Abstract
In this study, we describe the generation and characterization of mice in which GITR gene (TNFRSF18 [tumor necrosis factor receptor superfamily 18]), a member of the TNFRSF expressed mainly on T lymphocytes, has been ablated (GITR−/− mice). Results indicate that GITR inactivation does not impair the normal development of the lymphoid organs but modulates T-cell activation. In fact, whenGITR−/− T lymphocytes are activated by treatment with an anti-CD3 monoclonal antibody they proliferate more than wild-type cells. Moreover, activatedGITR−/− T lymphocytes express higher levels of interleukin-2 receptor, produce larger amounts of interleukin-2, and are more sensitive to activation-induced cell death than controls. These results suggest that GITR is involved in the regulation of T-cell receptor/CD3–driven T-cell activation and programmed cell death.
Introduction
Members of the tumor necrosis factor receptor superfamily (TNFRSF) regulate development and function of the immune system. In particular, some of them transduce signals involved in the regulation of apoptosis, while others participate in lymphocyte activation.1 GITR is a member of the TNFRSF (TNFRSF18) cloned in a glucocorticoid-treated T-cell hybridoma and up-regulated upon triggering of T-cell receptor (TCR).2,3 The cytoplasmic domain of GITR shows a striking homology with the corresponding region of other TNFRSF members, namely CD40,4,5 OX40,6 4-1BB,7 and CD27,8 defining a new subfamily within this superfamily.9 We have previously demonstrated that GITR-transfected cells were more resistant to anti-CD3–induced apoptosis as compared with untransfected cells, thus suggesting a role for GITR in the control of T-cell activation and death.
Here we show that GITR is involved in the regulation of TCR/CD3-driven T-cell activation and death.
Materials and methods
Generation of GITR-deficient mice
A genomic clone containing GITR was isolated as previously described.9 GITR was inactivated by homologous recombination as previously described.10 A GITR targeting vector was generated by inserting the 3.1-kilobase (kb)BamHI-StuI fragment located at the 5′ side ofGITR gene downstream the neomycin gene and the 1.4-kbXhoI-BamHI fragment located at the 3′ side of GITR gene upstream the neomycin gene into the pPNT vector.11 129-derived embryonic stem (ES) cells were electroporated with the GITR targeting vector and double selected by G418 (350 μg/mL) and gancyclovir (2 μM). The recombined clones were injected into C57BL/6 blastocysts, and the resulting male chimeras were bred to C57BL/6 females and analyzed for germline transmission.
Flow cytometry and ELISA
Cells (1 × 106) were stained with anti-CD4, anti-CD8, and anti-CD25 (interleukin-2 receptor [IL-2R]) phycoerythrin-conjugated mAbs and anti-B220, anti–Pgp-1 (CD44), and anti-Fas fluorescein isothiocyanate (FITC)–conjugated mAbs (Pharmingen, San Diego, CA). Apoptosis and cell cycle were evaluated as previously described.12 Briefly, cells were centrifuged, resuspended in hypotonic propidium iodide solution, and kept 1 hour at room temperature. Samples (5 × 104) were analyzed with a FACScan Lysis II and CellQuest software (both from Becton Dickinson) for immunostaining/apoptosis and cell cycle, respectively. The amount of IL-2 produced by activated cells was detected by an enzyme-linked immunosorbent assay (ELISA) (R&D Systems, Minneapolis, MN) following the manufacturer's instructions.
In vitro T-cell activation
Purified T lymphocytes (obtained by an immunostaining with anti-B220 FITC-conjugated antibody [Pharmingen] followed by a separation with Biomag sheep antifluorescein magnetic beads [PerSeptive Biosystems, Framingham, MA]), splenocytes, and total lymphocytes (5 × 105 cells/mL) from lymph nodes were cultured in the absence or presence of anti-CD3ε mAb (10 μg/mL). Soluble anti-CD3ε mAb was added together with cells at the indicated concentrations. Concanavalin A (ConA) (Sigma, St Louis, MO) was added to cells (2 × 106 cells/mL) at a concentration of 2 μg/mL, phorbol myristate acetate (Sigma) at 100 nM, and ionophore (Sigma) at 1 μM.
Results and discussion
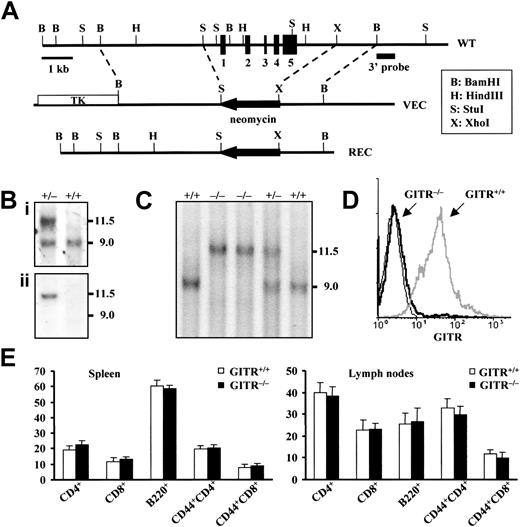
Generation of GITR−/− mice by gene targeting
The gene encoding GITR was inactivated by homologous recombination technique in murine ES cells as shown in Figure1A. The structure of the expected mutant allele in the ES clones and the genotype of the progeny (Sv129 × C57BL/6) were verified by Southern blotting using a 3′ probe (Figure 1Bi) and confirmed using a neomycin probe (Figure 1Bii). Genotypic frequencies (Figure 1C) were in accordance with the expected mendelian ratios, and the absence of GITR in mutant mice was also verified by flow cytometric analysis on purified T lymphocytes (Figure1D). The deletion of GITR had no detectable effect on the viability or fertility of mice. Because GITR is expressed mainly in lymphoid organs, we examined thymus, spleen, and lymph nodes. The weight of the lymphoid organs, cellularity, and expression of the main thymocyte (not shown) and peripheral lymphocyte differentiation markers, including CD3, CD4, CD8, and B220, were normal. In particular, results reported in Figure1E indicate that no significant differences were observed between lymphocyte subpopulations in the spleen and lymph nodes ofGITR+/+ and GITR−/−mice. Thus, the loss of GITR did not affect the physiologic development and homeostasis of T lymphocytes despite the basal expression of GITR in wild-type T cells.
Targeted disruption of GITR gene.
(A) Structure and restriction map of GITR gene locus (WT, top), targeting vector (VEC, middle), and predicted mutated allele (REC, bottom). The whole gene, about 600 base pairs of the promoter region and 1.2 kb of the 3′ side of GITR gene, were replaced by a neomycin resistance cassette (neor) in reverse orientation. Exons are represented by filled boxes. The targeting vector contains the neor cassette and the HSV-tk gene (TK) allowing the positive-negative selection of the ES clones. (Bi) Southern blot of ES DNA upon a BamHI digestion probed with the 3′ external probe indicated in panel A. The wild-type band is 9-kb long, while the mutated band is 11.5 kb. (Bii) The same Southern blot has been reprobed with a neomycin probe that hybridizes only to the mutated band. (C) Tail analysis of heterozygous (GITR+/−), homozygous knock-out (GITR−/−), and homozygous wild-type mice (GITR+/+) by the same Southern blot as for ES cells. (D) Flow cytometric analysis of purified T cells from GITR+/+ and GITR−/− mice with anti-GITR antibody. The anti-GITR antibody (R&D Systems) was used undiluted (10 μL per sample), and the antigoat IgG FITC-conjugated secondary antibody was used diluted 1:100. (E) Lymphocyte subpopulations of GITR−/− and GITR+/+ mice. Values represent the percentage ± SD (n > 6). Cervical, brachial, axillary, superficial inguinal, and mesenteric lymph nodes were analyzed.
Targeted disruption of GITR gene.
(A) Structure and restriction map of GITR gene locus (WT, top), targeting vector (VEC, middle), and predicted mutated allele (REC, bottom). The whole gene, about 600 base pairs of the promoter region and 1.2 kb of the 3′ side of GITR gene, were replaced by a neomycin resistance cassette (neor) in reverse orientation. Exons are represented by filled boxes. The targeting vector contains the neor cassette and the HSV-tk gene (TK) allowing the positive-negative selection of the ES clones. (Bi) Southern blot of ES DNA upon a BamHI digestion probed with the 3′ external probe indicated in panel A. The wild-type band is 9-kb long, while the mutated band is 11.5 kb. (Bii) The same Southern blot has been reprobed with a neomycin probe that hybridizes only to the mutated band. (C) Tail analysis of heterozygous (GITR+/−), homozygous knock-out (GITR−/−), and homozygous wild-type mice (GITR+/+) by the same Southern blot as for ES cells. (D) Flow cytometric analysis of purified T cells from GITR+/+ and GITR−/− mice with anti-GITR antibody. The anti-GITR antibody (R&D Systems) was used undiluted (10 μL per sample), and the antigoat IgG FITC-conjugated secondary antibody was used diluted 1:100. (E) Lymphocyte subpopulations of GITR−/− and GITR+/+ mice. Values represent the percentage ± SD (n > 6). Cervical, brachial, axillary, superficial inguinal, and mesenteric lymph nodes were analyzed.
Increased T-cell proliferation in the absence of GITR
We have previously demonstrated that the promoter region of GITR contains several binding sites for factors involved in T-cell activation9 and that GITR is up-regulated in activated T cells.3 To test how mature T cells coming from sex- and age-matched (6- to 12-week-old) GITR−/− mice respond to an activating stimulus, we treated either splenocytes, lymph node lymphocytes (Figure 2A), or purified T lymphocytes from lymph nodes (not shown) with plate-bound anti-CD3 mAb. In some experiments purified T lymphocytes were activated by soluble anti-CD3 mAb on a feeder of irradiated splenocytes (Figure 2B). In all the experimental conditions tested, the values of the [3H]thymidine uptake were significantly higher inGITR−/− T cells as compared with GITR+/+ controls, and the increase ranged from 1.6- to 4.1-fold after 36 hours of activation. The addition of exogenous IL-2 slightly enhanced the differences of [3H]thymidine incorporation between GITR+/+ andGITR−/− T cells (Figure 2B). Results in Figure2C show that the difference in [3H]thymidine uptake between GITR+/+ and GITR−/− cells is maintained within a wide range of anti-CD3 mAb concentrations and also when cells are stimulated by anti-CD3 plus anti-CD28 (Figure2D).
Higher [3H]thymidine uptake, proliferation, activation, and AICD sensitivity of GITR−/− cells upon anti-CD3 triggering.
(A) [3H]thymidine uptake of splenocytes and total lymphocytes from lymph nodes (5 × 105 cells/mL) activated by plate-bound anti-CD3 mAb (clone 145-2C-11, Pharmingen) is shown. GITR−/− cells (filled columns) have a [3H]thymidine uptake significantly higher thanGITR+/+ cells (empty columns) (**P < .01 and ***P < .001). Each value is the mean ± SD of at least 3 independent experiments. A total of 2.5 μCi [3H]thymidine (Amersham International, Amersham, United Kingdom) per well was added to the cultures. (B) One of 5 independent experiments of [3H]thymidine uptake of purified T lymphocytes activated by soluble anti-CD3 mAb (0.2 μg/mL) on a feeder of irradiated (20 Gy, 5 minutes) splenocytes is shown. GITR−/− cells (filled circles) have a [3H]thymidine uptake significantly higher than GITR+/+ cells (empty triangles) (***P < .001 and **P < .01), in the presence (dashed lines) and in the absence (solid lines) of murine IL-2 (50 U/mL). UntreatedGITR−/− and GITR+/+cells incorporate less than 1000 cpm and are not shown. Each value is the mean ± SD of 4 counts. (C) Dose dependence of anti-CD3 mAb activation of purified T lymphocytes cultured on a feeder of irradiated splenocytes for 36 hours. One of 3 independent experiments is shown. Each value is the mean ± SD of 4 counts. The difference of incorporation between GITR−/− (filled circles) and GITR+/+ (empty triangles) cells was significant (***P < .001 and **P < .01). (D) T lymphocytes cultured on a feeder of irradiated splenocytes activated with soluble anti-CD3 (0.01 μg/mL) or with soluble anti-CD3 plus anti-CD28 (Pharmingen) (0.01 μg/mL) for 24 hours. One of 3 independent experiments is shown. Each value is the mean ± SD of 4 counts. GITR−/−cells (filled columns) have a [3H]thymidine uptake significantly higher than GITR+/+ cells (empty columns) (*P < .05). (E) GITR−/−cells enter into the cell cycle as a significantly higher number (**P < .01 and *P < .05) upon activation. Purified T lymphocytes from lymph nodes were activated by plate-bound anti-CD3 mAb. The percentage of T cells in S/G2/M phases is shown. Activated splenocytes showed similar results (not shown). Empty and filled columns represent GITR+/+ andGITR−/− lymphocytes, respectively. The values represent the mean ± SD of 3 independent experiments. (F) IL-2R expression, revealed by anti-CD25 phycoerythrin-conjugated mAb (Pharmingen), is significantly higher inGITR−/−–activated T lymphocytes (40% inGITR−/− vs 30% inGITR+/+ cells). T lymphocytes were activated by plate-bound anti-CD3 mAb for 24 hours. The thin line curve represents control sample. Basal values in unstimulated T lymphocytes (5.0% ± 1.3% in wild-type vs 3.1% ± 1.1% in GITR−/−) were similar (P > .05) (not shown). One of 3 independent experiments with similar results is shown. (G) IL-2 produced by GITR−/− T cells (filled columns) activated by platebound anti-CD3 mAb (10 μg/mL) 48 hours after activation is significantly higher (**P < .01) compared with GITR+/+–activated T cells (empty columns). Values represent the mean ± SD of 3 ELISA assays performed for each experimental condition. (H) Higher AICD in GITR−/− T lymphocytes (*P < .05). Cells were stimulated for 2 days with ConA and then plated at a density of 1 × 106/mL on wells that had been coated with anti-CD3 mAb (10 μg/mL), either in the absence or presence of murine IL-2 (50 U/mL). After 24 hours cells were harvested and stained with propidium iodide for evaluation of apoptosis. Empty and filled columns representGITR+/+ and GITR−/−splenocytes, respectively. Values represent the mean ± SD of 3 independent experiments.
Higher [3H]thymidine uptake, proliferation, activation, and AICD sensitivity of GITR−/− cells upon anti-CD3 triggering.
(A) [3H]thymidine uptake of splenocytes and total lymphocytes from lymph nodes (5 × 105 cells/mL) activated by plate-bound anti-CD3 mAb (clone 145-2C-11, Pharmingen) is shown. GITR−/− cells (filled columns) have a [3H]thymidine uptake significantly higher thanGITR+/+ cells (empty columns) (**P < .01 and ***P < .001). Each value is the mean ± SD of at least 3 independent experiments. A total of 2.5 μCi [3H]thymidine (Amersham International, Amersham, United Kingdom) per well was added to the cultures. (B) One of 5 independent experiments of [3H]thymidine uptake of purified T lymphocytes activated by soluble anti-CD3 mAb (0.2 μg/mL) on a feeder of irradiated (20 Gy, 5 minutes) splenocytes is shown. GITR−/− cells (filled circles) have a [3H]thymidine uptake significantly higher than GITR+/+ cells (empty triangles) (***P < .001 and **P < .01), in the presence (dashed lines) and in the absence (solid lines) of murine IL-2 (50 U/mL). UntreatedGITR−/− and GITR+/+cells incorporate less than 1000 cpm and are not shown. Each value is the mean ± SD of 4 counts. (C) Dose dependence of anti-CD3 mAb activation of purified T lymphocytes cultured on a feeder of irradiated splenocytes for 36 hours. One of 3 independent experiments is shown. Each value is the mean ± SD of 4 counts. The difference of incorporation between GITR−/− (filled circles) and GITR+/+ (empty triangles) cells was significant (***P < .001 and **P < .01). (D) T lymphocytes cultured on a feeder of irradiated splenocytes activated with soluble anti-CD3 (0.01 μg/mL) or with soluble anti-CD3 plus anti-CD28 (Pharmingen) (0.01 μg/mL) for 24 hours. One of 3 independent experiments is shown. Each value is the mean ± SD of 4 counts. GITR−/−cells (filled columns) have a [3H]thymidine uptake significantly higher than GITR+/+ cells (empty columns) (*P < .05). (E) GITR−/−cells enter into the cell cycle as a significantly higher number (**P < .01 and *P < .05) upon activation. Purified T lymphocytes from lymph nodes were activated by plate-bound anti-CD3 mAb. The percentage of T cells in S/G2/M phases is shown. Activated splenocytes showed similar results (not shown). Empty and filled columns represent GITR+/+ andGITR−/− lymphocytes, respectively. The values represent the mean ± SD of 3 independent experiments. (F) IL-2R expression, revealed by anti-CD25 phycoerythrin-conjugated mAb (Pharmingen), is significantly higher inGITR−/−–activated T lymphocytes (40% inGITR−/− vs 30% inGITR+/+ cells). T lymphocytes were activated by plate-bound anti-CD3 mAb for 24 hours. The thin line curve represents control sample. Basal values in unstimulated T lymphocytes (5.0% ± 1.3% in wild-type vs 3.1% ± 1.1% in GITR−/−) were similar (P > .05) (not shown). One of 3 independent experiments with similar results is shown. (G) IL-2 produced by GITR−/− T cells (filled columns) activated by platebound anti-CD3 mAb (10 μg/mL) 48 hours after activation is significantly higher (**P < .01) compared with GITR+/+–activated T cells (empty columns). Values represent the mean ± SD of 3 ELISA assays performed for each experimental condition. (H) Higher AICD in GITR−/− T lymphocytes (*P < .05). Cells were stimulated for 2 days with ConA and then plated at a density of 1 × 106/mL on wells that had been coated with anti-CD3 mAb (10 μg/mL), either in the absence or presence of murine IL-2 (50 U/mL). After 24 hours cells were harvested and stained with propidium iodide for evaluation of apoptosis. Empty and filled columns representGITR+/+ and GITR−/−splenocytes, respectively. Values represent the mean ± SD of 3 independent experiments.
To assess whether other mitogen agents would have the same effect as anti-CD3 mAb triggering, we activated splenocytes and purified T cells with ConA or phorbol myristate acetate plus calcium-ionophore for 36 hours. Both cells fromGITR−/− and fromGITR+/+ mice show similar [3H]thymidine uptake (not shown).
We next addressed the question of whether the increased thymidine uptake matched with a cell cycle modulation. Flow cytometric analysis of the cell cycle showed that the percentage of purifiedGITR−/− T lymphocytes entering into the S/G2/M phases is higher than that ofGITR+/+ cells (Figure 2E).
Our results suggest that the abnormal behavior observed inGITR−/−–activated lymphocytes is restricted to the activation triggered through the TCR/CD3 complex.
Augmented expression of IL-2, IL-2R, and Fas
We next sought to verify whether GITR−/−T lymphocytes that proliferate more were also more activated. Specifically, we looked at the P55 receptor for IL-2 (IL-2R), the release of IL-2, and Fas expression. Results in Figure 2F,G indicate that TCR/CD3 triggering induced a higher IL-2R expression and a higher IL-2 production in activated GITR−/− T lymphocytes as compared with GITR+/+ mice (P < .01). Moreover, Fas expression, which is 5.1 ± 0.5 (median ± SD) and 5.3 ± 0.8, respectively, in unstimulated GITR+/+ and GITR−/− T lymphocytes, was increased to 19.0 ± 1.1 and 27.1 ± 3 in activated T lymphocytes (P < .05, comparing activatedGITR+/+ and GITR−/−cells), confirming our initial hypothesis.
Higher sensitivity of GITR−/− T cells to AICD
A portion of activated T cells undergoes apoptosis to limit clonal expansion (activation-induced cell death [AICD]).13 GITR has previously been involved in the control of this process.2 We therefore evaluated the sensitivity of activated GITR−/− T cells to AICD. For this purpose, we treated ConA-activated T lymphocytes with anti-CD3 mAb in the presence or absence of IL-2 and measured the apoptosis after 24 hours. GITR−/− cells proved to be more sensitive to AICD than GITR+/+ cells in both experimental conditions (Figure 2H). This effect can be ascribed to the ability of GITR−/− cells to become more activated upon anti-CD3 triggering. Taken together, these findings on the in vivo role of GITR are in line with the main feature of the TNFRSF members. In fact, some of them are important for positive regulation of T-cell immune response exerting a function similar to that of CD28 (eg, CD278,14 and OX406,15), while some others contribute to balance T-cell response either by inducing apoptosis (eg, Fas or TNFR1) or delivering a negative signal (eg, DR6 and CD40) similar to that of cytotoxic T lymphocyte antigen 4.16 In fact, CD4+ T cells from DR6-targeted mutant mice hyperproliferate in response to TCR-mediated stimulation,17 and T cells fromCD40−/− mice induce autoimmune diseases in nude mice.5 In conclusion, our study suggests that GITR is another TNFRSF member delivering a negative signal to activated cells. How GITR functions as a negative regulatory molecule is currently under investigation.
Prepublished online as Blood First Edition paper, April 30, 2002; DOI 10.1182/blood-2001- 12-0276.
Supported by the Italian Association for Cancer Research (AIRC), Milan, and Consiglio Nazionale delle Ricerche (CNR) Target Project on Biotechnology, Rome, Italy.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Carlo Riccardi, Dipartimento di Medicina Clinica e Sperimentale, Sezione di Farmacologia, Università di Perugia, Via del Giochetto, 06100 Perugia, Italy; e-mail: riccardi@unipg.it; Pier Paolo Pandolfi, Molecular Biology Program, Department of Pathology, Memorial Sloan-Kettering Cancer Center, 1275 York Ave, Box 110, New York, NY 10021; e-mail: p-pandolfi@ski.mskcc.org.

![Fig. 2. Higher [3H]thymidine uptake, proliferation, activation, and AICD sensitivity of GITR−/− cells upon anti-CD3 triggering. / (A) [3H]thymidine uptake of splenocytes and total lymphocytes from lymph nodes (5 × 105 cells/mL) activated by plate-bound anti-CD3 mAb (clone 145-2C-11, Pharmingen) is shown. GITR−/− cells (filled columns) have a [3H]thymidine uptake significantly higher thanGITR+/+ cells (empty columns) (**P < .01 and ***P < .001). Each value is the mean ± SD of at least 3 independent experiments. A total of 2.5 μCi [3H]thymidine (Amersham International, Amersham, United Kingdom) per well was added to the cultures. (B) One of 5 independent experiments of [3H]thymidine uptake of purified T lymphocytes activated by soluble anti-CD3 mAb (0.2 μg/mL) on a feeder of irradiated (20 Gy, 5 minutes) splenocytes is shown. GITR−/− cells (filled circles) have a [3H]thymidine uptake significantly higher than GITR+/+ cells (empty triangles) (***P < .001 and **P < .01), in the presence (dashed lines) and in the absence (solid lines) of murine IL-2 (50 U/mL). UntreatedGITR−/− and GITR+/+cells incorporate less than 1000 cpm and are not shown. Each value is the mean ± SD of 4 counts. (C) Dose dependence of anti-CD3 mAb activation of purified T lymphocytes cultured on a feeder of irradiated splenocytes for 36 hours. One of 3 independent experiments is shown. Each value is the mean ± SD of 4 counts. The difference of incorporation between GITR−/− (filled circles) and GITR+/+ (empty triangles) cells was significant (***P < .001 and **P < .01). (D) T lymphocytes cultured on a feeder of irradiated splenocytes activated with soluble anti-CD3 (0.01 μg/mL) or with soluble anti-CD3 plus anti-CD28 (Pharmingen) (0.01 μg/mL) for 24 hours. One of 3 independent experiments is shown. Each value is the mean ± SD of 4 counts. GITR−/−cells (filled columns) have a [3H]thymidine uptake significantly higher than GITR+/+ cells (empty columns) (*P < .05). (E) GITR−/−cells enter into the cell cycle as a significantly higher number (**P < .01 and *P < .05) upon activation. Purified T lymphocytes from lymph nodes were activated by plate-bound anti-CD3 mAb. The percentage of T cells in S/G2/M phases is shown. Activated splenocytes showed similar results (not shown). Empty and filled columns represent GITR+/+ andGITR−/− lymphocytes, respectively. The values represent the mean ± SD of 3 independent experiments. (F) IL-2R expression, revealed by anti-CD25 phycoerythrin-conjugated mAb (Pharmingen), is significantly higher inGITR−/−–activated T lymphocytes (40% inGITR−/− vs 30% inGITR+/+ cells). T lymphocytes were activated by plate-bound anti-CD3 mAb for 24 hours. The thin line curve represents control sample. Basal values in unstimulated T lymphocytes (5.0% ± 1.3% in wild-type vs 3.1% ± 1.1% in GITR−/−) were similar (P > .05) (not shown). One of 3 independent experiments with similar results is shown. (G) IL-2 produced by GITR−/− T cells (filled columns) activated by platebound anti-CD3 mAb (10 μg/mL) 48 hours after activation is significantly higher (**P < .01) compared with GITR+/+–activated T cells (empty columns). Values represent the mean ± SD of 3 ELISA assays performed for each experimental condition. (H) Higher AICD in GITR−/− T lymphocytes (*P < .05). Cells were stimulated for 2 days with ConA and then plated at a density of 1 × 106/mL on wells that had been coated with anti-CD3 mAb (10 μg/mL), either in the absence or presence of murine IL-2 (50 U/mL). After 24 hours cells were harvested and stained with propidium iodide for evaluation of apoptosis. Empty and filled columns representGITR+/+ and GITR−/−splenocytes, respectively. Values represent the mean ± SD of 3 independent experiments.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/100/1/10.1182_blood-2001-12-0276/7/m_h81322757002.jpeg?Expires=1769144561&Signature=NwavpajXnPnn1skSs-joGo9kEOvLjIU1wxkv~i9xeHc5RjS~QiX87dM-8JqWwkbrHMQnklyuT88pwQVdp4CCj~mikJDaRbmNidgygogU6sB0J1Xk5p304SFK19I4N2~OoFX6r7XxvhVmQYslQD2BxAwE~Gmdf0Ppt8e6uD7AaEtVbx~oSbOdlVFTXkKuMnQSH8pPeuW05Oy9HUOmvm9-mxpuYIhLHulfgLS545Fc2xkQU7lwCWH-jMpL3JS6vcxBcsYmnhJxK7xB3UcJbEzx6KnO9xxPMJYOGC2zhy~p0HxTKHORTKNaJztiWu2LaORz3RbW59SR1T5K3DfREdKGOA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal