Recent data suggest that vascular endothelial growth factor (VEGF), a cytokine involved in autocrine growth of tumor cells and tumor angiogenesis, is up-regulated and plays a potential role in myelogenous leukemias. In chronic myelogenous leukemia (CML), VEGF is expressed at high levels in the bone marrow and peripheral blood. We show here that the CML-associated oncogene BCR/ABL induces VEGF gene expression in growth factor–dependent Ba/F3 cells. Whereas starved cells were found to contain only baseline levels of VEGF mRNA, Ba/F3 cells induced to express BCR/ABL exhibited substantial amounts of VEGF mRNA. BCR/ABL also induced VEGF promoter activity and increased VEGF protein levels in Ba/F3 cells. Moreover, BCR/ABL was found to promote the expression of functionally active hypoxia-inducible factor-1 (HIF-1), a major transcriptional regulator of VEGF gene expression. BCR/ABL-induced VEGF gene expression was counteracted by the phosphoinositide 3-kinase (PI3-kinase) inhibitor LY294002 and rapamycin, an antagonist of mammalian target of rapamycin (mTOR), but not by inhibition of the mitogen-activated protein kinase pathway. Similarly, BCR/ABL-dependent HIF-1α expression was inhibited by the addition of LY294002 and rapamycin. Together, our data show that BCR/ABL induces VEGF- and HIF-1α gene expression through a pathway involving PI3-kinase and mTOR. BCR/ABL-induced VEGF expression may contribute to the pathogenesis and increased angiogenesis in CML.
Introduction
Chronic myelogenous leukemia (CML) is a neoplasm of myeloid progenitor cells expressing the 210-kDa form of BCR/ABL, an oncoprotein generated by the reciprocal translocation t(9;22).1-3 BCR/ABL exhibits constitutive tyrosine kinase activity leading to its autophosphorylation4 and to activation of multiple signaling molecules, including p21Ras,5 signal transducer and activator of transcription 5 (STAT5),6-8 and phosphoinositide 3-kinase (PI3-kinase).9 As a consequence, BCR/ABL leads to increased proliferation and inhibition of apoptosis.10,11Moreover, the expression of BCR/ABL converts growth factor–dependent cell lines to growth factor independence.12 However, though substantial progress has been made in the characterization of BCR/ABL-dependent signaling pathways, the exact mechanisms of transformation in vivo are not fully understood, and little is known about BCR/ABL-driven effector molecules contributing to the pathophysiology and clinical picture of CML.
Vascular endothelial growth factor (VEGF), also termed vascular permeability factor (VPF), was originally described as a major angiogenic and capillary leak–inducing cytokine.13 VEGF is expressed by a variety of human solid tumors and has been implicated in tumor-associated angiogenesis.14,15 Recent studies have shown that VEGF is also (over)expressed in neoplastic myeloid cells and may play a potential role in human leukemias.16 In particular, VEGF has been implicated in leukemia-associated angiogenesis and has been described as an autocrine growth factor for leukemic cells.17 In CML, increased serum levels of VEGF and expression of VEGF in the affected bone marrow have been reported.16 18 Thus, leukemia cell–derived VEGF may contribute, as an important effector molecule, to the pathogenesis of CML. Little is known so far about the mechanisms of VEGF expression in CML cells.
Numerous factors, including hypoxia and inflammation, are known to induce the expression of VEGF in diverse cells.19 Hypoxia, which is commonly observed in the microenvironment of solid tumors, has been implicated as a major stimulus of VEGF gene expression in neoplastic cells.19 However, recent studies have shown that several oncogenes, such as v-src and oncogenic forms of Ras, can induce VEGF production in tumor cells in the absence of hypoxia.20-22 Oncogene-dependent and hypoxia-induced expression of VEGF appear to involve similar signaling pathways and transcriptional regulators.23 A major regulator of VEGF gene expression is hypoxia inducible factor-1α (HIF-1α).24 This basic helix-loop-helix transcription factor not only contributes to hypoxia-induced VEGF production, it may also play a role in oncogene-dependent expression of VEGF.20 23
The aims of the present study were to examine whether the BCR/ABL oncogene induces VEGF gene expression and to characterize respective signal transduction pathways. To address these questions, Ba/F3 cells expressing BCR/ABL under the control of a doxycycline-inducible promoter, referred to as Ton.B210 cells,25 were used. To prolong survival under growth factor–free conditions, antiapoptotic Bcl-xL was coexpressed in Ton.B210 cells. Using this novel subtype of Ton.B210 cells (Ton.B210-X), we were able to compare BCR/ABL-dependent gene expression with gene expression in growth factor–deprived cells.
Materials and methods
Constructs
Plasmids used in this study were pMT-Ha-Ras-N17 (Mark Ewen, Dana Farber Cancer Institute, Boston, MA),26 pDCR-Ha-Ras-G12V (Yoel Kloog, Hadassah Medical Center, Jerusalem, Israel),27,28 pCMV5-Akt [-wt and -K179M] (Xiantao Wang, National Institute on Aging, National Institutes of Health, Baltimore, MD),29,30 and pCMV5-PKC-ζ-T410A (Alex Toker, Beth Israel Deaconess Medical Center, Boston, MA).31 Plasmids were cloned into the pcDNA3.1+ vector (Invitrogen, Carlsbad, CA). A VEGF-promoter luciferase plasmid (VEGF-Luc) was constructed by replacing the green fluorescent protein (GFP) gene within a pVEGF-GFP reporter gene construct32 (Brian Seed, Massachusetts General Hospital, Boston) by a luciferase gene fromPhotinus pyralis. The serum response element (SRE)–Luc plasmid was obtained from Clontech (Palo Alto, CA). The pRE-tk-Luc33 (hypoxia response element [HRE]-Luc) construct was provided by Steven McKnight (Southwestern Medical Center, Dallas, TX).
Cell lines and culture conditions
TonB.210 is an interleukin-3 (IL-3)–dependent Ba/F3-derived hematopoietic precursor cell line in which the 210-kDa form of BCR/ABL can conditionally be induced through the addition of doxycyline.25 TonB.210-X cells were generated by stable transfection of TonB.210 cells with a plasmid (pC1-EGFP) containing a cDNA encoding antiapoptotic Bcl-xL (kindly provided by Franck Gesbert, Boston, MA). After transfection, TonB.210-X cells were selected for their capacity to survive the withdrawal of IL-3. TonB.210-X cells were grown in RPMI 1640 medium (Mediatech Cellgro, Herndon, VA) with 10% (vol/vol) fetal calf serum (FCS) (Gibco BRL, Carlsbad, CA) and 10% (vol/vol) WEHI-3B conditioned medium (as a source of murine IL-3) in a humidified incubator at 37°C and 5% CO2. For starvation, cells were cultured in the absence of IL-3 for 16 to 72 hours. Expression of BCR/ABL was induced by the addition of doxycycline (1 μg/mL). In inhibition experiments, cells were incubated with the PI3-kinase inhibitor LY294002, MEK inhibitor PD98059, or rapamycin (all from Calbiochem, San Diego, CA). In typical experiments, 20 μM LY294002, 50 μM PD98059, and 20 nM rapamycin were applied for up to 24 hours. For induction of hypoxia, culture flasks were placed in sealed chambers containing GENbag anaer (bioMérieux, Marcy l'Etoile, France) for 12 hours. All experiments were performed in serum-free medium (UltraCulture, BioWhittaker, Walkersville, MD).
Isolation and culture of primary cells
Primary leukemic cells were obtained from 2 patients with untreated chronic-phase CML. Informed consent was obtained before blood donation. Peripheral blood mononuclear cells (PBMCs) were isolated by Ficoll density centrifugation. Isolated PBMCs were cultured in RPMI 1640 medium and 10% FCS in the presence of LY294002 (10 μM), PD98059 (10 μM), rapamycin (10 nM), or control medium at 37°C and 5% CO2 for 12 hours. After incubation, total RNA was isolated from cells and subjected to Northern blot analysis.
VEGF reporter gene assay
To determine VEGF promoter activity, the VEGF-Luc construct (12.5 μg) was transfected together with a pCMV-βGAL construct (12.5 μg) by electroporation (0.35 kV, 960 μF; Gene Pulser; Bio-Rad, Hercules, CA) into TonB.210-X cells. Thereafter, cells were maintained in the presence or absence of doxycycline (1 μg/mL) for 48 hours in serum-free medium. Cells were then harvested, and the pellets were resuspended in lysis buffer-E397A (Promega, Madison, WI). Lysates (20 μL) were incubated with 200 μL luciferase assay buffer (25 mM glycylglycine [pH 7.8], 15 mM potassium phosphate [pH 7.8], 15 mM MgSO4, 4 mM EGTA [ethyleneglycoltetraacetic acid], 2 mM adenosine triphosphate [ATP], 1 mM dithiothreitol [DTT]), and 100 μL D-luciferin (0.3 mg/mL; PharMingen, San Diego, CA). Luciferase activity was determined by the use of an automated luminometer (ML 3000 Microtiter Plate Luminometer; Dynatech Laboratories, Chantilly, VA). Plasmid pCMV-βGAL (Invitrogen) was used as a reporter for transfection efficiency. We used the βGAL Assay Kit (Invitrogen) to determine βGAL activity. VEGF reporter gene activity is given as a ratio of luciferase activity to βGAL activity. In separate experiments, various plasmids (Ras-N17, Ras-G12V, Akt-K179M, PKC-ζ–T410A; 8 μg each) were transfected together with VEGF-Luc (8 μg) and pCMV-βGAL (8 μg) into TonB.210-X cells.
Northern blot analysis
Total RNA was extracted from TonB.210-X cells or primary CML-derived PBMCs using Trizol (Gibco BRL) according to the manufacturer's instructions. Northern blotting was performed essentially as described.34 In brief, 20 μg RNA were size fractionated on 1.0% formaldehyde–agarose gels, transferred to synthetic membranes (Hybond N; Amersham, Aylesbury, United Kingdom), and cross-linked by UV irradiation (UV Stratalinker 1800; Stratagene, San Diego, CA). Membranes were prehybridized at 42°C for 4 hours in prehybridization buffer (50% formamide, 10% dextran sulfate, 5 × Denhardt solution (1 × Denhardt solution consists of 0.02% bovine serum albumin [BSA], 0.02% polyvinyl pyrrolidone, and 0.02% Ficoll), 0.8 M NaCl, 0.1% Na-pyrophosphate, 50 mM Tris, 0.1% sodium dodecyl sulfate (SDS) and salmon sperm DNA (10 μg/mL; Gibco BRL). Hybridization was performed with32P-labeled cDNA specific for VEGF, HIF-1α, and β-actin. Primers for polymerase chain reaction (PCR) amplification of probes were as follows: murine VEGF, 5′-TGTGCAGGCTGCTGTAACGA-3′ (forward) and 5′-CGCCTTGGCTTGTCACATCT-3′ (reverse); murine β-actin, 5′-GACGGCCAGGTCATCACTAT-3′ (forward) and 5′-AGGGAGACCAAAGCCTTCAT-3′ (reverse); murine HIF-1α, 5′-AAACACTCCTAACTTTTCCCAGC-3′ (forward) and 5′-AGGAATGAGATTAGGAAACGCTC-3′ (reverse); human VEGF, 5′-ATGAACTTTCTGCTGTCTTGGG-3′ (forward) and 5′-CCGCCTCGGCTTGTCACATCTGC-3′ (reverse); human β-actin, 5′-ATGGATGATGATATCGCCGCG-3′ (forward) and 5′-CTAGAAGCATTTGCGGTGGACGATGGAGGGGCC-3′ (reverse). Labeling was performed using the Megaprime kit (Amersham). Blots were washed in 2 × SSC (1 × SSC consists of 150 mM NaCl and 15 mM sodium citrate, pH 7.0) with 0.1% SDS at room temperature for 1 hour and thereafter in 0.2 × SSC and 0.1% SDS at 42°C for 30 minutes and at 62°C for another 30 minutes. Bound radioactivity was visualized by exposure to Biomax MS film (Eastman Kodak, Rochester, NY) at −80°C using intensifying screens. mRNA expression levels were quantified by densitometry of autoradiograms using E.A.S.Y. Win32 software (Herolab, Wiesloch, Germany).
Immunometric detection of VEGF
To determine the levels of VEGF protein secreted from TonB.210-X cells, a commercial enzyme-linked immunosorbent assay (ELISA; Quantikine M mouse VEGF; R&D Systems, Minneapolis, MN) was used. ELISA was performed following the recommendations of the manufacturer. The detection limit of the ELISA amounted to 3 pg/mL VEGF.
Results
Generation of Ba/F3 cells inducibly expressing BCR/ABL with a prolonged capacity for starvation
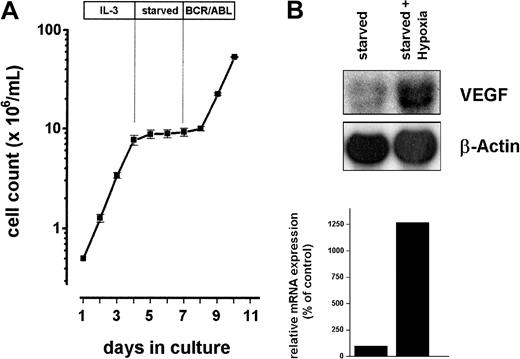
Ba/F3 is a murine hematopoietic cell line that requires IL-3 for growth and viability and that undergoes apoptosis after growth factor withdrawal lasting more than 24 hours.12 However, Ba/F3 cells transformed with an oncogene such as BCR/ABL become IL-3 independent.12 Therefore, Ba/F3 cells inducibly expressing BCR/ABL (Ton.B210 cells) are frequently used for the investigation of early BCR/ABL-dependent signaling events.12 25 To study BCR/ABL-induced protein expression, longer starvation periods (longer than 24 hours) are required. We generated TonB.210 cells stably expressing antiapoptotic Bcl-xL. This new clone of TonB.210 exhibits a prolonged capacity for starvation and is referred to as TonB.210-X throughout this article. Like TonB.210, TonB.210-X cells could be kept in IL-3, and they expressed BCR/ABL on exposure to doxycycline. When deprived of IL-3, TonB.210-X cells were found to survive for several days without proliferation and without significant loss of viability. In particular, even after prolonged starvation, TonB.210-X cells resumed growth on the induction of BCR/ABL, similar to the growth kinetics observed with IL-3 (Figure 1A). Moreover, Ton.B210-X cells starved for 2 days were still able to up-regulate VEGF-mRNA when subjected to hypoxia (Figure 1B). Together, TonB.210-X cells represent a useful tool for the investigation of BCR/ABL-dependent protein expression.
Growth kinetics and induction of VEGF in Ton.B210-X cells.
(A) Ton.B210-X cells were grown in IL-3 for 3 days, starved of IL-3 for another 3 days, and then induced to express BCR/ABL by the addition of doxycycline. (B) Ton.B210-X cells were starved for 48 hours and were maintained under hypoxic conditions (starved + hypoxia) or normal atmosphere (starved) overnight. Total RNA was extracted and subjected to Northern blot analysis using a murine VEGF probe. The β-actin loading control and densitometric evaluation of VEGF mRNA expression relative to β-actin mRNA expression (relative mRNA expression) is shown below the blot analyses.
Growth kinetics and induction of VEGF in Ton.B210-X cells.
(A) Ton.B210-X cells were grown in IL-3 for 3 days, starved of IL-3 for another 3 days, and then induced to express BCR/ABL by the addition of doxycycline. (B) Ton.B210-X cells were starved for 48 hours and were maintained under hypoxic conditions (starved + hypoxia) or normal atmosphere (starved) overnight. Total RNA was extracted and subjected to Northern blot analysis using a murine VEGF probe. The β-actin loading control and densitometric evaluation of VEGF mRNA expression relative to β-actin mRNA expression (relative mRNA expression) is shown below the blot analyses.
BCR/ABL induces expression of VEGF mRNA and activates the VEGF promoter in Ton.B210-X cells
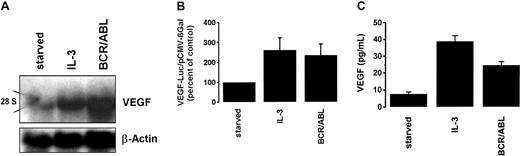
To investigate whether BCR/ABL would induce VEGF gene expression, TonB.210-X cells grown in the presence of doxycycline were compared with IL-3–deprived cells. Whereas starved cells were found to contain only baseline levels of VEGF mRNA, Ton.B210-X cells induced to express BCR/ABL exhibited substantial amounts of VEGF mRNA (Figure2A). A similar increase in VEGF mRNA levels was seen when cells were maintained in IL-3 (Figure 2A). We next asked whether the induction of VEGF mRNA by BCR/ABL would depend on transcriptional activation of the VEGF promoter. For this purpose, Ton.B210-X cells were transfected with a VEGF promoter construct (VEGF-Luc). After transfection, cells were split and were maintained in the presence or absence of doxycycline. As shown in Figure 2B, the induction of BCR/ABL was associated with a 2- to 3-fold increase in VEGF reporter gene activity compared with starved (IL-3–deprived) cells. A similar effect of IL-3 on VEGF reporter gene activity was observed (Figure 2B). These data show that the BCR/ABL oncogene induces VEGF expression through transcriptional activation of the VEGF promoter in TonB.210-X cells. To confirm the effects of BCR/ABL on VEGF expression at the protein level, we measured immunogenic VEGF in cell culture supernatants by ELISA. In these experiments, the induction of BCR/ABL resulted in a substantial increase of VEGF secreted from Ton.B210-X cells. A similar effect was obtained with IL-3 (Figure2C).
BCR/ABL- and IL-3–induced VEGF gene expression in Ton.B210-X cells.
(A) Northern blot analysis. Starved Ton.B210-X cells were maintained in the presence (BCR/ABL) or absence (starved) of doxycycline and then subjected to Northern blotting. In addition, Ton.B210-X cells cultured in IL-3 were examined. Northern blot analysis was performed using a murine VEGF probe. After stripping the blot, a β-actin probe was applied. 28S indicates the position of 28S rRNA. (B) VEGF promoter activity. Ton.B210-X cells were transfected with the VEGF-Luc and pCMV-βGal plasmids and were grown in serum-free medium in the presence (BCR/ABL) or absence (starved) of doxycycline for 48 hours. In addition, cells were maintained in IL-3. After incubation, cells were harvested and assayed for the expression of luciferase and βGal activities. Luciferase activity was reported as the ratio VEGF-Luc/pCMV-βGal and was expressed as a percentage of control (starved cells). (C) VEGF ELISA. Supernatants of Ton.B210-X cells incubated with (BCR/ABL) or without (starved) doxycycline or cultured in IL-3 for 24 hours were collected and examined for the levels of secreted VEGF by ELISA. Results represent the means ± SD of 3 independent experiments.
BCR/ABL- and IL-3–induced VEGF gene expression in Ton.B210-X cells.
(A) Northern blot analysis. Starved Ton.B210-X cells were maintained in the presence (BCR/ABL) or absence (starved) of doxycycline and then subjected to Northern blotting. In addition, Ton.B210-X cells cultured in IL-3 were examined. Northern blot analysis was performed using a murine VEGF probe. After stripping the blot, a β-actin probe was applied. 28S indicates the position of 28S rRNA. (B) VEGF promoter activity. Ton.B210-X cells were transfected with the VEGF-Luc and pCMV-βGal plasmids and were grown in serum-free medium in the presence (BCR/ABL) or absence (starved) of doxycycline for 48 hours. In addition, cells were maintained in IL-3. After incubation, cells were harvested and assayed for the expression of luciferase and βGal activities. Luciferase activity was reported as the ratio VEGF-Luc/pCMV-βGal and was expressed as a percentage of control (starved cells). (C) VEGF ELISA. Supernatants of Ton.B210-X cells incubated with (BCR/ABL) or without (starved) doxycycline or cultured in IL-3 for 24 hours were collected and examined for the levels of secreted VEGF by ELISA. Results represent the means ± SD of 3 independent experiments.
Induction of VEGF by BCR/ABL depends on PI3-kinase but not on p21Ras or MAPK/ERK-kinase
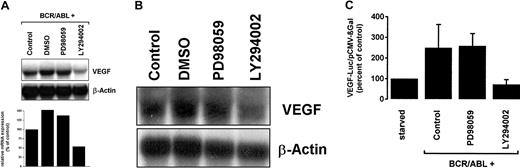
To investigate the signaling pathways contributing to BCR/ABL-dependent VEGF mRNA expression, we applied inhibitors of MEK (MAPK/ERK-kinase [mitogen-activated protein kinase/extracellular signal–regulated kinase-kinase]; PD98059) and PI3-kinase (LY294002) on Ton.B210-X cells. As shown in Figure3A, treatment of BCR/ABL-expressing Ton.B210-X cells with LY294002 resulted in a considerable decrease in VEGF mRNA levels. In contrast, treatment with the MEK inhibitor PD98059 showed no inhibitory effect on BCR/ABL-induced VEGF mRNA expression in Ton.B210-X cells (Figure 3A).
Effects of PD98059 and LY294002 on BCR/ABL-induced VEGF gene expression.
(A) Ton.B210-X cells grown in the presence of doxycycline were incubated with MEK inhibitor PD98059 (50 μM), PI3-kinase inhibitor LY294002 (20 μM), dimethyl sulfoxide (DMSO; solvent control), or control medium (control) for 24 hours. After incubation, cells were harvested and subjected to Northern blotting using a VEGF cDNA probe. The β-actin loading control and a densitometric quantification of VEGF mRNA levels (relative to β-actin) are shown below the blot analyses. (B) Primary leukemic peripheral blood cells derived from a patient with chronic-phase BCR/ABL+ CML were incubated with PD98059 (10 μM), LY294002 (10 μM), DMSO, or control medium for 12 hours and then subjected to Northern blotting. The β-actin loading control is also shown. (C) Ton.B210-X cells induced to express BCR/ABL by the addition of doxycycline were incubated with PD98059 (50 μM), LY294002 (20 μM), or control medium for 24 hours. Induced and starved Ton.B210-X cells (not expressing BCR/ABL) were examined for VEGF reporter gene activity. Results represent the means ± SD of 3 independent experiments.
Effects of PD98059 and LY294002 on BCR/ABL-induced VEGF gene expression.
(A) Ton.B210-X cells grown in the presence of doxycycline were incubated with MEK inhibitor PD98059 (50 μM), PI3-kinase inhibitor LY294002 (20 μM), dimethyl sulfoxide (DMSO; solvent control), or control medium (control) for 24 hours. After incubation, cells were harvested and subjected to Northern blotting using a VEGF cDNA probe. The β-actin loading control and a densitometric quantification of VEGF mRNA levels (relative to β-actin) are shown below the blot analyses. (B) Primary leukemic peripheral blood cells derived from a patient with chronic-phase BCR/ABL+ CML were incubated with PD98059 (10 μM), LY294002 (10 μM), DMSO, or control medium for 12 hours and then subjected to Northern blotting. The β-actin loading control is also shown. (C) Ton.B210-X cells induced to express BCR/ABL by the addition of doxycycline were incubated with PD98059 (50 μM), LY294002 (20 μM), or control medium for 24 hours. Induced and starved Ton.B210-X cells (not expressing BCR/ABL) were examined for VEGF reporter gene activity. Results represent the means ± SD of 3 independent experiments.
We next asked whether VEGF expression in primary leukemic cells would also involve PI3-kinase. For this purpose, pharmacologic inhibitors were applied on leukemic cells derived from patients with BCR/ABL+ CML. As expected, these cells were found to express VEGF mRNA in a constitutive manner. Addition of LY294002 for 12 hours resulted in decreased expression of VEGF mRNA in CML cells, whereas no effect was seen with PD98059 (Figure 3B).
Next we investigated the effects of inhibitors on BCR/ABL-induced VEGF promoter activity in Ton.B210-X cells. As shown in Figure 3C, LY294002 completely abolished BCR/ABL-induced VEGF promoter activity in these cells, whereas the addition of PD98059 showed no inhibitory effect.
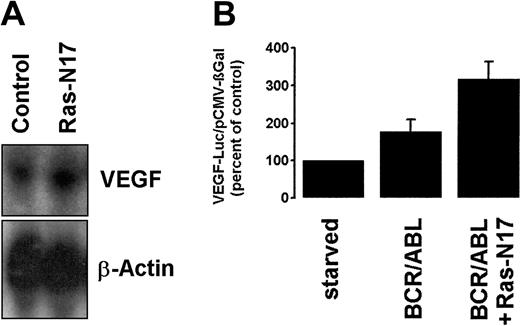
Because Ras activation is an important event in BCR/ABL-dependent signaling,5 we also investigated the role of p21Ras in BCR/ABL-induced VEGF gene expression. For this purpose, a dominant-negative form of Ras (Ras-N17) was applied. However, transfection of BCR/ABL-expressing Ton.B210-X cells with Ras-N17 did not result in a decrease of VEGF mRNA expression (Figure4A). Moreover, the Ras-N17 mutant failed to inhibit BCR/ABL-induced VEGF promoter activity in Ton.B210-X cells (Figure 4B). In control experiments, Ras-N17 decreased BCR/ABL-dependent SRE transcriptional activity in the same cells (not shown). Together, our data suggest that BCR/ABL-induced VEGF mRNA expression and VEGF promoter activity involve the PI3-kinase pathway but are not dependent on signaling through MAPKs.
Effect of a dominant-negative Ras mutant (Ras-N17) on BCR/ABL- induced VEGF gene expression.
(A) Ton.B210-X cells induced to express BCR/ABL were transfected with Ras-N17 or a control plasmid (control) and were subjected to Northern blot analysis. The blot was probed for VEGF and reprobed for β-actin. (B) VEGF promoter activity. Ton.B210-X cells were cotransfected with VEGF-Luc/pCMV-βGAL and Ras-N17 or a control plasmid. Results represent the means ± SD of 3 independent experiments.
Effect of a dominant-negative Ras mutant (Ras-N17) on BCR/ABL- induced VEGF gene expression.
(A) Ton.B210-X cells induced to express BCR/ABL were transfected with Ras-N17 or a control plasmid (control) and were subjected to Northern blot analysis. The blot was probed for VEGF and reprobed for β-actin. (B) VEGF promoter activity. Ton.B210-X cells were cotransfected with VEGF-Luc/pCMV-βGAL and Ras-N17 or a control plasmid. Results represent the means ± SD of 3 independent experiments.
Induction of VEGF reporter gene activity through oncogenic Ras is down-regulated by the PI3-kinase inhibitor LY294002
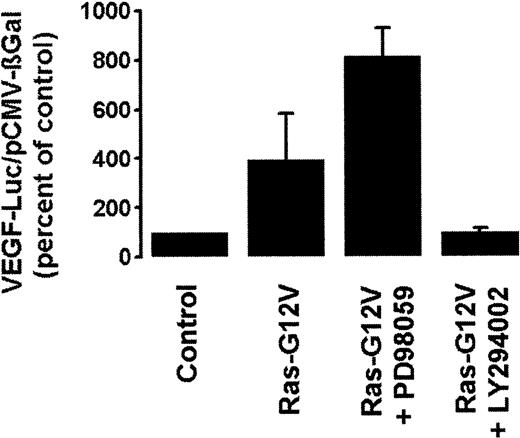
Oncogenic forms of Ras have been shown to induce VEGF expression in diverse cell types.21 22 However, as described above, the dominant-negative Ras-N17 mutant failed to inhibit BCR/ABL-dependent VEGF gene expression in Ton.B210-X cells. We therefore asked whether Ras activation can lead to VEGF gene expression in Ton.B210-X cells, and we compared respective signaling pathways. For this purpose, Ton.B210-X cells were transfected with the Ras-G12V mutant. Expression of Ras-G12V resulted in an approximately 4-fold increase in VEGF reporter gene activity when compared with control. Interestingly, this effect of oncogenic Ras could not be inhibited by the addition of PD98059, but it was abolished by LY294002 (Figure 5). These data suggest that oncogenic Ras induces transcriptional activation of the VEGF gene through the activation of PI3-kinase (but not through the MAPK pathway), similar to BCR/ABL-induced VEGF gene expression.
Effect of pharmacologic inhibitors on Ras-G12V–induced VEGF reporter gene activity.
Ton.B210-X cells were transfected with the oncogenic Ras-G12V mutant or a control plasmid (together with VEGF-Luc/pCMV-βGal) and incubated with PD98059 (25 μM), LY294002 (20 μM), or control medium. After 48 hours, cells were harvested and assayed for luciferase and βGal activities. VEGF promoter activity was reported as the ratio VEGF-Luc/pCMV-βGal and was expressed as a percentage of control (transfection with control plasmid; Control). Results represent the means ± SD of 3 independent experiments.
Effect of pharmacologic inhibitors on Ras-G12V–induced VEGF reporter gene activity.
Ton.B210-X cells were transfected with the oncogenic Ras-G12V mutant or a control plasmid (together with VEGF-Luc/pCMV-βGal) and incubated with PD98059 (25 μM), LY294002 (20 μM), or control medium. After 48 hours, cells were harvested and assayed for luciferase and βGal activities. VEGF promoter activity was reported as the ratio VEGF-Luc/pCMV-βGal and was expressed as a percentage of control (transfection with control plasmid; Control). Results represent the means ± SD of 3 independent experiments.
Role of Akt and PKC-ζ in BCR/ABL-dependent VEGF gene expression
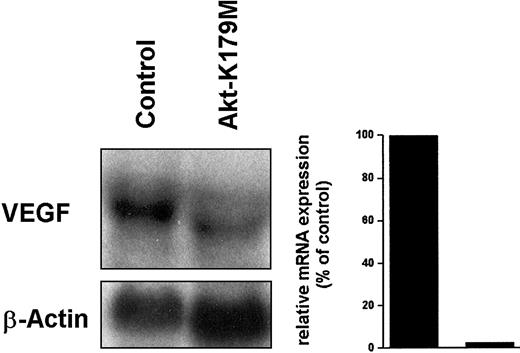
The generation of 3′-phosphorylated phosphoinositides through activated PI3-kinase recruits a number of kinases to the cell membrane, including PDK-1, Akt, and PKC-ζ.31,35 We attempted to identify PI3-kinase downstream targets possibly involved in BCR/ABL-induced VEGF expression. We investigated the effects of a kinase-dead mutant of Akt, Akt-K179M, that acts in a dominant-negative manner.36 For this purpose, Ton.B210-X cells induced to express BCR/ABL were transfected with Akt-K179M. As shown in Figure6, the expression of Akt-K179M resulted in a decrease of VEGF mRNA expression.
Effect of a dominant-negative Akt mutant on BCR/ABL-dependent VEGF mRNA expression.
Ton.B210-X cells induced to express BCR/ABL were transfected with the dominant-negative Akt-K179M mutant or a control plasmid (Control) and were subjected to Northern blotting. The blot was probed for VEGF and reprobed for β-actin. Densitometric evaluation of VEGF mRNA expression is shown below the blot analyses.
Effect of a dominant-negative Akt mutant on BCR/ABL-dependent VEGF mRNA expression.
Ton.B210-X cells induced to express BCR/ABL were transfected with the dominant-negative Akt-K179M mutant or a control plasmid (Control) and were subjected to Northern blotting. The blot was probed for VEGF and reprobed for β-actin. Densitometric evaluation of VEGF mRNA expression is shown below the blot analyses.
Another downstream effector of PI3-kinase is PKC-ζ, an atypical member of the PKC family.31 This kinase is activated through PDK-1 and has recently been shown to play a role in VEGF gene expression in renal cancer cells.22 However, transfection of Ton.B210-X cells with the dominant-negative T410A mutant of PKC-ζ31 did not result in the inhibition of BCR/ABL-induced VEGF reporter gene activity (not shown). These data suggest that BCR/ABL induces VEGF gene expression through a PI3-kinase/Akt-dependent pathway in Ton.B210-X cells.
Rapamycin inhibits BCR/ABL-induced expression of VEGF
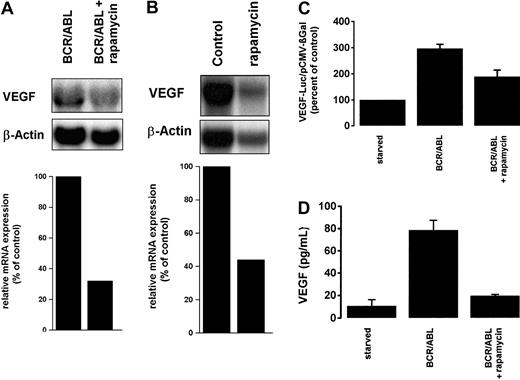
Signaling molecules located downstream of Akt (also known as protein kinase B) include the mammalian target of rapamycin (mTOR) and p70 S6-kinase.37-39 To investigate whether mTOR and p70 S6 kinase are involved in BCR/ABL-dependent VEGF expression, we conducted experiments using the specific inhibitor rapamycin.40Treatment of Ton.B210-X cells with rapamycin resulted in a substantial decrease in BCR/ABL-dependent expression of VEGF mRNA (Figure7A). Moreover, rapamycin was found to down-regulate the constitutive expression of VEGF mRNA in primary BCR/ABL+ CML cells (Figure 7B). We next asked whether rapamycin would influence BCR/ABL-dependent VEGF promoter activity and VEGF protein expression. As shown in Figure 7C-D, exposure of Ton.B210-X cells to rapamycin resulted in a decrease of VEGF reporter gene activity and in the inhibition of BCR/ABL-induced secretion of VEGF in Ton.B210-X cells.
Effects of rapamycin on BCR/ABL-induced VEGF gene expression.
(A) Ton.B210-X cells induced to express BCR/ABL were treated with rapamycin (20 nM) (BCR/ABL + rapamycin) or control medium (BCR/ABL) for 24 hours and then subjected to Northern blot analysis using a murine VEGF probe. (B) Primary peripheral blood mononuclear cells obtained from a patient with BCR/ABL+ CML were cultured in the presence (rapamycin) or absence (Control) of rapamycin (10 nM) for 12 hours. Cells were subjected to Northern blot analysis using a human VEGF cDNA probe. The β-actin loading control and densitometric quantifications of VEGF mRNA expression are shown below the blot analyses. (C) VEGF reporter gene activity was measured in Ton.B210-X cells. Cells induced to express BCR/ABL were incubated with rapamycin (20 nM) or control medium for 24 hours. Induced and starved Ton.B210-X cells (not expressing BCR/ABL) were examined for VEGF reporter gene activity. Results represent the means ± SD of 3 independent experiments. (D) Ton.B210-X cells induced to express BCR/ABL were cultured in the presence (BCR/ABL + rapamycin) or absence (BCR/ABL) of rapamycin (20 nM) for 36 hours. Starved Ton.B210-X cells were also examined. After incubation, cell-free supernatants were collected and examined for secreted VEGF by ELISA. Results represent the means ± SD of 3 independent experiments.
Effects of rapamycin on BCR/ABL-induced VEGF gene expression.
(A) Ton.B210-X cells induced to express BCR/ABL were treated with rapamycin (20 nM) (BCR/ABL + rapamycin) or control medium (BCR/ABL) for 24 hours and then subjected to Northern blot analysis using a murine VEGF probe. (B) Primary peripheral blood mononuclear cells obtained from a patient with BCR/ABL+ CML were cultured in the presence (rapamycin) or absence (Control) of rapamycin (10 nM) for 12 hours. Cells were subjected to Northern blot analysis using a human VEGF cDNA probe. The β-actin loading control and densitometric quantifications of VEGF mRNA expression are shown below the blot analyses. (C) VEGF reporter gene activity was measured in Ton.B210-X cells. Cells induced to express BCR/ABL were incubated with rapamycin (20 nM) or control medium for 24 hours. Induced and starved Ton.B210-X cells (not expressing BCR/ABL) were examined for VEGF reporter gene activity. Results represent the means ± SD of 3 independent experiments. (D) Ton.B210-X cells induced to express BCR/ABL were cultured in the presence (BCR/ABL + rapamycin) or absence (BCR/ABL) of rapamycin (20 nM) for 36 hours. Starved Ton.B210-X cells were also examined. After incubation, cell-free supernatants were collected and examined for secreted VEGF by ELISA. Results represent the means ± SD of 3 independent experiments.
BCR/ABL induces HIF-1α mRNA expression and transcriptional activity
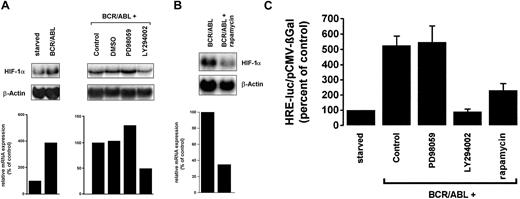
HIF-1, a heterodimeric basic helix-loop-helix transcription factor, is a major regulator of VEGF gene expression.41,42We investigated the effect of BCR/ABL on HIF-1α expression in TonB.210-X cells. In these experiments, BCR/ABL was found to up-regulate HIF-1α mRNA expression (Figure8A). As was BCR/ABL-dependent VEGF expression, the induction of HIF-1α mRNA expression by BCR/ABL was inhibitable by the PI3-kinase inhibitor LY294002 (Figure 8A) and by rapamycin (Figure 8B), but not by PD98059. We next asked whether the BCR/ABL-induced increase in HIF-1α mRNA expression in Ton.B210-X cells would also result in enhanced transcriptional HIF-1 activity. For this purpose, a reporter gene construct containing the hypoxia response element (HRE), which represents the HIF-1α–binding site within the VEGF promoter, was applied.33 As shown in Figure 8C, the expression of BCR/ABL in Ton.B210-X cells led to an approximately 5-fold increase in HRE-reporter gene activity. The addition of LY294002 or rapamycin inhibited BCR/ABL-induced HRE reporter gene activity, whereas the MEK inhibitor PD98059 showed no effect (Figure 8C). These data suggest that binding of HIF-1 to HRE sites within the VEGF promoter contributes to BCR/ABL-induced VEGF gene expression.
Effects of pharmacologic inhibitors on BCR/ABL-dependent expression of HIF-1α mRNA and HIF-1α transcriptional activity.
(A) Ton.B210-X cells were starved or grown in the presence of doxycycline and were incubated with the MEK-inhibitor PD98059 (25 μM), the PI3-kinase inhibitor LY294002 (20 μM), DMSO, or control medium for 16 hours. A densitometric evaluation of HIF-1α mRNA is shown below. (B) The effect of rapamycin (20 nM) on BCR/ABL-dependent expression of HIF-1α mRNA in Ton.B210-X cells is shown. Northern blotting was performed using a murine HIF-1α probe. After reprobing for β-actin, autoradiograms were subjected to densitometry, the results of which are shown below the blot analyses. (C) Effect of inhibitors on BCR/ABL-induced HRE-promoter activity. Ton.B210-X cells were starved or induced to express BCR/ABL and were incubated with inhibitors as indicated (50 μM PD98059; 20 μM LY294002; 20 nM rapamycin). After 48 hours, cells were assayed for luciferase and βGal activities. HRE transcriptional activity was reported as the ratio HRE-Luc/pCMV-βGal and was expressed as percentage of control (starved cells). Results represent the means ± SD of 3 independent experiments.
Effects of pharmacologic inhibitors on BCR/ABL-dependent expression of HIF-1α mRNA and HIF-1α transcriptional activity.
(A) Ton.B210-X cells were starved or grown in the presence of doxycycline and were incubated with the MEK-inhibitor PD98059 (25 μM), the PI3-kinase inhibitor LY294002 (20 μM), DMSO, or control medium for 16 hours. A densitometric evaluation of HIF-1α mRNA is shown below. (B) The effect of rapamycin (20 nM) on BCR/ABL-dependent expression of HIF-1α mRNA in Ton.B210-X cells is shown. Northern blotting was performed using a murine HIF-1α probe. After reprobing for β-actin, autoradiograms were subjected to densitometry, the results of which are shown below the blot analyses. (C) Effect of inhibitors on BCR/ABL-induced HRE-promoter activity. Ton.B210-X cells were starved or induced to express BCR/ABL and were incubated with inhibitors as indicated (50 μM PD98059; 20 μM LY294002; 20 nM rapamycin). After 48 hours, cells were assayed for luciferase and βGal activities. HRE transcriptional activity was reported as the ratio HRE-Luc/pCMV-βGal and was expressed as percentage of control (starved cells). Results represent the means ± SD of 3 independent experiments.
Discussion
Recent data suggest that VEGF plays a role as an autocrine growth factor and a mediator of tumor-associated angiogenesis in various hematopoietic neoplasms.16-18 Chronic myelogenous leukemia is a hematopoietic neoplasm characterized by the presence of the BCR/ABL oncogene.1 2 In this study, we show that the expression of BCR/ABL leads to the induction of VEGF in Ton.B210-X cells. BCR/ABL-induced VEGF gene expression was found to be down-regulated by inhibition of PI3-kinase and by rapamycin, an inhibitior of mTOR. Moreover, BCR/ABL was found to up-regulate HIF-1α, a major regulator of VEGF gene expression.
Recent data suggest that BCR/ABL-dependent expression of Bcl-xL counteracts apoptosis and thus contributes to the viability of CML cells.7,43 44 In the present study, we selected Bcl-xL as a survival factor of Ton.B210 cells. Because BCR/ABL itself induces Bcl-xL, the activation of additional BCR/ABL-independent signaling pathways that would have influenced VEGF gene expression in our experimental system could be excluded. In this regard it was important to show that starved Ton.B210-X cells contained only baseline levels of VEGF compared with Ton.B210-X cells maintained in IL-3. The expression of Bcl-xL in Ton.B210-X cells enhanced viability but did not seem to up-regulate VEGF gene expression. Thus, Ton.B210-X cells appeared to be a suitable cell system in which to analyze BCR/ABL-dependent expression of VEGF.
A number of previous studies have shown that BCR/ABL-dependent signaling involves the PI3-kinase pathway as well as Ras and downstream MAPKs (Raf, MEK, ERK).5,45,46 In the present study, the PI3-kinase inhibitor LY294002 was found to down-modulate BCR/ABL-induced VEGF gene expression in Ton.B210-X cells, whereas no effect was observed with the MEK inhibitor PD98059. These observations suggest that the BCR/ABL-induced expression of VEGF in Ton.B210-X cells involves the PI3-kinase pathway, but not the Raf-MEK-ERK cascade. These data are in line with studies performed on epidermal growth factor (EGF) receptor–mediated expression of VEGF in human glioblastoma and prostate cancer cell lines.47,48 In addition, it has been shown that oncogenic Ras induces VEGF expression through a PI3-kinase–dependent pathway in human epithelial breast cancer cell lines,23 corresponding with our results obtained from oncogenic Ras expressed in Ton.B210-X cells. In other studies, however, it was found that v-Ras– and v-Raf–induced expression of VEGF in NIH-3T3 fibroblasts is suppressed by the MEK inhibitor PD98059 but not by LY294002.49 Thus, oncogene-dependent VEGF expression may involve variable signaling pathways, depending on the cell type analyzed. It was therefore of considerable importance to investigate signaling pathways contributing to VEGF expression in primary BCR/ABL+ human CML cells. In these experiments, the PI3-kinase inhibitor LY294002 was found to down-regulate the expression of VEGF mRNA in leukemic cells, whereas no effect was observed with PD98059. These data suggest that the BCR/ABL-dependent expression of VEGF is mediated, at least in part, through PI3-kinase. On the other hand, the PI3-kinase inhibitor LY294002 did not completely inhibit BCR/ABL-dependent expression of VEGF mRNA. This may point to additional pathways potentially involved in BCR/ABL-dependent VEGF induction. In fact, BCR/ABL-dependent signaling has recently been shown to involve the STAT5 pathway.6-8 In this regard it is noteworthy that a possible link between STATs, angiogenesis, and VEGF has recently been proposed.50 Whether signaling through STATs can indeed contribute to BCR/ABL-dependent expression of VEGF remains to be determined.
Given that oncogenic Ras up-regulates VEGF gene expression in various cells and that BCR/ABL is well known to activate Ras,5,21we also asked whether Ras contributes to BCR/ABL-dependent VEGF expression in Ton.B210-X cells. For this purpose, the dominant-negative Ras-N17 mutant was applied.26 However, Ras-N17 failed to counteract BCR/ABL-induced expression of VEGF mRNA and VEGF promoter activity in Ton.B210-X cells, though Ras-N17 was found to inhibit SRE-reporter gene activity in the same cells (not shown). These data suggest that VEGF induction by BCR/ABL in Ton.B210-X cells is mediated by PI3-kinase but does not require BCR/ABL-dependent signaling through Ras, which itself can activate PI3 kinase51-54 (Figure 9). To exclude a general defect in Ras–PI3-kinase signaling leading to VEGF expression in Ton.B210-X cells, we conducted experiments using the oncogenic Ras-G12V mutant. As did BCR/ABL, oncogenic Ras substantially up-regulated VEGF promoter activity in Ton.B210-X cells, and LY294002 was found to inhibit Ras-G12V–dependent activation of the VEGF promoter.
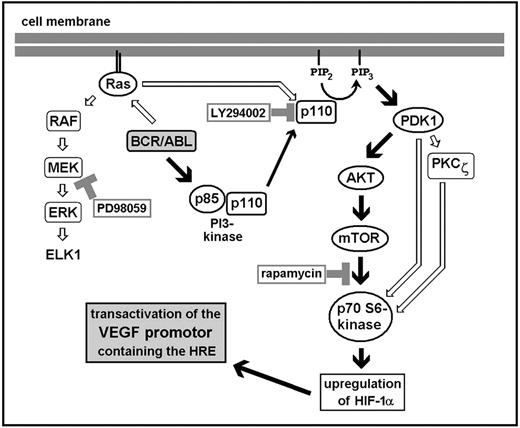
BCR/ABL-dependent VEGF gene expression: proposed signaling pathways.
Activation of Ras and PI3-kinase by BCR/ABL leads to MAPK signaling (Raf, MEK, ERK) and to activation of signaling molecules downstream of PI3-kinase. Based on results obtained with Ton.B210-X cells and primary CML-derived cells, it is hypothesized that VEGF induction by BCR/ABL is mediated through sequential activation of PI3-kinase, phosphoinositide-dependent kinase 1 (PDK1), Akt, mTOR, and p70 S6 kinase. As a result, the transcription factor HIF-1α is up-regulated. HIF-1, in turn, binds to HRE sites within the VEGF promoter and transcriptionally activates the VEGF gene.
BCR/ABL-dependent VEGF gene expression: proposed signaling pathways.
Activation of Ras and PI3-kinase by BCR/ABL leads to MAPK signaling (Raf, MEK, ERK) and to activation of signaling molecules downstream of PI3-kinase. Based on results obtained with Ton.B210-X cells and primary CML-derived cells, it is hypothesized that VEGF induction by BCR/ABL is mediated through sequential activation of PI3-kinase, phosphoinositide-dependent kinase 1 (PDK1), Akt, mTOR, and p70 S6 kinase. As a result, the transcription factor HIF-1α is up-regulated. HIF-1, in turn, binds to HRE sites within the VEGF promoter and transcriptionally activates the VEGF gene.
The PI3-kinase pathway includes a number of downstream effector molecules, among them Akt, PKC-ζ, and mTOR31,35 (Figure9). With regard to stress-induced VEGF gene expression, Akt and PKC-ζ have already been implicated as critical signaling molecules.55 In the present study, we were able to show that BCR/ABL-induced VEGF gene expression involves Akt and mTOR, but not PKC-ζ. In particular, the dominant-negative Akt-K179M mutant inhibited BCR/ABL-induced VEGF mRNA expression in Ton.B210-X cells, whereas a dominant-negative PKC-ζ construct showed no inhibitory effect. These data suggest that BCR/ABL induces VEGF expression in Ton.B210-X cells in a PI3-kinase– and an Akt-dependent manner. Corresponding results have been reported for epidermal growth factor receptor–dependent VEGF expression in glioblastoma and prostate cancer cell lines48 and for oncogenic Ras-induced VEGF expression in human breast cancer cells.23
mTOR, a member of the PI3–kinase related kinase family, is an important signaling molecule downstream of PI3-kinase and Akt37,38 (Figure 9). In particular, mTOR activates p70 S6 kinase and eIF-4E binding protein 1 (4E-BP1), thereby controlling the translation of specific subsets of mRNA.56,57 We sought to determine whether BCR/ABL-induced VEGF gene expression in Ton.B210-X cells would depend on signaling through mTOR. In respective experiments, application of the specific mTOR/p70 S6 kinase inhibitor rapamycin led to a substantial decrease in BCR/ABL-induced VEGF expression at the promoter, mRNA, and protein level in Ton.B210-X cells. Moreover, we were able to show that rapamycin inhibits VEGF mRNA expression in primary leukemic cells derived from patients with BCR/ABL+ CML. These data suggest that BCR/ABL-dependent VEGF gene expression requires mTOR and p70 S6 kinase activity. Interestingly, the HER2 (neu)-induced expression of VEGF in breast cancer cells is also dependent on signaling through mTOR.58 These data point to a novel emerging concept involving oncogene-induced VEGF gene expression and specific pharmacologic inhibitors targeting mTOR.
The basic helix-loop-helix transcription factor HIF-1α is a major regulator of VEGF gene expression.41,42 In fact, HIF-1α mediates hypoxia-induced and oncogene-induced expression of VEGF in diverse cells.23 Likewise, HIF-1α has been implicated in Ras-G12V–induced expression of VEGF in breast cancer cells.23 In addition, HIF-1α has recently been shown to contribute to VEGF induction by HER2 in human breast cancer cells.58,59 Interestingly, in breast cancer cells, HER2-dependent up-regulation of HIF-1α expression was reported to occur at the level of HIF-1α protein synthesis, with no change in HIF-1α mRNA expression.58 By contrast, in the present study, we show that the BCR/ABL oncogene augments the expression of HIF-1α mRNA in Ton.B210-X cells. A corresponding observation has been reported for the v-Src oncogene that was found to up-regulate the expression of HIF-1α mRNA in a rat hepatoma cell line.20 As was BCR/ABL-induced VEGF expression, BCR/ABL-induced HIF-1α expression was inhibitable by LY294002 and rapamycin. Because HIF-1α is a well-known transcriptional regulator of VEGF, our data suggest that BCR/ABL-dependent VEGF gene expression is mediated through HIF-1α. This is further supported by our finding that a reporter construct containing HIF-1α–binding sites (present within the VEGF promoter) was activated upon expression of BCR/ABL. Recent data suggest that apart from HIF-1α, other transcriptional regulators might be involved in VEGF gene expression in neoplastic cells.47 48 Therefore, it remains unknown whether HIF-1α is indeed the only transcription factor involved in BCR/ABL-dependent VEGF gene expression.
Recent data suggest that VEGF plays a role as an autocrine growth factor and an angiogenesis-promoting cytokine in myeloid leukemias.17 In CML, overexpression of VEGF and increased angiogenesis are consistent findings.16,18 However, little is known about biochemical mechanisms leading to VEGF expression in CML cells. We show that primary leukemic cells obtained from patients with BCR/ABL+ CML express VEGF mRNA in a constitutive manner. Furthermore, we were able to demonstrate that BCR/ABL-induced expression of VEGF is dependent on PI3-kinase and mTOR activity. An interesting aspect of the present study was that targeting mTOR by rapamycin not only suppressed BCR/ABL-induced VEGF gene expression in Ton.B210-X cells, but also inhibited the constitutive expression of VEGF mRNA in primary cells derived from patients with CML. Whether mTOR-dependent expression of VEGF in CML cells plays a role in leukemia cell growth and is inhibited by rapamycin in vivo in patients with CML remains to be determined. In this regard it is also of interest that mTOR has been implicated as a major regulator of cell-cycle progression and tumor-cell growth.37 38
In summary, our data show that BCR/ABL induces VEGF gene expression through a pathway involving PI3-kinase, Akt, mTOR, and HIF-1α. These observations may have implications for the pathogenesis of CML and for the development of new treatment strategies.
We thank Yasamin Majlesi and Hans Semper for skillful technical assistance and Winfried F. Pickl, Richard Moriggl, Edgar Selzer, and Reinhard Kodym for helpful discussion. We thank Dr George Q. Daley (Boston, MA) for providing TonB.210 cells and the following people for sharing plasmids: Drs Mark Ewen, Yoel Kloog, Xiantao Wang, Alex Toker, Brian Seed, and Steven McKnight.
Prepublished online as Blood First Edition Paper, July 18, 2002; DOI 10.1182/blood-2002-01-0109.
Supported by the Fonds zur Förderung der Wissenschaftlichen Forschung in Österreich grants P-15487, P-14031, and SFB 005/01.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Christian Sillaber, Department of Internal Medicine I, Division of Hematology and Hemostaseology, University of Vienna, AKH-Wien, Währinger Gürtel 18-20, A-1097 Vienna, Austria; e-mail: christian.sillaber@univie.ac.at.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal