A small proportion of patients with deep vein thrombosis develop recurrent venous thromboembolic complications or bleeding during anticoagulant treatment. These complications may occur more frequently if these patients have concomitant cancer. This prospective follow-up study sought to determine whether in thrombosis patients those with cancer have a higher risk for recurrent venous thromboembolism or bleeding during anticoagulant treatment than those without cancer. Of the 842 included patients, 181 had known cancer at entry. The 12-month cumulative incidence of recurrent thromboembolism in cancer patients was 20.7% (95% CI, 15.6%-25.8%) versus 6.8% (95% CI, 3.9%- 9.7%) in patients without cancer, for a hazard ratio of 3.2 (95% CI, 1.9-5.4) The 12-month cumulative incidence of major bleeding was 12.4% (95% CI, 6.5%-18.2%) in patients with cancer and 4.9% (95% CI, 2.5%-7.4%) in patients without cancer, for a hazard ratio of 2.2 (95% CI, 1.2-4.1). Recurrence and bleeding were both related to cancer severity and occurred predominantly during the first month of anticoagulant therapy but could not be explained by sub- or overanticoagulation. Cancer patients with venous thrombosis are more likely to develop recurrent thromboembolic complications and major bleeding during anticoagulant treatment than those without malignancy. These risks correlate with the extent of cancer. Possibilities for improvement using the current paradigms of anticoagulation seem limited and new treatment strategies should be developed.
Introduction
Based on studies performed in the 1970s, it was established that patients with deep vein thrombosis (DVT) should receive anticoagulants for a period of 3 months to prevent recurrent venous thromboembolism (VTE).1 Further studies demonstrated that the presence of active cancer or thrombophilic conditions were persisting risk factors for recurrent DVT.2-5 Subsequently, longer durations of anticoagulation became gradually adopted in these patient groups.6,7Despite effective treatment, still approximately 5% to 7% of patients with DVT have recurrent venous thromboembolic complications during heparinization or the subsequent 3-month period of oral anticoagulant therapy.6,7 Patients with DVT who also have cancer seem to be at a higher risk for recurrent venous thromboembolic complications during anticoagulation.8-10 This risk may be related to release of procoagulants by tumor cells that could make patients with cancer resistant to the usual intensities of anticoagulant drugs.11-13 Also the risk of anticoagulant-induced bleeding seems to be enhanced in patients with cancer,9,14-16 although this is not consistently found.17 18 Little is known about whether the perceived higher risks for recurrent VTE and bleeding during anticoagulation apply to all patients with cancer or to specific groups.
We analyzed the data of our cohort of consecutive patients with a first episode of symptomatic DVT with specific attention to the period of anticoagulation. The aim of this analysis was to assess the relative risk for recurrent VTE and major bleeding during anticoagulant treatment in patients with and without cancer. We also addressed whether or not these risks were related to the stage of the malignant disease.
Patients and methods
Inception cohort
All outpatients referred to the thrombosis unit of the University of Padua with clinically suspected DVT were potentially eligible for the study if compression ultrasound or venography confirmed the presence of the disease.2 Patients were excluded from the study if they had had a previous episode of VTE, if anticoagulant therapy was not instituted, if they lived too far from the study center to return for follow-up sessions, or if informed consent was refused. Long-term follow-up data from part of this cohort have been published earlier.2 4
At the time of referral, demographic details were recorded and a medical history was taken. Patients with known cancer at baseline but also those who had cancer diagnosed during the initial hospital stay constituted the cancer group. The type and stage of the malignant disease were classified according to standard guidelines by a panel of 3 independent physicians. Patients with solid cancer were categorized as having extensive, moderately extensive, or less extensive malignancy according to the tumor-node-metastasis (TNM) classification.19 Thus, patients with stage IV disease were classified as having extensive malignancy, patients with stage III disease as moderately extensive malignancy, and patients with stage I or II disease as less extensive malignancy. For the purpose of this study, solid cancers were grouped as cancer of breast, gastrointestinal tract, genitourinary tract, lung, or other (rare) conditions. Patients with brain cancers and those with myeloproliferative or lymphoproliferative disorders were categorized as having extensive, moderately, or less extensive malignant disease according to standardized guidelines.20-24
Anticoagulant treatment and follow-up
Patients were treated with an initial course of high-dose adjusted intravenous standard heparin or body weight–adjusted low-molecular-weight heparin (LMWH).25,26 The use of unfractionated or LMWH depended on the availability of either compound and the preference of the treating physician, except for periods of inclusion of patients in clinical trials.8,25 27 Sodium warfarin was started during the first week of treatment and continued for a period of at least 3 months. Then, the duration of anticoagulation varied according to initial risk factors, usually being prolonged in patients with cancer or hereditary or acquired hypercoagulable states (antithrombin, protein C deficiency, protein S deficiency, or antiphospholipid antibody syndrome). The dose of oral anticoagulant therapy was monitored intensively during the first weeks until the international normalized ratio (INR) was stable between 2.0 and 3.0. Thereafter, the INR was checked regularly with a frequency of approximately twice a month. Heparin treatment was discontinued if it was given for at least 5 days and 2 consecutive INR values higher than 2.0 were obtained. The individual quality of warfarin anticoagulation was considered satisfactory if the INR was within or above the therapeutic range in more than 70% of determinations. The INR was temporarily reduced to 1.5 to 2.0 in patients with chemotherapy- or radiation-induced thrombocytopenia (ie, platelet count < 100 × 109/L).
Patients were instructed to attend the follow-up visits at 3, 6, and 12 months from the initial referral. However, patients were asked to return immediately to the thrombosis unit if they developed bleeding or symptoms suggestive of recurrent venous thromboembolism. At each follow-up visit, the medical history concerning general health, symptoms of recurrent VTE, and bleeding events were obtained using a standardized form. Patients who were not able to attend the follow-up sessions were visited at home. For all patients who died during follow-up, the date and cause of death were documented. An autopsy was performed for all patients in whom a pulmonary embolism could not be excluded as the cause of death.
Diagnosis of recurrent VTE and major bleeding
Recurrent DVT was diagnosed by venography or compression ultrasound.28,29 Patients with suspected pulmonary embolism underwent ventilation/perfusion lung scanning, followed by pulmonary angiography in case of indeterminate findings.30A diagnosis of fatal pulmonary embolism was based on the findings of autopsy or, if not done, on the opinion of an independent physician. All suspected venous thromboembolic complications were adjudicated by an independent committee. Bleeding was defined as major if it was overt and associated with either a decrease in the hemoglobin level (at least 2.0 g/dL) or the need for transfusion (2 units of blood or more), if it was retroperitoneal or intracranial, or if the treatment had to be discontinued permanently. If bleeding or thrombosis occurred, a set of laboratory tests was performed to assess the presence of disseminated intravascular coagulation. This diagnosis was made in case of a decrease in antithrombin, fibrinogen, and platelet count associated with a positive d-dimer test and a prolongation of both activated partial thromboplastin time (APTT) and prothrombin time (PT).31
Analysis
The period of follow-up considered for this analysis was the duration of anticoagulant therapy with a maximum of 1 year. Demographic and clinical characteristics of study patients were compared with the use of χ2 or Fisher exact test, as appropriate. The cumulative incidence of first episodes of recurrent thromboembolic events and that of major bleeding in patients with and without cancer was estimated according to the method of Kaplan and Meier. The hazard ratios and 95% CI of presence of cancer, and its severity categories, for the development of VTE, or bleeding were estimated by the Cox proportional hazard model and tested by the Wald statistic after checking the proportional assumption. Hazard ratios were adjusted for effects of age. Patients in whom anticoagulant therapy was stopped were censored for the analysis at the time of treatment cessation. Likewise, patients who developed malignant disease after hospital discharge remained in the group of patients classified as having no cancer but were censored for the analysis at the time of cancer diagnosis.P < .05 was considered to indicate statistical significance.
Results
Characteristics of study patients
Between January 1986 and December 1997, 1050 patients were potentially eligible for the study. Of these, 208 were excluded because of previous VTE (n = 129), geographic inaccessibility (n = 41), failure to institute proper anticoagulation (n = 28), or refusal to participate in the study (n = 10). Thus, 842 patients were included in the study. Of these patients, 181 had cancer already known at the time of referral or detected during admission, whereas the remaining 661 patients were apparently free from cancer. The demographic and clinical characteristics of study patients are shown in Table1. Patients with cancer were older and had a lower frequency of other risk factors for venous thrombosis than patients without cancer. The types of cancer are shown in Table2. Cancer in these 181 patients was extensive in 67 (37.0%), moderately extensive in 40 (22.1%), and less extensive in 74 (40.9%). Eight of the initial 661 cancer-free patients who developed cancer during follow-up were censored for the analysis at the time of diagnosis of the malignancy.
Demographic and clinical characteristics of study patients
| Characteristics . | Patients with cancer (%) n = 181 . | Patients without cancer (%) n = 661 . | P . |
|---|---|---|---|
| Age (mean ± SD) | 64.7 ± 11.4 | 60.1 ± 18.2 | .003 |
| Range | 31-90 | 15-96 | |
| Sex, M/F | 84/97 | 325/336 | |
| Patient-doctor delay* (d; median, range) | 7 (1-30) | 7 (1-30) | |
| Extent of thrombosis | |||
| Isolated calf | 11 (6.1) | 42 (6.3) | |
| Calf + popliteal | 15 (8.3) | 60 (9.1) | |
| Calf + popliteal + femoral | 63 (34.8) | 247 (37.4) | |
| All proximal veins | 82 (45.3) | 266 (40.2) | |
| Femoral and/or iliac | 10 (5.5) | 46 (6.9) | |
| Concomitant pulmonary embolism | 30 (16.6) | 92 (13.9) | |
| Additional risk factors | |||
| Chemotherapy | 36 (19.9) | NA | |
| Radiotherapy | 10 (5.5) | NA | |
| Recent trauma or surgery (<3 mo) | 55 (30.4) | 240 (36.3) | |
| Estrogen therapy | |||
| Pregnancy/childbirth | 6 (3.3) | 39 (5.9) | |
| 0 | 14 (2.1) | ||
| Initial treatment | |||
| Unfractionated heparin | 122 (67.4) | 442 (66.9) | |
| LMWH | 58 (32.0) | 216 (32.7) | |
| Thrombolytic drugs | 1 (0.5) | 3 (0.4) | |
| Length of oral anticoagulation | |||
| 6 mo or shorter | 79 (43.6) | 488 (73.8) | .001 |
| Longer than 6 mo | 102 (56.3) | 173 (26.2) | |
| Quality of oral anticoagulation | |||
| High quality | 137 (75.7) | 494 (74.7) | |
| Low quality | 44 (24.3) | 167 (25.3) |
| Characteristics . | Patients with cancer (%) n = 181 . | Patients without cancer (%) n = 661 . | P . |
|---|---|---|---|
| Age (mean ± SD) | 64.7 ± 11.4 | 60.1 ± 18.2 | .003 |
| Range | 31-90 | 15-96 | |
| Sex, M/F | 84/97 | 325/336 | |
| Patient-doctor delay* (d; median, range) | 7 (1-30) | 7 (1-30) | |
| Extent of thrombosis | |||
| Isolated calf | 11 (6.1) | 42 (6.3) | |
| Calf + popliteal | 15 (8.3) | 60 (9.1) | |
| Calf + popliteal + femoral | 63 (34.8) | 247 (37.4) | |
| All proximal veins | 82 (45.3) | 266 (40.2) | |
| Femoral and/or iliac | 10 (5.5) | 46 (6.9) | |
| Concomitant pulmonary embolism | 30 (16.6) | 92 (13.9) | |
| Additional risk factors | |||
| Chemotherapy | 36 (19.9) | NA | |
| Radiotherapy | 10 (5.5) | NA | |
| Recent trauma or surgery (<3 mo) | 55 (30.4) | 240 (36.3) | |
| Estrogen therapy | |||
| Pregnancy/childbirth | 6 (3.3) | 39 (5.9) | |
| 0 | 14 (2.1) | ||
| Initial treatment | |||
| Unfractionated heparin | 122 (67.4) | 442 (66.9) | |
| LMWH | 58 (32.0) | 216 (32.7) | |
| Thrombolytic drugs | 1 (0.5) | 3 (0.4) | |
| Length of oral anticoagulation | |||
| 6 mo or shorter | 79 (43.6) | 488 (73.8) | .001 |
| Longer than 6 mo | 102 (56.3) | 173 (26.2) | |
| Quality of oral anticoagulation | |||
| High quality | 137 (75.7) | 494 (74.7) | |
| Low quality | 44 (24.3) | 167 (25.3) |
Empty cells indicate not significant; NA, not applicable.
Delay from onset of DVT symptoms to start of anticoagulant therapy.
Hazard ratios of recurrent thromboembolism and major bleeding in the 181 patients with malignancy as compared to the 661 cancer-free patients according to the site of malignancy
| Site of cancer . | No. (%) . | Follow-up, patient-y . | Recurrent VTE hazard ratio (95% CI) . | Bleeding hazard ratio (95%CI) . |
|---|---|---|---|---|
| Genitourinary* | 47 (26.0) | 26.4 | 3.7 (1.7-8.0) | 4.5 (2.1-9.9) |
| Gastrointestinal† | 37 (20.4) | 18.4 | 5.1 (2.3-11.3) | 1.3 (0.3-5.6) |
| Breast | 27 (15.0) | 18.5 | 0.7 (0.1-4.9) | 2.3 (0.7-7.9) |
| Myelolymphoproliferative‡ | 24 (13.3) | 18.9 | 2.3 (0.7-7.5) | 1.6 (0.4-6.9) |
| Lung | 24 (13.3) | 14.9 | 6.9 (3.0-15.9) | 0.9 (0.1-7.2) |
| Brain | 14 (7.7) | 6.2 | 3.7 (0.8-14.1) | 0.0 |
| Other2-153 | 8 (4.4) | 5.0 | 2.3 (0.3-16.7) | 0.0 |
| Site of cancer . | No. (%) . | Follow-up, patient-y . | Recurrent VTE hazard ratio (95% CI) . | Bleeding hazard ratio (95%CI) . |
|---|---|---|---|---|
| Genitourinary* | 47 (26.0) | 26.4 | 3.7 (1.7-8.0) | 4.5 (2.1-9.9) |
| Gastrointestinal† | 37 (20.4) | 18.4 | 5.1 (2.3-11.3) | 1.3 (0.3-5.6) |
| Breast | 27 (15.0) | 18.5 | 0.7 (0.1-4.9) | 2.3 (0.7-7.9) |
| Myelolymphoproliferative‡ | 24 (13.3) | 18.9 | 2.3 (0.7-7.5) | 1.6 (0.4-6.9) |
| Lung | 24 (13.3) | 14.9 | 6.9 (3.0-15.9) | 0.9 (0.1-7.2) |
| Brain | 14 (7.7) | 6.2 | 3.7 (0.8-14.1) | 0.0 |
| Other2-153 | 8 (4.4) | 5.0 | 2.3 (0.3-16.7) | 0.0 |
Uterus (13 patients [numbers in parentheses are numbers of patients]), kidney (9), ovary or testicle (8), bladder (9), prostate (8).
Colorectal (19), stomach or esophagus (7), pancreas (7), liver or gallbladder (4).
Chronic leukemia (11), lymphoma (8), myeloma (5).
Melanoma (2), muscle sarcoma (2), rhinopharyngeal (1), laryngeal (1), vulvar (1), adrenal gland (1).
Anticoagulant treatment
The type of initial treatment and the quality of warfarin anticoagulation during follow-up were similar in patients with and without cancer. The duration of oral anticoagulation was shorter than 6 months in 79 (44%) cancer patients and in 488 (74%) cancer-free patients. In the remaining patients of both groups oral anticoagulation was continued for more than 6 months. The median duration of anticoagulation was 224 days (range, 4-360 days) in patients with cancer and 90 days (range, 3-360 days) in those without. The total duration of anticoagulation was 111.3 patient-years in cancer patients and 270.1 patient-years in cancer-free patients. In patients with extensive, moderately extensive, and less extensive cancer, the overall duration of anticoagulation was 25.1, 26.8, and 59.3 patient-years, respectively.
Recurrent VTE
Recurrent VTE occurred in 30 of the 181 patients with cancer (30.0/100 patient-years), and in 33 of the 661 patients without cancer (12.8/100 patient-years). Objective confirmation of VTE was obtained in all patients except 2 (one in each group) who died suddenly and did not undergo autopsy. Both patients were classified as having had pulmonary embolism.
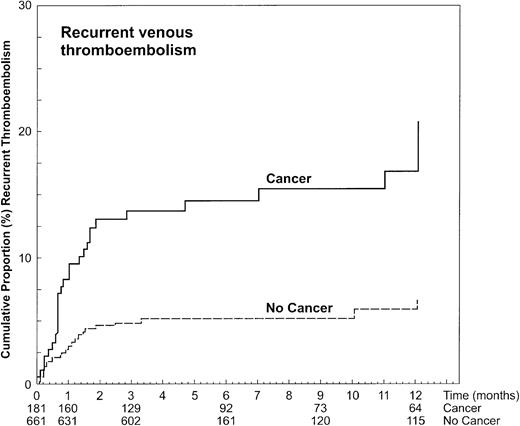
The 12-month cumulative incidence of recurrent thromboembolism in cancer patients was 20.7% (95% CI, 15.6%-25.8%) versus 6.8% (95% CI, 3.9%-9.7%; Figure 1) in patients without cancer. Adjusting for age, a hazard ratio of 3.2 (95% CI, 1.9-5.4) for recurrences in patients with cancer was observed. At the time of recurrence, the level of anticoagulation was within or above the therapeutic range in a higher proportion of patients with cancer (25 of 30 events, 83.3%) than in patients without cancer (19 of 33 events, 57.6%; P = .03).
The frequency of recurrent VTE per 100 patient-years in patients with extensive, moderately extensive, or less extensive disease was 54.1, 44.1, and 14.5, respectively. Compared to the rate observed in patients without cancer, the hazard ratios for recurrent thromboembolism of the respective groups were 4.6 (95% CI, 2.3-9.0), 5.3 (95% CI, 2.5-10.9), and 1.9 (95% CI, 0.8-4.2; Table 3). In 2 (6.7%; 95% CI, 0.8-22.1) of the 30 cancer patients with recurrent VTE, the episode occurred during or in direct conjunction (ie, within 3 weeks) with administration of chemotherapy.
Occurrence of recurrent venous thromboembolic and major bleeding complications in 181 patients with extensive, moderately extensive, or less extensive cancer and 661 venous thrombosis patients without cancer
| . | Extent of cancer . | ||||
|---|---|---|---|---|---|
| No cancer, n = 661 . | All cancer, n = 181 . | Extensive, n = 67 (37.0%) . | Moderately extensive, n = 40 (22.1%) . | Less extensive, n = 74 (40.9%) . | |
| Overall duration of anticoagulation (patient-y) | 270.1 | 111.3 | 25.1 | 26.8 | 59.3 |
| Recurrent VTE/100 patient-y | 12.8 | 30.0 | 54.1 | 44.1 | 14.5 |
| Hazard ratio (95% CI)3-150 | 3.2 (1.9-5.4) | 4.6 (2.3-9.0) | 5.3 (2.5-10.9) | 1.9 (0.8-4.2) | |
| Major bleeding/100 patient-y | 8.6 | 15.7 | 42.8 | 19.1 | 3.4 |
| Hazard ratio (95% CI)3-150 | 2.2 (1.2-4.1) | 4.8 (2.3-10.1) | 2.5 (0.9-6.7) | 0.5 (0.1-2.1) | |
| . | Extent of cancer . | ||||
|---|---|---|---|---|---|
| No cancer, n = 661 . | All cancer, n = 181 . | Extensive, n = 67 (37.0%) . | Moderately extensive, n = 40 (22.1%) . | Less extensive, n = 74 (40.9%) . | |
| Overall duration of anticoagulation (patient-y) | 270.1 | 111.3 | 25.1 | 26.8 | 59.3 |
| Recurrent VTE/100 patient-y | 12.8 | 30.0 | 54.1 | 44.1 | 14.5 |
| Hazard ratio (95% CI)3-150 | 3.2 (1.9-5.4) | 4.6 (2.3-9.0) | 5.3 (2.5-10.9) | 1.9 (0.8-4.2) | |
| Major bleeding/100 patient-y | 8.6 | 15.7 | 42.8 | 19.1 | 3.4 |
| Hazard ratio (95% CI)3-150 | 2.2 (1.2-4.1) | 4.8 (2.3-10.1) | 2.5 (0.9-6.7) | 0.5 (0.1-2.1) | |
As compared with patients without cancer.
Of the 30 recurrences in patients with cancer, 11 occurred in the ipsilateral leg and 12 in the contralateral leg, whereas 7 were isolated pulmonary embolism. Six of these 30 episodes occurred during the initial heparin treatment (1 in the 58 patients treated with LMWH, and 5 in the 122 patients treated with unfractionated heparin), whereas the remaining 25 occurred during the subsequent period of oral anticoagulation. Of the 33 recurrent events in patients without cancer, 3 occurred in the 8 patients who were later diagnosed with cancer. Of these recurrences in patients without cancer, 10 occurred in the ipsilateral leg and 12 in the contralateral leg, whereas 11 were isolated pulmonary embolism. Twelve of these 33 episodes occurred during the initial heparin treatment (2 in the 216 patients treated with LMWH, and 10 in the 442 patients treated with unfractionated heparin), whereas the remaining 22 occurred during the subsequent period of oral anticoagulation.
Major bleeding
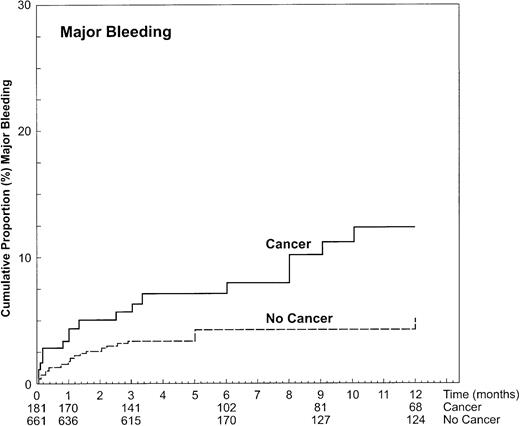
Major bleeding occurred in 17 of the 181 patients with cancer (15.7/100 patient-years), and in 23 of the 661 patients without cancer (8.6/100 patient-years). Of these latter 23 events, 1 occurred in the 8 patients who were later diagnosed with cancer. The 12-month cumulative incidence of major bleeding was 12.4% (95% CI, 6.5%-18.2%) in patients with cancer and 4.9% (95% CI, 2.5%-7.4%) in patients without cancer, for an age-adjusted hazard ratio of 2.2 (95% CI, 1.2-4.1; Figure 2). At the time of bleeding, the level of anticoagulation was above the therapeutic range in similar proportions of patients with cancer (4 of 17 events, 23.5%) and without cancer (8 of 23 events, 34.8%;P = .5), and was seen to a similar extent during initial heparinization and oral anticoagulant therapy.
Cumulative incidence of clinically important bleeding during anticoagulant therapy in DVT patients with and without cancer.
Cumulative incidence of clinically important bleeding during anticoagulant therapy in DVT patients with and without cancer.
The frequency of major bleeding per 100 patient-years was 42.8, 19.1, and 3.4 in patients with severe, moderately severe, and less severe cancer, respectively. Compared to the rate observed in patients without cancer, the hazard ratios for major bleeding was 4.8 (95% CI, 2.3-10.1), 2.5 (95% CI, 0.9-6.7), and 0.5 (95% CI, 0.1-2.1; Table 3), respectively. Two (11.8%; 95 CI, 1.5-36.4) of the 17 bleeds in cancer patients occurred during chemotherapy-induced thrombocytopenia (platelet counts < 50 × 109/L in both), despite an achieved low INR (ie, 1.6 and 1.8, respectively). In cancer patients, 5 of the 17 bleeds occurred during the initial heparin treatment (all of them in the 122 patients treated with unfractionated heparin, and none in the 58 patients treated with LMWH), whereas the remaining 12 occurred during the subsequent period of oral anticoagulation). In patients without cancer, 8 of the 23 bleeds occurred during the initial heparin treatment (1 in the 216 patients treated with LMWH, and 7 in the 442 patients treated with unfractionated heparin), whereas the remaining 14 occurred during the subsequent period of oral anticoagulation.
Additional observations
In Table 2, the hazard ratios of recurrent VTE and major bleeding are reported for patients with cancer at various sites as compared with patients without cancer. The point estimates of the obtained hazard ratios showed a comparable increased risk for recurrent VTE in all subgroups, with the exception of patients with breast cancer. However, in the latter group a 5-fold risk increase could still not be excluded. Similar results were obtained for the risk of major bleeding among the subgroups. Bleeding or thrombosis associated with diffuse intravascular coagulation was not observed.
Discussion
Our study demonstrates that patients with cancer have a higher risk for recurrent thromboembolic events and bleeding during anticoagulation than patients without cancer. The risk for recurrent VTE in cancer patients was increased around 4 times, both during the initial heparin treatment and the following course of oral anticoagulants. This translates to an on-treatment cumulative incidence of 20% after 1 year in patients with cancer. Likewise, the risk of bleeding during anticoagulation was approximately twice as high in cancer patients, both during initial heparinization and subsequent oral anticoagulation, for a 12-month cumulative incidence of 12%. This increased risk for recurrent VTE and bleeding in cancer patients was not due to more frequent anticoagulant intensities outside the therapeutic range. In fact, at the time of the recurrent event, anticoagulation was subtherapeutic in a smaller proportion of patients with cancer than in patients without cancer. Likewise, at the time of bleeding a smaller proportion of patients with cancer had supratherapeutic levels of anticoagulation. However, there appeared to be an association between the extent of cancer at baseline and the subsequent risks of complications. Overall, the risk for recurrences was 2- to 3-fold increased in patients with less extensive cancer, whereas in patients with extensive and moderately extensive cancer this increase was almost 5-fold. Similarly, although the risk of major bleeding was not increased among patients with less extensive cancer, it was increased by a factor of 2 to 3 in patients with moderately extensive cancer and by a factor of 5 in patients with extensive cancer. Pathophysiologic mechanisms that explain these associations include bleeding at the site of cancer, procoagulant states induced by cancer, and decline in general condition leading to immobilization.
We believe that our observations reflect the true risk for recurrent thromboembolism and bleeding during anticoagulation in cancer patients. Selection bias was avoided by including consecutive patients with objectively confirmed DVT who were referred to our center, which serves as a diagnostic facility for most general practitioners in the area of Padua. The demographic and clinical characteristics of our patients were indeed comparable to those from other large series of patients with symptomatic DVT.6 7 Patients were treated according to standard practice and prospective follow-up was complete. Control patients received similar treatment, had a comparable follow-up, and differed from study patients only with regard to the absence of cancer. A diagnostic work-up for VTE was performed only in patients with a clinical suspicion. Screening for venous thrombosis was not performed.
State-of-the-art, objective criteria were strictly applied for diagnosis of recurrent VTE, including contrast venography and pulmonary angiography if indicated, and major bleeding was classified according to widely accepted and validated criteria. Follow-up was confined to the first year after inclusion in the study because the expected high mortality rate in cancer patients would have precluded a subsequent reliable estimation of the risk of recurrent thromboembolism and bleeding in comparison with cancer-free patients beyond the first year.
Because of the relatively low number of patients with cancer, the relationship between site of malignancy and the patient's outcome was determined by assembling groups of neoplastic diseases. The point estimates of the obtained hazard ratios demonstrated a comparable increased risk for recurrent VTE in all subgroups, with the exception of patients with breast cancer. However, in the latter group a 5-fold risk increase could still not be excluded. Similar results were obtained for the risk of major bleeding among the subgroups.
The risk of bleeding in patients with and without cancer varied over time. Thus, one third of all bleeding occurred during the 5- to 10-day period of heparinization with an additional 30% bleeding during the following 4 weeks with oral anticoagulants. This increased risk is consistent with that observed by others,17 32 and could be a consequence of less well-controlled therapy at the start of treatment in combination with the presence of lesions predisposing to bleeding. Therefore, physicians should be aware of this risk profile and carefully monitor the intensity of anticoagulant therapy, especially during the first treatment weeks when the risk of bleeding is greatest.
A complicating factor in improving anticoagulant therapy in cancer patients is the occurrence of excess bleeding in combination with excess recurrent venous thromboembolic complications. Although some improvements can be expected from optimizing laboratory monitoring of anticoagulant therapy, most bleeding and thrombotic complications occurred in patients with anticoagulant parameters within the therapeutic range. Therefore, a change in anticoagulant intensity is a case of Hobson's choice where it is likely to achieve fewer thrombotic complications for the price of more bleeding or less bleeding for more thrombotic complications. An exception might be the group of patients with less extensive cancer because in this group the higher incidence of recurrent VTE is not associated with an increased bleeding risk. This suggests that in this group, approximately 40% of cancer patients with venous thrombosis, a more intense anticoagulant regimen might be considered. It is questionable whether the suggested replacement of oral anticoagulants by long-term heparin treatment,33 will provide substantial benefit, because the 5- to 10-day period of initial heparinization was associated with one third of all bleeding and one fourth of all thrombotic complications. Clearly, the use of inferior caval vein filters without anticoagulation in this patient group would eliminate the increased bleeding risk. However, the risk for recurrent leg vein thrombosis is then likely extremely high.34 The combination of caval vein filters and anticoagulants would maintain the increased bleeding risk but substantially reduce the risk for pulmonary embolism. However, because pulmonary embolism constitutes only 30% of recurrent events during anticoagulation and use of caval filters is associated with increased risk for recurrent leg vein thrombosis, the overall risk for recurrent events would remain high.
In conclusion, cancer patients with established venous thrombosis are more likely to develop recurrent thromboembolic complications and major bleeding than venous thrombosis patients without malignancy. These risks correlate with the extent of cancer. Possibilities for improvement using the current paradigms of anticoagulation seem limited and new treatment strategies should be developed.
Prepublished online as Blood First Edition Paper, July 12, 2002; DOI 10.1182/blood-2002-01-0108.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Anthonie W. A. Lensing, Center for Vascular Medicine, Academic Medical Center F4-237, Meibergdreef 9, 1105 AZ Amsterdam, The Netherlands.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal