ratios. As shown in Figure 2, transgenic expression of CD40L did not significantly influence the proliferative activity of either MPC-11 (Figure 2C) or S107 (Figure 2D) cell lines.
Transgenic expression of CD40L induces immune-mediated tumor protection
To evaluate the role of T lymphocytes in reducing tumor growth in animals receiving tumor cells and CL7.1/mCD40L fibroblasts, we selectively depleted CD8+ or CD4+ T lymphocytes in vivo before injection of the tumor cells. The results shown in Table2 demonstrate that both CD8+and CD4+ T lymphocytes contributed to the protective response. All animals (5 of 5) receiving MPC-11 or S107 cells mixed with CL7.1/mCD40L fibroblasts and depleted of CD8+ T lymphocytes by IP injection of 2.43 mAb were killed within 6 weeks because of massive tumor growth (Table 2). Similarly, when CD4+ T lymphocytes were depleted by IP injection of Gk1.5 mAb, the protective effect of CD40L transgenic expression against tumor growth was abrogated (Table 2).
Effect of 2.43 (anti-CD8) and Gk1.5 (anti-CD4) monoclonal antibodies on tumor growth 6 weeks after inoculation of CD40+ plasmacytoma cell lines mixed with CL7.1/mCD40 ligand fibroblasts
| Mice . | MPC-11 cell line . | S107 cell line . | ||||
|---|---|---|---|---|---|---|
| No mAb . | 2.43 mAb . | Gk1.5 mAb . | No mAb . | 2.43 mAb . | Gk1.5 mAb . | |
| Inoculated | 8 | 5 | 5 | 8 | 5 | 5 |
| Developed tumor after inoculation | 2 | 5 | 4 | 2 | 5 | 5 |
| Mice . | MPC-11 cell line . | S107 cell line . | ||||
|---|---|---|---|---|---|---|
| No mAb . | 2.43 mAb . | Gk1.5 mAb . | No mAb . | 2.43 mAb . | Gk1.5 mAb . | |
| Inoculated | 8 | 5 | 5 | 8 | 5 | 5 |
| Developed tumor after inoculation | 2 | 5 | 4 | 2 | 5 | 5 |
mAb indicates monoclonal antibody.
The generation of CD4/CD8–mediated immunity is protective against subsequent tumor challenge
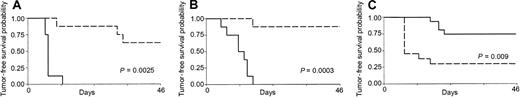
Mice receiving 2 consecutive injections of sublethally irradiated tumor cells mixed with CL7.1/mCD40L fibroblasts were challenged with live tumor cells (5 × 105 cells) 2 weeks after the second injection. As shown in Figure 3, mice immunized in the presence of transgenically expressed CD40L had significantly increased tumor-free survival compared with controls receiving either MPC-11 (Figure 3A) or S107 (Figure 3B) cell lines (P = .0025 and P = .0003). All control mice (8 of 8) injected SC with live MPC-11 tumor cells developed fast-growing tumors and were killed within 3 weeks of the tumor injection, while only 3 of 8 mice vaccinated with CD40L had discernible tumor after challenge. Similarly, only 1 of 8 mice vaccinated with the S107 cell line and CL7.1/mCD40L fibroblasts developed tumor after challenge. Immunization with CD40L fibroblasts alone had no protective effect (data not shown). Cross-challenge experiments were also performed to analyze the specificity of the antitumor response. Of 13 mice injected twice with sublethally irradiated S107 cell line mixed with CL7.1/CD40L fibroblasts, 10 developed a discernable tumor when challenged with live MPC-11 cells; this was statistically significant compared with animals rechallenged with the S107 cell line (P = .009) (Figure 3C).
Effect of transgenic expression of CD40L on systemic immune protection in CD40+ plasmacytoma cell lines.
Transgenic expression of CD40L generates systemic immune protection in CD40+ plasmacytoma cell lines. Mice were vaccinated twice with sublethally irradiated MPC-11 (panel A) or S107 (panel B) cells mixed with CL7.1/mCD40L fibroblasts and challenged 2 weeks later at a distant site with live tumor cells. As a control, live tumor cells were injected in the back of nonimmunized animals. Transgenic expression of CD40L (broken lines) significantly increases tumor-free survival compared with controls (solid lines) for both MPC-11 and S107 cell lines. Data are derived from 8 animals in each group. (C) The immune response mediated by CD40L was specific. Mice vaccinated twice with S107 cells mixed with CL7.1/mCD40L fibroblasts and challenged 2 weeks later at a distant site with live MPC-11 cells (broken line) had poor tumor-free survival compared with animals challenged with the S107 cell line (solid line).
Effect of transgenic expression of CD40L on systemic immune protection in CD40+ plasmacytoma cell lines.
Transgenic expression of CD40L generates systemic immune protection in CD40+ plasmacytoma cell lines. Mice were vaccinated twice with sublethally irradiated MPC-11 (panel A) or S107 (panel B) cells mixed with CL7.1/mCD40L fibroblasts and challenged 2 weeks later at a distant site with live tumor cells. As a control, live tumor cells were injected in the back of nonimmunized animals. Transgenic expression of CD40L (broken lines) significantly increases tumor-free survival compared with controls (solid lines) for both MPC-11 and S107 cell lines. Data are derived from 8 animals in each group. (C) The immune response mediated by CD40L was specific. Mice vaccinated twice with S107 cells mixed with CL7.1/mCD40L fibroblasts and challenged 2 weeks later at a distant site with live MPC-11 cells (broken line) had poor tumor-free survival compared with animals challenged with the S107 cell line (solid line).
Tumor protection is not mediated by the acquisition of antigen-presenting function by CD40 activation of plasmacytoma cell lines
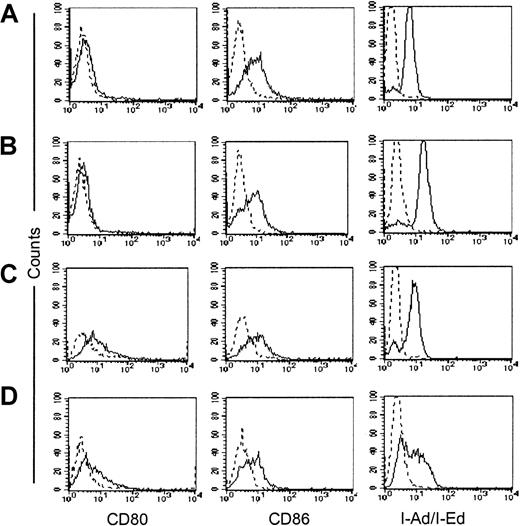
CD40-stimulated lymphoma/CLL cells acquire improved antigen-presenting function.27 28 To evaluate whether the immune protection obtained against plasmacytoma cells following exposure to transgenic CD40L was also due to the acquisition of antigen-presenting function, we measured the expression of costimulatory molecules on plasmacytoma cells. Tumor cells and normal splenocytes were cocultured in vitro with fibroblasts expressing CD40L and analyzed daily for up-regulation of CD80 and CD86 costimulatory molecules and of MHC class II molecules. As shown in Figure4 and Table3, neither CD80/CD86 nor MHC class II molecules were up-regulated on either the MPC-11 or the S107 cell lines. In contrast, significant up-regulation of the CD80 molecule was observed in normal splenocytes cultured in the presence of CD40L-expressing fibroblasts, demonstrating the functionality of the transgenic molecule (Table 3).
Effect of CD40L expression on expression of costimulatory molecules and MHC class II molecules in the CD40+ plasmacytoma cell lines.
CD40L expression does not up-regulate expression of costimulatory molecules and MHC class II molecules in either of the CD40+plasmacytoma cell lines. MPC-11 (panels A-B) and S107 (panels C-D) cells were cocultured in vitro with either CL7.1/neo (panels A,C) or CL7.1/mCD40L (panels B,D) fibroblasts. Cells were analyzed after 24, 48, and 72 hours. The expression of CD80 and CD86 costimulatory molecules as well as of MHC class II molecules is unaffected by transgenic CD40L. Data are representative of 3 independent experiments on each line.
Effect of CD40L expression on expression of costimulatory molecules and MHC class II molecules in the CD40+ plasmacytoma cell lines.
CD40L expression does not up-regulate expression of costimulatory molecules and MHC class II molecules in either of the CD40+plasmacytoma cell lines. MPC-11 (panels A-B) and S107 (panels C-D) cells were cocultured in vitro with either CL7.1/neo (panels A,C) or CL7.1/mCD40L (panels B,D) fibroblasts. Cells were analyzed after 24, 48, and 72 hours. The expression of CD80 and CD86 costimulatory molecules as well as of MHC class II molecules is unaffected by transgenic CD40L. Data are representative of 3 independent experiments on each line.
Phenotype of plasmacytoma cells cocultured with CL7.1/neo or CL7.1/mCD40L fibroblasts
| Cell type and coculture . | Time of coculture, h . | % of positive cells3-150 . | ||
|---|---|---|---|---|
| CD80 . | CD86 . | I-Ad/I-Ed . | ||
| MPC-11 | Fresh cells | 6 ± 1.3 | 26 ± 20 | 59 ± 19 |
| CL7.1/neo | 24 | 9 ± 4.2 | 27 ± 17 | 83 ± 9.3 |
| 48 | 4 ± 2.7 | 33 ± 16.2 | 65 ± 28.5 | |
| 72 | 2 ± 1.9 | 29 ± 7.2 | 79 ± 4.5 | |
| CL7.1/mCD40L | 24 | 16 ± 9 | 22 ± 18 | 70 ± 24.6 |
| 48 | 7 ± 3.4 | 44 ± 26 | 85 ± 2.9 | |
| 72 | 2 ± 1 | 21 ± 6.1 | 82 ± 8.5 | |
| S107 | Fresh cells | 35 ± 28 | 12 ± 6 | 41 ± 20 |
| CL7.1/neo | 24 | 35 ± 34 | 24 ± 11 | 78 ± 6 |
| 48 | 21 ± 7.3 | 28 ± 24 | 78 ± 17.9 | |
| 72 | 19 ± 8.1 | 29 ± 9.7 | 73 ± 24.1 | |
| CL7.1/mCD40L | 24 | 31 ± 20 | 18 ± 18 | 72 ± 17 |
| 48 | 25 ± 11 | 22 ± 19 | 72 ± 12 | |
| 72 | 22 ± 12 | 28 ± 14 | 69 ± 25 | |
| B splenocytes | Fresh cells | 33 ± 14 | 56.8 ± 25 | 98 ± 1.3 |
| CL7.1/neo | 24 | 13 ± 3 | 39 ± 7 | 92 ± 4 |
| 48 | 17.7 ± 5 | 43.1 ± 32.6 | 80.4 ± 14 | |
| 72 | 21.4 ± 3 | 67.5 ± 24.7 | 84.6 ± 6.5 | |
| CL7.1/mCD40L | 24 | 25.7 ± 4.7 | 89.6 ± 7.9 | 83.5 ± 19 |
| 48 | 74.1 ± 10.13-151 | 78.5 ± 2 | 81 ± 3 | |
| 72 | 62 ± 17 | 88.1 ± 11 | 79.5 ± 5 | |
| Cell type and coculture . | Time of coculture, h . | % of positive cells3-150 . | ||
|---|---|---|---|---|
| CD80 . | CD86 . | I-Ad/I-Ed . | ||
| MPC-11 | Fresh cells | 6 ± 1.3 | 26 ± 20 | 59 ± 19 |
| CL7.1/neo | 24 | 9 ± 4.2 | 27 ± 17 | 83 ± 9.3 |
| 48 | 4 ± 2.7 | 33 ± 16.2 | 65 ± 28.5 | |
| 72 | 2 ± 1.9 | 29 ± 7.2 | 79 ± 4.5 | |
| CL7.1/mCD40L | 24 | 16 ± 9 | 22 ± 18 | 70 ± 24.6 |
| 48 | 7 ± 3.4 | 44 ± 26 | 85 ± 2.9 | |
| 72 | 2 ± 1 | 21 ± 6.1 | 82 ± 8.5 | |
| S107 | Fresh cells | 35 ± 28 | 12 ± 6 | 41 ± 20 |
| CL7.1/neo | 24 | 35 ± 34 | 24 ± 11 | 78 ± 6 |
| 48 | 21 ± 7.3 | 28 ± 24 | 78 ± 17.9 | |
| 72 | 19 ± 8.1 | 29 ± 9.7 | 73 ± 24.1 | |
| CL7.1/mCD40L | 24 | 31 ± 20 | 18 ± 18 | 72 ± 17 |
| 48 | 25 ± 11 | 22 ± 19 | 72 ± 12 | |
| 72 | 22 ± 12 | 28 ± 14 | 69 ± 25 | |
| B splenocytes | Fresh cells | 33 ± 14 | 56.8 ± 25 | 98 ± 1.3 |
| CL7.1/neo | 24 | 13 ± 3 | 39 ± 7 | 92 ± 4 |
| 48 | 17.7 ± 5 | 43.1 ± 32.6 | 80.4 ± 14 | |
| 72 | 21.4 ± 3 | 67.5 ± 24.7 | 84.6 ± 6.5 | |
| CL7.1/mCD40L | 24 | 25.7 ± 4.7 | 89.6 ± 7.9 | 83.5 ± 19 |
| 48 | 74.1 ± 10.13-151 | 78.5 ± 2 | 81 ± 3 | |
| 72 | 62 ± 17 | 88.1 ± 11 | 79.5 ± 5 | |
See Table 2 for abbreviations.
Data are represented as a percentage of positive cells ± SD of 3 independent experiments.
Results statistically significant; P = .02.
Transgenic expression of CD40L mediates tumor protection in a CD40− plasmacytoma cell line
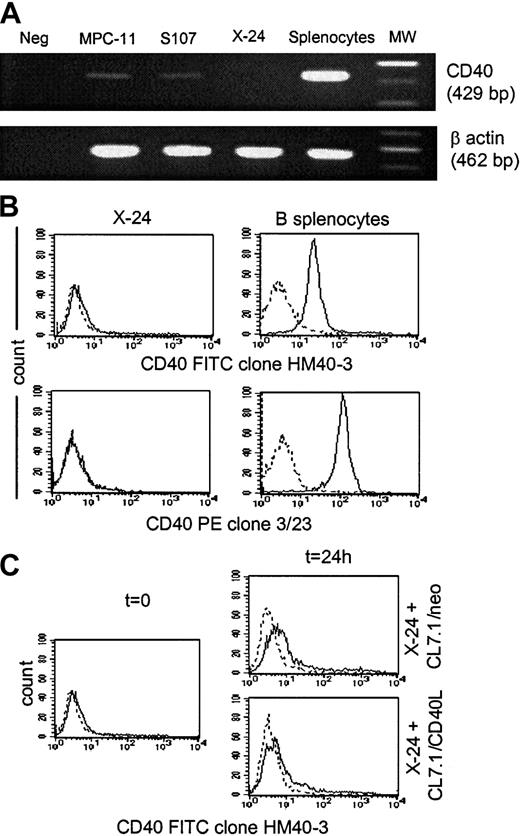
To further demonstrate that the antitumor effect of CD40L in our MM model is independent of the acquisition of antigen-presenting function capability by plasmacytoma cells, we evaluated whether similar protection could be obtained in a CD40− plasmacytoma cell line. As shown in Figure 5, the X-24 plasmacytoma cell line is negative for CD40 messenger RNA (mRNA) expression as assessed by reverse-transcription PCR analysis (Figure5A) and for surface expression of the CD40 molecule as assessed by the use of 2 different anti-CD40 mAbs (clones HM40-3 and 3/23) (Figure 5B). The expression of CD40 on X-24 cells growing in vivo in BALB/CBYJ mice was also undetectable (data not shown), and there was no measurable up-regulation of CD40 from the addition of CD40L-expressing fibroblasts (Figure 5C). As observed for the MPC-11 and S107 cells, transgenic expression of CD40L by fibroblasts significantly reduced the local growth of X-24 cells in BALB/CBYJ mice (Figure6A) although transgenic expression of CD40L did not significantly influence the proliferative activity in vitro of X-24 cells compared with control cells cultured in the presence of CL7.1/neo fibroblasts (data not shown). The local inhibitory effect produced in vivo by transgenic CD40L expression was immune mediated, since it induced significant protection when animals were challenged 2 weeks later with live tumor cells (Figure 6B). As expected, no significant modulation of the costimulatory molecules (CD80 and CD86) was observed after coculture of CD40− X-24 cells with fibroblasts expressing CD40L (data not shown).
X-24 plasmacytoma cell line.
X-24 plasmacytoma cell line does not express the CD40 molecule. Data are representative of 3 independent experiments. (A) The X-24 cell line (upper row) was negative for expression of CD40 mRNA as assessed by reverse transcription–PCR analysis. The amplification of the β-actin shows the integrity of reverse-transcribed RNA (lower row). (B) Exponentially growing X-24 murine plasmacytoma cells were negative for CD40 molecule expression as measured by staining with the anti-CD40–FITC (clone HM40-3)–conjugated mAb (upper panels, solid lines) or the anti-CD40–PE (clone 3/23)–conjugated mAb (lower panels, solid lines). Dashed lines represent the profile of the isotype-matched antibody. (C) Expression of CD40 by X-24 cells was not significantly enhanced by exposure to transgenic CD40L as assessed by coculture for 24 hours with either CL7.1/mCD40L or CL7.1/neo fibroblasts.
X-24 plasmacytoma cell line.
X-24 plasmacytoma cell line does not express the CD40 molecule. Data are representative of 3 independent experiments. (A) The X-24 cell line (upper row) was negative for expression of CD40 mRNA as assessed by reverse transcription–PCR analysis. The amplification of the β-actin shows the integrity of reverse-transcribed RNA (lower row). (B) Exponentially growing X-24 murine plasmacytoma cells were negative for CD40 molecule expression as measured by staining with the anti-CD40–FITC (clone HM40-3)–conjugated mAb (upper panels, solid lines) or the anti-CD40–PE (clone 3/23)–conjugated mAb (lower panels, solid lines). Dashed lines represent the profile of the isotype-matched antibody. (C) Expression of CD40 by X-24 cells was not significantly enhanced by exposure to transgenic CD40L as assessed by coculture for 24 hours with either CL7.1/mCD40L or CL7.1/neo fibroblasts.
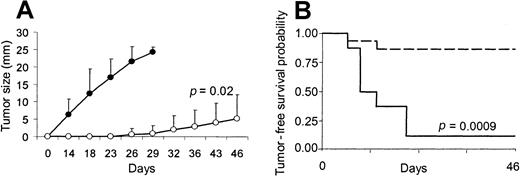
Effect of transgene expression of CD40L on the CD40− X-24 plasmacytoma cell line.
Transgene expression of CD40L generates immune protection in the CD40− X-24 plasmacytoma cell line. To evaluate the in vivo effect of CD40L on X-24 cell growth, mice were inoculated SC with X-24 cells (6 × 106) mixed with CL7.1/mCD40L (1 × 106) (white boxes) or CL7.1/neo (1 × 106) (black boxes) fibroblasts. Transgenic expression of CD40L significantly reduces local tumor growth compared with animals receiving tumor cells mixed with CL7.1/neo fibroblasts (A). The tumor size of 6 animals in each group is reported as mean mm ± SD of the 2 maximum diameters. Mice that were vaccinated twice with X-24 cells mixed with CL7.1/mCD40L fibroblasts and challenged 2 weeks later at a distant site with live tumor cells (6 × 106 cells) (broken lines) showed increased tumor-free survival compared with controls (solid lines) (B). Data are derived from 8 animals in the control group and 15 animals in the vaccinated group.
Effect of transgene expression of CD40L on the CD40− X-24 plasmacytoma cell line.
Transgene expression of CD40L generates immune protection in the CD40− X-24 plasmacytoma cell line. To evaluate the in vivo effect of CD40L on X-24 cell growth, mice were inoculated SC with X-24 cells (6 × 106) mixed with CL7.1/mCD40L (1 × 106) (white boxes) or CL7.1/neo (1 × 106) (black boxes) fibroblasts. Transgenic expression of CD40L significantly reduces local tumor growth compared with animals receiving tumor cells mixed with CL7.1/neo fibroblasts (A). The tumor size of 6 animals in each group is reported as mean mm ± SD of the 2 maximum diameters. Mice that were vaccinated twice with X-24 cells mixed with CL7.1/mCD40L fibroblasts and challenged 2 weeks later at a distant site with live tumor cells (6 × 106 cells) (broken lines) showed increased tumor-free survival compared with controls (solid lines) (B). Data are derived from 8 animals in the control group and 15 animals in the vaccinated group.
Immune-mediated tumor protection is determined predominantly by in vivo activation of professional APCs
Because the protective effect induced by transgenic expression of CD40L was independent of the acquisition of antigen-presenting capability by plasmacytoma cells, we investigated whether this effect could be mediated by activation of professional APCs cross-primed with tumor-derived antigens. We injected animals with plasmacytoma cells previously exposed to UV radiation and cultured overnight in serum-free medium. Staining with annexin-V and propidium iodide and FACS analysis showed that more than 60% of cells were apoptotic and 25% necrotic, with fewer than 15% viable (negative for both annexin-V and propidium iodide). After mixing with CL7.1/mCD40L fibroblasts, this cell suspension was injected as previously described for viable tumor cells. Mice were challenged 2 weeks later with live tumor cells, and as shown in Figure 7, transgenic expression of CD40L produced the same systemic tumor protection observed in mice that had received viable tumor cells. Thus, 3 of 8 mice vaccinated with apoptotic MPC-11 cells and CL7.1/mCD40L fibroblasts developed tumor after challenge as compared with 8 of 8 controls (P = .0024) (Figure 7A). Similar levels of protection were observed when the S107 cell line (3 of 8 versus 8 of 8,P = .0003) (Figure 7B) or X-24 cell line (2 of 15 versus 7 of 8, P = .001) (Figure 7C) were substituted. Mice injected with the identical tumor cell suspensions mixed with CL7.1/neo fibroblast were not protected, because all mice developed tumor at the site of injection (data not shown).
Effect of apoptotic tumor cells combined with transgenic CD40L expression on immune-mediated tumor protection.
Apoptotic tumor cells combined with transgenic CD40L expression produce immune-mediated tumor protection. Mice were vaccinated twice with apoptotic MPC-11 (A), S107 (B), or X-24 (C) cells mixed with CL7.1/mCD40L fibroblasts and challenged 2 weeks later at a distant site with live plasmacytoma cells. Transgenic expression of CD40L (broken lines) significantly increases tumor-free survival compared with controls (solid lines) for all plasmacytoma cell lines. Data are representative of 8 animals for each group for the MPC-11 and S107 cell lines. For the X-24 cell line, the data are representative of 8 animals for the control group and 15 animals for the vaccinated group.
Effect of apoptotic tumor cells combined with transgenic CD40L expression on immune-mediated tumor protection.
Apoptotic tumor cells combined with transgenic CD40L expression produce immune-mediated tumor protection. Mice were vaccinated twice with apoptotic MPC-11 (A), S107 (B), or X-24 (C) cells mixed with CL7.1/mCD40L fibroblasts and challenged 2 weeks later at a distant site with live plasmacytoma cells. Transgenic expression of CD40L (broken lines) significantly increases tumor-free survival compared with controls (solid lines) for all plasmacytoma cell lines. Data are representative of 8 animals for each group for the MPC-11 and S107 cell lines. For the X-24 cell line, the data are representative of 8 animals for the control group and 15 animals for the vaccinated group.
Discussion
In this report, we have shown that transgenic expression of CD40L in the proximity of MM cells can generate both local and systemic tumor protection. This effect is probably mediated by in vivo recruitment/activation of professional APCs able to take up antigens released by tumor cells, rather than by the acquisition of APC function by MM cells, and requires both CD4+ and CD8+ T lymphocytes.
Vaccination with whole tumor cells represents an attractive strategy for human cancer immunotherapy.12 Although the substitution of defined tumor-associated antigens may ultimately be desirable, human tumors are extremely heterogeneous, making the identification of one or more “universal” tumor antigens extremely difficult for many tumors. In addition, many potential tumor-associated antigens have yet to be identified.32 Finally, it is becoming increasingly evident that the use of defined antigens may be able to recruit only a limited humoral or cellular immune response, one that is inadequate to produce sustained and effective antitumor activity in humans. Instead, it is necessary to use antigenic stimuli that recruit both CD8+ and CD4+ T lymphocytes that can each recognize their relevant class I and class II MHC–restricted tumor epitopes residing on a single or proximate APC.33 These critical cognate interactions between helper and cytotoxic T cells may be difficult to achieve with a single defined tumor antigen, which may lack peptide sequences capable of being presented by both class I and class II MHC molecules on the patient's APCs.
Transgenic expression of CD40L is one means of enhancing the immune response to whole tumor cells, and the molecule may be provided on the tumor cell itself or, as here, by bystander cells in the vicinity of the injected tumor.21 CD40L is particularly attractive for the immunotherapy of B-cell tumors because these are often CD40+. It has been suggested that in this setting, CD40 activation by CD40L up-regulates the costimulatory molecules (CD80/B7.1 and CD86/B7.2) and class II MHC expression on the malignant cells, enhancing their inherent antigen-presenting capabilities.21,27,28 This in turn increases the possibility of generating specific cytotoxic T lymphocytes.34 Our results with MM show that transgenic expression of CD40L by syngeneic fibroblasts in the tumor microenvironment reduces the local tumor growth of plasmacytoma cell lines. This local effect is not dependent on any direct inhibitory effect of CD40L expression on the growth of either tumor.35-37 Instead, it is immune mediated, because vaccination inhibited not only local tumor growth, but also tumor growth from a subsequent tumor challenge at a distal site. Importantly, immune protection requires both CD4+ and CD8+ T lymphocytes, because depletion of either subset abolishes protection. These results indicate that transgenic CD40L expression has been associated with the processing and subsequent presentation of peptides derived from antigens released by tumor cells to both CD4+and CD8+ effector cells. This combination of cellular responses may particularly favor the broad and sustained antitumor responses required for the treatment of human tumors.
Transgenic CD40L had no effect on MHC or costimulatory molecule (CD80 and CD86) expression by CD40+ plasmacytoma cells, indicating that the immune effects were unlikely to be mediated by an increase in the ability of the tumor to function as an APC consequent upon CD40 activation. CD40L also fails to enhance tumor IL-6 release in both the MPC-11 and the S107 cell line, even when the transgenic CD40L highly up-regulates this cytokine production when added to spleen cells (data not shown). This contrasts with findings observed in other B-cell malignancies, such as lymphoma/CLL,27,28 and suggests that these murine MM cells could be less responsive to CD40-CD40L signaling. This deficit may be a general phenomenon in MM since CD40 activation by transgenic CD40L also fails to up-regulate B7 molecules in human MM cell lines35,36 and primary MM cells.38Although a normal capacity to up-regulate alternative costimulatory molecules on plasmacytoma cells cannot be ruled out, MM cells appear to have a defect in the CD40 transduction pathway. Cross-linking of CD40 on normal B lymphocytes induces the activation of several pathways, including Rel/nuclear factor–κB factors, a family of transcription factors involved in the control of several genes crucial for lymphoid cell functions.39,40 The signaling competence of CD40 after engagement with CD40L is compromised in S107 and MPC-11 cell lines owing to defective RelB RNA transcription (Neumann et al41 and data not shown). Signaling may also be impaired secondarily to mutational or conformational abnormalities in the tumor CD40 antigen. The existence of such changes may be inferred from abnormal patterns of reactivity with anti-CD40 mAbs—for example, the failure of line S107 to bind to a second anti-CD40 mAb clone, 3/23 (data not shown). But although the molecular mechanism(s) of the absent up-regulation of CD80 and CD86 costimulatory molecules after CD40-CD40L engagement in plasmacytoma may be complex and varied, the absence of these effects and the ability to use transgenic CD40L to obtain protection from a CD40− plasmacytoma further indicate that CD40 activation on MM cells is not a requirement for inducing an antitumor immune response. Instead, the immunologic effects produced by transgenic CD40L are more likely to be mediated by recruitment/activation of professional APCs, enhancing their ability to take up and present antigens released by tumors or tumor apoptotic bodies.42,43 This suggestion is supported by our observation that injection of apoptotic/necrotic tumor cells is as effective as injection of viable tumor cells for induction of a protective immune response. In our model, the nature of the antigen(s) involved in tumor protection remains to be identified. Although cross-challenge experiments demonstrated significant specificity of the antitumor response, limited protection was also obtained against a second plasmacytoma cell line. Hence, at least some of the protective immune response may be targeted against common passenger viruses in murine cell lines,44 which may be absent from human tumors.
In conclusion, we have shown that transgenic CD40L expression induces a protective response against myeloma. Both CD4+ and CD8+ tumor-specific T cells are induced, a combination likely to be desirable for the immunotherapy of human malignancy.12,32 Moreover, because our results show that the interaction with CD40 antigen on tumor cells is neither universal nor required for the immune enhancement mediated by transgenic CD40L, the molecule may be of therapeutic value in both CD40+ and CD40− forms of the disease.29
We thank Susan Smith and Tatiana Gotsolova for excellent technical assistance. We also thank Gloria Levin for help in preparing this manuscript.
Supported by National Institutes of Health Grants RO1 CA75014 and RO1 CA78792; G.D. was supported by a grant from Associazione Paolo Belli—lotta alla leucemia.
G.D. and B.S. equally contributed to this work.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Malcolm K. Brenner, Center for Cell and Gene Therapy, Baylor College of Medicine, 6621 Fannin St, MC 3-3320, Houston, TX 77030; e-mail: mbrenner@bcm.tmc.edu.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal