The adhesion of sickle erythrocytes to vascular endothelium is important to the generation of vascular occlusion. Interactions between sickle cells and the endothelium use several cell adhesion molecules. We have reported that sickle cell adhesion to endothelial cells under static conditions involves P-selectin. Others have shown that sickle cell adhesion is decreased by unfractionated heparin, but the molecular target of this inhibition has not been defined. We postulated that the adhesion of sickle cells to P-selectin might be the pathway blocked by unfractionated heparin. In this report we demonstrate that the flow adherence of sickle cells to thrombin-treated human vascular endothelial cells also uses P-selectin and that this component of adhesion is inhibited by unfractionated heparin. We also demonstrate that sickle cells adhere to immobilized recombinant P-selectin under flow conditions. This adhesion too was inhibited by unfractionated heparin, in a concentration range that is clinically attainable. These findings and the general role of P-selectin in initiating adhesion of blood cells to the endothelium suggest that unfractionated heparin may be useful in preventing painful vascular occlusion. A clinical trial to test this hypothesis is indicated.
Introduction
Vascular occlusion is responsible for much of the morbidity associated with sickle cell disease.1,2 Although the underlying cause of sickle cell disease is a single nucleotide mutation that directs the production of an easily polymerized hemoglobin protein,3,4 both the erythrocyte sickling caused by hemoglobin polymerization and the interactions between a proadhesive population of sickle cells and the vascular endothelium are essential to vascular occlusion.5 6
The correlation between sickle cell adhesiveness and the severity of vascular occlusion7 led to extensive studies of the molecular mechanisms of adhesion. These pathways recently have been reviewed8-12 and are briefly described here. Sickle red cells express adhesion molecules including integrin α4β1, CD36, band 3 protein, sulfated glycolipid, Lutheran protein, phosphatidylserine, and integrin-associated protein. The proadhesive sickle cells may bind to endothelial cell P-selectin, E-selectin, vascular cell adhesion molecule-1 (VCAM-1), CD36, and integrins. Activation of endothelial cells by specific agonists enhances adhesion by inducing the expression of cellular adhesion molecules and by causing cell contraction, which exposes extracellular matrix proteins, such as thrombospondin (TSP), laminin, and fibronectin. Bridging molecules from the plasma such as von Willebrand factor (VWF) and TSP also participate in certain of these adhesive interactions.
Sickle cell adhesion has been studied under both static and flow conditions.13 The relevance of both types of adhesion is suggested by in vivo observations of laminar, intermittent, and antegrade-retrograde flow in the microvasculature of sickle cell subjects and sickle cell mouse models.14-16 Initial events likely involve the adhesion of sickle erythrocytes to activated endothelial cells under laminar flow. The resultant adhesion of cells to the vascular wall creates nonlaminar and arrested flow, which propagates vascular occlusion by both static and flow adhesion mechanisms. It is likely too that the distinct mechanisms of adhesion and of regulation of endothelial cell adhesivity pertain under dissimilar types of flow.17
The expression of adhesion molecules by endothelial cells is affected by cell agonists such as thrombin, histamine, tumor necrosis factor α (TNF-α), interleukin 1 (IL-1), platelet activating factor (PAF), erythropoietin, and vascular endothelial growth factor (VEGF), and by local environmental factors such as hypoxia, reperfusion, flow, as well as by sickle erythrocytes themselves.18,19 An important effector in sickle cell vascular occlusion is thrombin. Increased thrombin activity correlates with sickle cell disease pain episodes.20,21 In addition to generating fibrin clot, thrombin also acts on specific thrombin receptors on endothelial cells and platelets.22,23 Work from our laboratory has demonstrated that thrombin treatment causes a rapid increase of endothelial cell adhesivity for sickle erythrocytes under static conditions.24
More recently we have demonstrated that P-selectin participates in this thrombin-enhanced adhesion of sickle cells to endothelial cells under static conditions.25 Although P-selectin plays a major role in the tethering, rolling, and firm adhesion of leukocytes to activated endothelial cells,26,27 its contribution to the initial steps is singular and essential to the overall adhesion process. Upon stimulation of endothelial cells by thrombin, P-selectin rapidly translocates from Weibel-Palade bodies to the luminal surface of the cells.28,29 In addition to thrombin, the agonists histamine, hypoxia, and reperfusion also have been documented to induce the expression of P-selectin on human endothelial cell surfaces.28-31 While each of these is germane to the activation of endothelial cells in sickle cell disease, we have chosen thrombin as the agonist of P-selectin expression for our studies. In addition to our report of sickle red cells binding to endothelial cell P-selectin, others have shown a role for P-selectin in the adhesion of neutrophils in sickle cell mouse models.32 33
Heparin is known to block certain types of tumor cell adherence, TSP-independent sickle cell adherence, and coagulation processes that are active in sickle cell disease.34-38 In one uncontrolled study, prophylactic administration of heparin reduced the frequency of sickle cell pain crises.39 The role of P-selectin in the endothelial adhesion of sickle red blood cells,25 the capacity of heparin to block selected P-selectin–mediated adhesive events,34 and the effect of heparin on sickle cell adhesion suggest an association among these findings.35 36 We postulated that heparin can be used to block adhesion of sickle cells to P-selectin under flow conditions. We tested this hypothesis by studying the adhesion of sickle erythrocytes to thrombin-treated endothelium and to immobilized P-selectin under flow conditions in vitro. Others have used this approach to measure the firm adherence of sickle cells. We, however, because of the critical importance of initial adhesive interactions to overall cell adherence, utilized the full potential of the method to measure both early and late adhesive interactions. Our studies suggest potential new therapeutic approaches to treating sickle cell disease.
Materials and methods
Preparation of erythrocytes
Blood samples obtained from subjects with sickle cell disease and from healthy control subjects, as approved by the Committee on Human Research at the University of California-San Francisco (UCSF), were drawn into citrate. The buffy coat was removed after the initial centrifugation and after each of 3 subsequent washes of the remaining erythrocytes in phosphate buffered saline (PBS) and one wash in HAH buffer (Hanks balanced salt solution [HBSS; UCSF Cell Culture Facility, San Francisco, CA], 1% bovine serum albumin [BSA, Fraction V; Sigma, St Louis, MO], 50 mM HEPES [N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid; Sigma], pH 7.40). Erythrocytes to be used in flow adhesion studies were suspended to a 0.5% hematocrit. To assess for leukocyte contamination, the erythrocyte preparation was exposed to 10 μg/mL rhodamine 6G, which stains leukocytes but not erythrocytes, and washed 3 times in HAH.40 No leukocytes were detected in the erythrocyte suspensions prepared using the above protocol.
Cell culture
Human umbilical vein endothelial cells (HUVECs; Clonetics, San Diego, CA) were grown in endothelial growth medium (EGM; Clonetics) at 37°C in 5% CO2 on gelatin-coated glass slides. HUVECs used for the adhesion experiment were no more than third passage and 90% to 95% confluent. To rid HUVECs of the heparin and fetal bovine serum, before treating the cells or assaying for adhesivity, we washed the monolayers 3 times with PBS and once with HAH.
Preparation of immobilized adhesion molecules
For studies of adherence to immobilized adhesion protein, 400 ng BSA, recombinant human Siglec-6-Ig chimera (hereafter referred to as recombinant Siglec), or recombinant human P-selectin–Ig chimera (hereafter referred to as recombinant P-selectin) in 10 mM carbonate buffer were applied to slides overnight.34 41Slides were washed with carbonate buffer 3 times, then blocked with 0.5% BSA in HBSS (UCSF Cell Culture Facility).
Parallel plate flow adhesion
Because of the vital importance of tethering and rolling adhesion to overall cell adhesion,27,42 we elected to measure the parameters of these early adhesive events rather than firm adhesion in our studies of P-selectin. Parallel plate flow adhesion experiments were performed as previously described.43Slides containing HUVECs or immobilized protein were attached to the base of a CytoShear parallel plate chamber (CytoDyne, San Diego, CA). All experiments were performed with cells and reagents in HAH. A shear stress of 1 dyne/cm2, which is estimated as that of normal postcapillary venular flow,44 was maintained using a Sage syringe pump (Orion, Beverly, MA). Temperature was maintained at 37°C using Heating Tape (Fisher, Pittsburgh, PA) and a Temperature Controller (Cole-Parmer, Vernon Hills, IL). Rolling cells were defined as those sickle cells that were in the same microscopic focal plane as the immobile surface or endothelial monolayer and moving at a distinctly slower velocity than the bulk flow. Those cells determined to be rolling were in contact with the substrata throughout their entire transit through the field of observation, which excludes those cells in brief transient contact with the substrate. Data on rolling adherence are expressed as the mean numbers of rolling red blood cells (cells/mm2) and the mean rolling velocity of these cells (mm/s) from multiple experiments; error is expressed as SEM. The microscopic field in which adherent cells were measured has an area of 0.15 mm2 and a volume of 0.038 mm3. In serial assessments of adhesion under different experimental conditions, only rare firmly adherent sickle cells were encountered. In such instances, a different microscopic field was selected randomly for determining rolling adhesion. The velocity of rolling cells was determined by the time required for a cell to roll across the 0.5-mm field (0.5 mm/s). Firmly adherent cells were defined as those cells whose location, under flow conditions, do not change over a 1-second (30-frame) observation time. Data on firm adhesion are expressed as the mean numbers of firmly adherent red blood cells in the area described above. Statistical significance was determined using the Student t test.
Endothelial cell activation and adhesion-blocking treatments
In some experiments, HUVECs were treated with 0.1 U/mL thrombin by exposure for 5 minutes in the parallel plate chamber. For blocking experiments, a 1:200 dilution of adhesion-blocking anti–P-selectin monoclonal antibody (mAb) 9E1 (R&D Systems, Minneapolis, MN), a 1:200 dilution of nonblocking control anti–P-selectin mAb AC1.2 (BD Pharmingen, San Diego, CA), 400 μg/mL laboratory-grade unfractionated porcine intestine heparin (Sigma), or 400 μg/mL laboratory-grade low-molecular-weight porcine intestinal heparin (Sigma) was infused into the parallel plate chamber over thrombin-treated HUVECs or immobilized recombinant P-selectin. This concentration of heparin is similar to published concentrations used in adhesion-blocking experiments36 and is equivalent to 50 U/mL. We also compared the antiadhesion effects of laboratory-grade (Sigma) and clinical-grade (Elkins-Sinn, Cherry Hill, NJ) unfractionated heparins at concentrations encompassing 4 orders of magnitude.
These interventions and the measurements of flow adhesion were made serially, in the following order: control adhesion to unstimulated endothelial monolayers, thrombin infusion, adhesion to activated endothelial monolayers, adhesion-blocking agent (mAb and/or heparin), and adhesion to blocked monolayers. In the case of immobilized adhesion proteins, thrombin infusion was not used.
Results
Published work from our laboratory has demonstrated that sickle cells are more adherent to P-selectin than nonsickle red cells under static conditions.25 Others have shown that sickle cells also have greater adhesivity for endothelium under flow conditions.36 Because of the vital importance of initial tethering and rolling adhesion to overall cell adhesion,27 42 we chose to emphasize parameters of rolling rather than firm adhesion in our experiments.
The flow adhesion of sickle cells is mediated by P-selectin in vitro
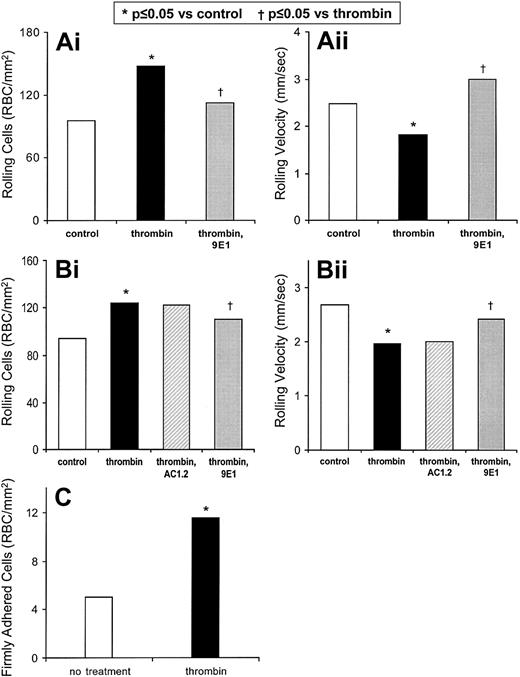
To test whether P-selectin has a role in the adhesion of sickle cells to thrombin-treated endothelium under flow conditions in vitro, we used a parallel plate chamber under ambient oxygen conditions. Under these conditions, the polymerization of hemoglobin S is not induced, and the sickle cells retain their antecedent shape. In 10 replicate experiments, we found that the flow adhesion of sickle erythrocytes was indeed increased after thrombin treatment of the HUVECs (Figure1A). All adherent cells had the shape of normal biconcave discs. Treatment of HUVECs with 0.1 U/mL thrombin for 5 minutes increases the number of rolling sickle cells by 54% (P < .001, n = 10; Figure 1Ai) and decreases their rolling velocities by 26% (P < .001, n = 10; Figure 1Aii). P-selectin antibody treatment of endothelial cells decreased thrombin-enhanced rolling adhesion in vitro (Figure 1A). P-selectin mAb 9E1 reduces thrombin-enhanced adhesion of rolling cells by 68% (P = .002, n = 10; Figure 1Ai) and increases their rolling velocities by 72% (P < .001, n = 10; Figure 1Aii). The nonblocking P-selectin mAb AC1.2 (isotype matched to 9E1) does not significantly affect the thrombin-enhanced number of rolling sickle cells (Figure 1Bi) or their rolling velocities (Figure 1Bii). Compared with mAb AC1.2, mAb 9E1 reduces the thrombin effect on number of rolling cells (P = .030, n = 3) and the rolling velocity (P = .007). These results indicate that the thrombin-enhanced component of rolling adhesion of sickle cells to endothelial cells primarily involves P-selectin.
P-selectin is important in the flow adhesion of sickle erythrocytes to thrombin-stimulated endothelium in vitro.
The number of sickle cells adhering to HUVECs (i) and the rolling velocities of the adhering cells (ii) were examined. (A) In 10 experiments, the rolling adhesion of sickle cells to HUVECs was examined prior to and after treatment of HUVECs with thrombin. The rolling adhesion of sickle cells to thrombin-treated HUVECs was then examined in the presence of anti–P-selectin mAb 9E1. Statistically significant differences compared with untreated HUVECs (*) and to thrombin-treated HUVECs (††) are indicated. (B) In 3 experiments the rolling adhesion of sickle cells to thrombin-treated HUVECs also was examined in the presence of nonblocking anti–P-selectin mAb AC1.2. Statistically significant differences compared with untreated HUVECs (*) and to thrombin-treated HUVECs (†) are indicated. (C) In 10 experiments the firm adhesion of sickle cells to HUVECs was examined prior to and after treatment of HUVECs with thrombin. Statistically significant differences (*) are indicated.
P-selectin is important in the flow adhesion of sickle erythrocytes to thrombin-stimulated endothelium in vitro.
The number of sickle cells adhering to HUVECs (i) and the rolling velocities of the adhering cells (ii) were examined. (A) In 10 experiments, the rolling adhesion of sickle cells to HUVECs was examined prior to and after treatment of HUVECs with thrombin. The rolling adhesion of sickle cells to thrombin-treated HUVECs was then examined in the presence of anti–P-selectin mAb 9E1. Statistically significant differences compared with untreated HUVECs (*) and to thrombin-treated HUVECs (††) are indicated. (B) In 3 experiments the rolling adhesion of sickle cells to thrombin-treated HUVECs also was examined in the presence of nonblocking anti–P-selectin mAb AC1.2. Statistically significant differences compared with untreated HUVECs (*) and to thrombin-treated HUVECs (†) are indicated. (C) In 10 experiments the firm adhesion of sickle cells to HUVECs was examined prior to and after treatment of HUVECs with thrombin. Statistically significant differences (*) are indicated.
Firm adhesion of sickle cells to HUVECs was also enhanced by thrombin treatment. Compared with the 95.4 sickle cells rolling per millimeters squared on untreated HUVECs, only 5.0 sickle cells were firmly adhered (n = 10). Thrombin treatment of HUVECs increases the number of firmly adhered cells by 130% (P < .001, n = 10; Figure 1C). Treatment with mAb 9E1 causes no statistically significant change in the number of cells firmly adhered to thrombin-treated HUVECs. These results indicate that thrombin-enhanced firm adhesion involves molecular pathways in addition to P-selectin.
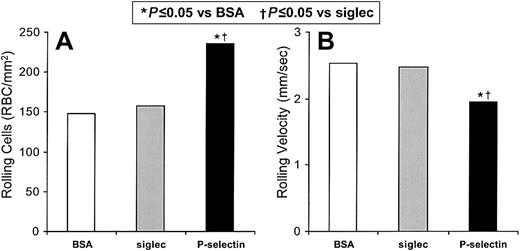
We independently verified that sickle cell flow adhesion involves P-selectin in studies with immobilized recombinant P-selectin in vitro (Figure 2). Immobilized BSA supported the rolling of 148 sickle cells/mm2 (1.17% of the total sickle cells) at a velocity of 2.54 mm/s, as well as 0.81 firmly adhered sickle cells/mm2. This level of adhesion to BSA reflects the innate adhesivity of proadhesive sickle cells. Immobilized recombinant Siglec (which does not recognize ligands on erythrocytes and has a human Ig-Fc tail) gives similar results, supporting the rolling of 157 sickle cells/mm2 (1.24%) at a velocity of 2.48 mm/s. Immobilized recombinant P-selectin supports 50% more rolling sickle cells than does the immobilized recombinant Siglec control (P = .005, n = 5) and 59% more than does the immobilized BSA control (P = .009, n = 5; Figure 2A). The rolling velocity of sickle cells on recombinant P-selectin is 21% slower than on immobilized recombinant Siglec (P = .004, n = 5) and 23% slower than on BSA (P = .026, n = 5; Figure 2B). Sickle erythrocyte rolling on immobilized recombinant Siglec is not significantly different than erythrocyte rolling on immobilized BSA. The number of rolling nonsickle erythrocytes increased by only 14.8% on immobilized recombinant P-selectin compared with BSA (P = .019, n = 4); there was no statistically significant change in their velocities. The firm adhesion to immobilized recombinant P-selectin compared with BSA was not significantly different for either sickle or nonsickle erythrocytes. This lesser level of firm adherence on recombinant P-selectin, compared with that observed on thrombin-treated HUVECs, again reflects the participation of endothelial cell adhesion mechanisms other than P-selectin. These results confirm that P-selectin can mediate specifically the rolling adhesion of sickle red cells.
Sickle cells adhere to immobilized P-selectin under flow conditions in vitro.
The number of sickle cells adhering to immobilized protein and the rolling velocities of the adhering cells were examined. In 5 experiments the rolling adhesion of sickle cells to BSA, immobilized recombinant Siglec, or immobilized recombinant P-selectin were examined. Statistically significant differences compared to immobilized BSA (*) and to immobilized recombinant Siglec (†) are indicated.
Sickle cells adhere to immobilized P-selectin under flow conditions in vitro.
The number of sickle cells adhering to immobilized protein and the rolling velocities of the adhering cells were examined. In 5 experiments the rolling adhesion of sickle cells to BSA, immobilized recombinant Siglec, or immobilized recombinant P-selectin were examined. Statistically significant differences compared to immobilized BSA (*) and to immobilized recombinant Siglec (†) are indicated.
Heparin inhibits the thrombin-enhanced flow adhesion of sickle erythrocytes to endothelial cell P-selectin in vitro
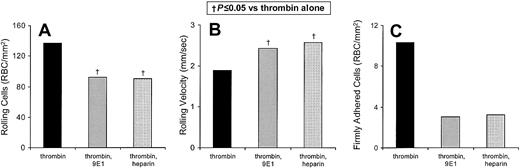
Heparin has been suggested to reduce the morbidity of sickle cell disease.39 In 6 replicate experiments, we tested the efficacy of heparin in blocking the thrombin-enhanced component of rolling adhesion of sickle cells to HUVECs in vitro (Figure3). Unfractionated laboratory-grade heparin reduces the number of sickle cells rolling on thrombin-treated HUVECs by 93% (P = .004; Figure 3A) and increases the velocity of these cells by 113% (P < .001; Figure 3B). There is no significant difference between inhibition by mAb 9E1 and unfractionated heparin for either the number of rolling cells or their rolling velocities, which is consistent with each eliciting its inhibitory effect on P-selectin. We also found that the combination of anti–P-selectin mAb 9E1 and heparin is not more effective than either agent alone (data not shown). These results indicate that heparin is blocking primarily the P-selectin–mediated in vitro flow adhesion and is not blocking P-selectin–independent mechanisms.
Heparin inhibits the flow adhesion of sickle erythrocytes to thrombin-stimulated endothelium in vitro.
(A,B) The number of sickle cells adhering to HUVECs and the rolling velocities of the adhering cells were examined. In 6 experiments the rolling adhesion of sickle cells to thrombin-treated HUVECs was examined in the presence of anti–P-selectin mAb 9E1 or in the presence of unfractionated heparin. Statistically significant differences compared to thrombin-treated HUVECs (†) are indicated. (C) In 3 experiments the firm adhesion of sickle cells to thrombin-treated HUVECs was examined in the presence of anti–P-selectin mAb 9E1 or in the presence of unfractionated heparin. Statistically significant differences (†) are indicated.
Heparin inhibits the flow adhesion of sickle erythrocytes to thrombin-stimulated endothelium in vitro.
(A,B) The number of sickle cells adhering to HUVECs and the rolling velocities of the adhering cells were examined. In 6 experiments the rolling adhesion of sickle cells to thrombin-treated HUVECs was examined in the presence of anti–P-selectin mAb 9E1 or in the presence of unfractionated heparin. Statistically significant differences compared to thrombin-treated HUVECs (†) are indicated. (C) In 3 experiments the firm adhesion of sickle cells to thrombin-treated HUVECs was examined in the presence of anti–P-selectin mAb 9E1 or in the presence of unfractionated heparin. Statistically significant differences (†) are indicated.
We also found that unfractionated heparin reduces the thrombin-enhanced number of rolling cells by 110% and increases their velocities by 78%, but that low-molecular-weight heparin reduces the thrombin-enhanced number of rolling cells by only 58% and increases their velocities by just 10%. This result is consistent with unfractionated heparin having a greater effect than low-molecular-weight heparin on the flow adhesion of sickle red cells to P-selectin, as also is true for the binding of P-selectin to immobilized sLeX.34
The low level of firm adherence of sickle cells to thrombin-treated HUVECs is reduced by either P-selectin blocking mAb 9E1 (n = 3;P = .058) or unfractionated heparin (n = 3;P = .057; Figure 3C). The similar levels of inhibition suggest that both agents are blocking P-selectin. However, neither of these inhibitory effects achieved statistical significance.
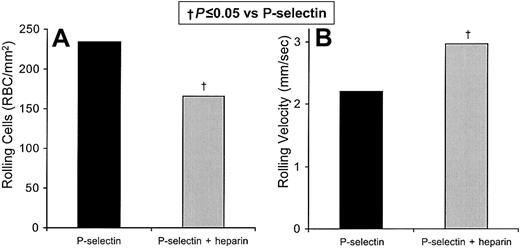
We also directly verified that sickle cell adhesion to P-selectin is inhibited by unfractionated laboratory-grade heparin by testing for in vitro flow adhesion to immobilized recombinant P-selectin (Figure4). In 3 replicate experiments, we tested the efficacy of heparin in blocking the flow adhesion of sickle cell to immobilized recombinant P-selectin in vitro (Figure 4). Unfractionated heparin reduces the number of rolling cells by 29% (P = .051; Figure 4A) and increased the velocity by 34% (P = .045; Figure 4B). As with rolling adhesion to thrombin-activated endothelial cells, we found that the combination of unfractionated heparin and anti–P-selectin mAb 9E1 decreased the number and increased the velocity of rolling cells on immobilized recombinant P-selectin to a similar extent (by 27% and by 57% respectively). These results are similar to those with heparin alone, which decreased the number of rolling cells by 35% and increased their rolling velocity by 30%, and to those with anti–P-selectin mAb 9E1 alone, which decreased the number of rolling cells by 39% and increased the rolling velocity by 58%. The low level of firm adherence of sickle cells to recombinant P-selectin is not significantly altered by mAb 9E1 or unfractionated heparin. Neither the rolling nor the firm adhesion of nonsickle erythrocytes on recombinant P-selectin is significantly affected by mAb 9E1 or unfractionated heparin. Overall, these findings indicate that the main role of P-selectin is to mediate the initial tethering and rolling adhesion of sickle cells, rather than their firm adhesion.
Heparin inhibits the flow adhesion of sickle erythrocytes to immobilized P-selectin in vitro.
The number of sickle cells adhering to immobilized protein and the rolling velocities of the adhering cells were examined. In 3 experiments the rolling adhesion of sickle cells to immobilized recombinant P-selectin was examined in the presence and absence of unfractionated heparin. Statistically significant differences compared with immobilized recombinant P-selectin (†) are indicated.
Heparin inhibits the flow adhesion of sickle erythrocytes to immobilized P-selectin in vitro.
The number of sickle cells adhering to immobilized protein and the rolling velocities of the adhering cells were examined. In 3 experiments the rolling adhesion of sickle cells to immobilized recombinant P-selectin was examined in the presence and absence of unfractionated heparin. Statistically significant differences compared with immobilized recombinant P-selectin (†) are indicated.
We also compared the effects of laboratory-grade unfractionated and low-molecular-weight heparins to immobilized recombinant P-selectin. We found that unfractionated heparin reduces the number of rolling cells by 27% and increases their velocity by 24%, whereas low-molecular-weight heparin reduces the number of rolling cells by only 6% and increases their velocity by just 6%. These results confirm that heparin can block P-selectin–mediated in vitro flow and are consistent with unfractionated heparin having a greater effect than low-molecular-weight heparin on the flow adhesion of sickle red cells to recombinant P-selectin. However, in this experiment we tested only one of the many types of low-molecular-weight heparins currently available.
The background adherence seen in all experiments could represent other biologically relevant adhesion systems that are not affected by thrombin activation. The background adhesion detected in studies using pure molecules (including BSA), suggests that much of the baseline adhesion is due to nonspecific stickiness of sickle erythrocytes. Regardless, the thrombin-dependent component with HUVECs is clearly shown to be due to P-selectin and this is confirmed by the studies with recombinant P-selectin. Likewise, the inhibitory effects of heparin can be explained by P-selectin blockade.
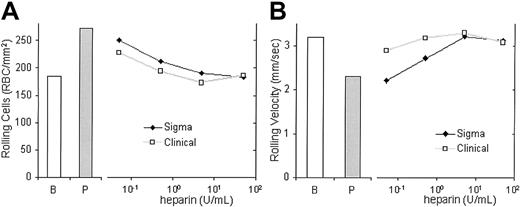
In the above studies, we had used high concentrations of unfractionated laboratory-grade heparin, similar to those described in the literature.36 To assess the clinical relevance for heparin therapy, we also compared the capacity of several concentrations of clinical-grade (Elkins-Sinn) and laboratory-grade (Sigma) unfractionated heparin to block the adhesion of sickle erythrocytes to immobilized recombinant P-selectin. Both types of heparin reduce the adhesion of sickle cells to immobilized recombinant P-selectin (Figure5). Both also cause a decrease in the number of rolling cells and an increase in their rolling velocities at all concentrations tested, including concentrations attained in the plasma during clinical administration (ie, 0.2-0.4 U/mL).37 At 5 U/mL to 50 U/mL, rolling adhesion was approximately the same as that of the basal adhesion to BSA. These results indicate a similarity in the effects of laboratory and clinical grades of heparin with regard to the inhibition of P-selectin–dependent sickle cell–endothelial cell adhesion and indicate their potential therapeutic relevance.
Clinically obtainable concentrations of clinical-grade heparin inhibit the adhesion of sickle cells to P-selectin.
The number of sickle cells adhering to immobilized protein (A) and the rolling velocities (B) of the adherent sickle cells on immobilized P-selectin were examined in the presence of 0.05, 0.5, 5, or 50 U/mL of laboratory-grade heparin (Sigma) or clinical-grade heparin (Clinical). Adherent sickle cells also were examined for number of cells rolling on BSA (B) or on immobilized P-selectin (P) and their velocities in the absence of heparin.
Clinically obtainable concentrations of clinical-grade heparin inhibit the adhesion of sickle cells to P-selectin.
The number of sickle cells adhering to immobilized protein (A) and the rolling velocities (B) of the adherent sickle cells on immobilized P-selectin were examined in the presence of 0.05, 0.5, 5, or 50 U/mL of laboratory-grade heparin (Sigma) or clinical-grade heparin (Clinical). Adherent sickle cells also were examined for number of cells rolling on BSA (B) or on immobilized P-selectin (P) and their velocities in the absence of heparin.
Discussion
In this report, we demonstrate that P-selectin mediates the flow adhesion of sickle erythrocytes to thrombin-activated endothelial cells in vitro. Furthermore, this thrombin-enhanced adhesion can be inhibited by antibodies to P-selectin or by unfractionated heparin. We reported previously that thrombin causes a rapid increase in endothelial cell adhesivity for sickle erythrocytes. Within 5 minutes of thrombin stimulation, the adhesion of sickle cells to endothelial cells markedly increases.24 Upon thrombin stimulation, P-selectin in Weibel-Palade bodies rapidly translocates and is rapidly expressed on the luminal surface of the endothelial cell. Previously, we have shown that P-selectin mediates thrombin-enhanced static adhesion of sickle erythrocytes to endothelial cells in vitro.25 This static adhesion is mediated by an unknown ligand on sickle erythrocytes. The susceptibility of this adhesion to sialidase treatment of erythrocytes indicates that the unknown ligand bears critical sialic acid residues. The lack of inhibition by trypsin treatment of erythrocytes suggests that the unknown ligand may not be a protein. Furthermore, the variable detection of sLeX on some samples of sickle cells (discussed in Matsui et al25) also suggests that in some cases a sLeX moiety may have a role.
Our results show that the number of rolling sickle cells increases and that their velocity decreases as a result of thrombin treatment of HUVECs. Studies with blocking and nonblocking anti–P-selectin mAb confirm that this enhancement is P-selectin–mediated under flow conditions.
Our findings that both unfractionated heparin and anti–P-selectin mAb 9E1 reduce thrombin-enhanced rolling adhesion of sickle cells to HUVECs and adhesion to immobilized recombinant P-selectin indicate that heparin is acting on P-selectin in this capacity. Low-molecular-weight heparin also causes a decrease in P-selectin–dependent sickle cell adhesion to HUVECs, but to a much lesser extent than does unfractionated heparin. The limited inhibition of sickle cell adhesion by low-molecular-weight heparin that we report here is based on the use of only one of the many types of low-molecular-weight heparins currently available. Given the generally more favorable pharmacology and toxicity profiles of low-molecular-weight heparin, these preparations still deserve further evaluation.
Our data are consistent with published findings that adhesive mechanisms other than P-selectin are involved in sickle cell adhesion to activated endothelium.8,9,45 In the current model of neutrophil adhesion to endothelium, selectins mediate the initial steps as they are well suited for tethering rapidly flowing cells and slowing them down as they associate with the vascular wall.46According to this model, activated endothelium expressing intercellular adhesion molecule-1 (ICAM-1) can then mediate firm adhesion by binding neutrophil β2 integrins. We postulate that, in a manner similar to that seen for neutrophil adhesion, P-selectin may play a role in the tethering and rolling adhesion of sickle cells (Figures 1and 2). As with neutrophils, integrins may then mediate the firm adhesion of rolling sickle erythrocytes. The integrin α4β1 is expressed on sickle reticulocytes and can mediate adhesion to endothelial cells, possibly via endothelial VCAM-4.47-49 The endothelial integrin, αVβ3, also mediates sickle cell adhesion to endothelial cells.50 Other β1 and β3 integrins may also fulfill this role.51 A more thorough investigation of cooperation between multiple adhesion mechanisms will be required to confirm our prediction that, like neutrophils, sickle erythrocytes too utilize a multistep model of adhesion to initiate vascular occlusion.
In addition to the above-mentioned pathways, other sickle cell–endothelial cell adhesion mechanisms have been described. Recently, integrin-associated protein (CD47) has been demonstrated to activate adhesivity in sickle reticulocyte as well as mediate adhesion to TSP.52,53 Whereas CD36 (GPIV) has been implicated in earlier studies to mediate adhesion of sickle cells, the presence or absence of CD36 on sickle reticulocytes and erythrocytes does not affect the clinical course.54,55 Band 3 protein also can mediate sickle cell–endothelial cell adhesion.56
Heparin has traditionally been used as an anticoagulant.37 Its effects against P-selectin–mediated tumor cell adhesion and inflammation also have been described.34,57 The TSP-mediated adhesion of sickle cells to endothelial cells and to the mesocecal vasculature of rats can be blocked by heparin or heparan sulfate.35,36 We found that heparin also can inhibit adhesion in the absence of plasma or added soluble ligands (Figures 3 and 4). These effects of heparin gain perspective from the suggestion that heparin therapy is beneficial as prophylaxis for patients with recurrent painful sickle cell crises.39
In addition to its anticoagulant, TSP-blocking, and selectin-blocking effects, heparin has numerous other actions.58 It is known to bind to and inhibit the action of IL-8 and other chemokines, which, in effect, reduces endothelial integrin activation. It also competes with hyaluronate binding of CD44. Damage by reactive oxygen species is reduced by heparin; this may indirectly affect the expression of endothelial adhesion molecules.
Several approaches to interfering with P-selectin–mediated adhesion are under study or development. Experiences with blocking selectin-ligand binding using antibodies against P-selectin such as 9E1, recombinant selectin-Ig chimeras, oligosaccharide components of natural selectin ligands such as sLeX or the amino-terminal domain of PSGL-1, or oligopeptides derived from P-selectin sequences have been reviewed.59 A powerful new strategy for developing polyvalent synthetic selectin-binding ligands that are orders of magnitude more potent than their monosaccharide components has capitalized on the much greater binding strength of polyvalent cell surface glycoprotein structures compared with their monomeric oligosaccharide constituents.60 Another suggested strategy relies upon small synthetic oligosaccharides, glycoconjugates, glycomimics, and unnatural substrates to modulate metabolically the biosynthesis, processing, assembly, or structure of adhesive glycoconjugates on cell surfaces.61
In addition to these elegant new molecular strategies, heparin has much to recommend it as an agent for inhibiting P-selectin–mediated sickle cell binding. The extensive clinical experience with its use, side effects, and dosing37 make it a compelling candidate for clinical trials of the prevention of painful vascular occlusion in sickle cell disease. The potential role of P-selectin as the initial adhesive process that initiates vascular occlusion suggests that heparin therapy would be more effective as prophylaxis than as treatment for established pain crises. We found that clinical-grade unfractionated heparin can inhibit partially P-selectin–dependent adhesion of sickle cells at concentrations attained in the plasma during clinical use (Figure 5). Significant concerns with prolonged heparin therapy are abnormal bleeding, heparin-induced thrombocytopenia, and inconvenience of administration. While the risk of bleeding is to some degree circumvented by the substantial clinical experience with heparin dosing, the issues of thrombocytopenia and convenience of administration remain. The use of subcutaneous low-molecular-weight heparin would permit more convenient administration and is associated with a lower incidence of heparin-induced thrombocytopenia, but its potential for preventing vascular occlusion may be diminished by its limited efficacy in blocking P-selectin binding, peculiar inability to increase tissue factor pathway inhibitor levels in sickle cell disease, and requirement for parenteral administration.34,62,63 The discomfort and inconvenience associated with long-term parenteral therapies lessens enthusiasm for the prophylactic administration of heparin. Despite assertions of the lack of absorption of orally administered heparin because of its large molecular weight, strong negative charge, and hydrophilicity,37,64 there are published reports that unfractionated heparin administered orally to laboratory animals is absorbed, binds avidly to the endothelium, and has antithrombotic activity.65-67 One particular formulation of heparin with an agent that promotes its oral absorption has been reported to have antithrombotic activity and possibly to be associated with a lower incidence of heparin-associated thrombocytopenia.68 These issues taken together with the findings we have presented herein indicate that the time has come for a clinical trial of the of unfractionated heparin, possibly administered by the oral route, for the prevention of painful vascular occlusion in sickle cell disease.
The use of heparin to inhibit the adhesive events important to sickle cell vascular occlusion may affect also multicellular events, such as those described for carcinoma emboli.69 There is evidence that both platelets and leukocytes facilitate carcinoma cell metastasis, that both P- and L-selectin participate in the process, and that heparin can inhibit both selectin molecules.70,71Regarding multicellular interactions in sickle cell vascular occlusion, it has been reported that the addition of platelets enhances the static adhesion of sickle cells to the vascular endothelium in vitro72 and that in mouse models of sickle cell disease, leukocyte adhesion may precede that of sickle erythrocytes.33 It also has been reported that in sickle cell disease platelets express P-selectin and adhere to sickle red cells in circulating clumps.73,74 Additionally, neutrophils are found in increased numbers in sickle cell disease, are often activated, and have been reported to bind to both endothelial cells and sickle erythrocytes.75 76 These findings are consistent with multicellular adhesion involving endothelial cells, sickle cells, platelets, and neutrophils in vascular occlusion, with a potential role for P- and/or L-selectin in such processes. These proposed interactions provide further support for a therapeutic trial of heparin in sickle cell disease.
We acknowledge the valuable advice of Drs Lubor Borsig and Steven D. Rosen. We appreciate the cooperation and support of our patients, without whom this study would not have been possible.
Prepublished online as Blood First Edition Paper, July 5, 2002; DOI 10.1182/blood-2002-02-0626.
Supported in part by grants from the National Institutes of Health to N.M. (Sickle Cell Scholar's Award HL20985), A.V. (R01-CA38701) and S.E. (RO1-HL64396).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Stephen H. Embury, Building 100, Room 263, San Francisco General Hospital, 1001 Potrero Ave, San Francisco, CA 94110; e-mail: sembury@itsa.ucsf.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal