Methotrexate (MTX) is a key compound of chemotherapeutic regimens used in the treatment of childhood acute lymphoblastic leukemia (ALL). Resistance to this drug may arise by, among other factors, altered cellular uptake that may hamper the efficacy of the treatment. Recently, a G80A polymorphism has been described in the reduced folate carrier gene (RFC1), which encodes the major MTX transporter. Here, we assessed the association between the genetic polymorphisms G80A and both MTX plasma levels and childhood ALL outcome. Children with the A80 variant had worse prognoses than patients with the GG genotype (P = .04), as shown by event-free survival estimates. Patients homozygous for A80 had higher levels of MTX (P = .004) than the other genotype groups. Possible explanations for observed associations are discussed; however, additional experiments are required to achieve understanding of the underlying mechanism.
Introduction
Methotrexate (MTX) is a key component in the treatment of childhood acute lymphoblastic leukemia (ALL), the most frequent malignancy in the pediatric population.1,2 The reduced folate carrier gene (RFC1) is a major MTX transporter whose impaired function was recognized as a frequent mechanism of antifolate resistance.3,4 Different gene alterations affecting RFC1 transport properties were found in cell lines selected for antifolate resistance and in patient lymphoblasts.5-8 Recently, a G80A polymorphism, which replaces His by Arg at position 27 of the RFC1 protein, was identified.9 The G variant correlated with lower plasma folate and higher homocysteine levels in healthy persons9 and was found at higher frequency in children with neural tube defects.10,11 Because folate and homocysteine homeostasis are affected by MTX action,12 13it is possible that RFC1 G80A may also modulate the outcome in patients treated with this drug. In the present study, we analyzed the association between the RFC1 G80A polymorphism and both disease outcome and MTX plasma level in children with ALL.
Study design
Patients
The patients (n = 204) included in this study were children of French Canadian origin who had ALL and were treated at the Sainte-Justine Hospital in Montreal, QC, Canada between 1988 and 2000. Multiagent chemotherapeutic protocols used were DFCI 87-01, DFCI 91-01, and DFCI 95-01, all developed by the Dana-Farber Cancer Institute.14 15 Patient samples were obtained at diagnosis after informed consent was provided according to the Declaration of Helsinki. Approval for this study was obtained from the institutional review board of the Hôpital Sainte-Justine. Patient characteristics and clinical prognostic factors at diagnosis (sex, age, white blood cell count [WBC], ALL cell type, treatment protocol, risk group, and DNA index) are given in Table1. Each patient enrolled in this retrospective study received a high MTX dose (4 g/m2) during the induction phase. MTX was also given intrathecally for central nervous system treatment (6-12 mg, depending on age) and at a once-a-week dose of 30 mg/m2during the maintenance phase. Children who had relapses or fatal outcomes from the disease were defined as having an event. To reduce MTX toxicity, leucovorin rescue therapy (200 mg/m2 followed by 24 mg/m2 every 6 hours until routinely measured plasma MTX levels were 0.1 μM or lower) was given after high-dose MTX. MTX plasma levels were thus available at 3 time points (24, 36, and 48 hours) for the 73 children for whom RFC1 genotyping was also performed.
Characteristics of children with and without event following treatment for ALL
| . | No. subjects and frequency (%) . | |||||
|---|---|---|---|---|---|---|
| Event . | Nonevent . | P* . | P† . | |||
| Sex | .7 | .2 | ||||
| Female | 15 | (42.9) | 77 | (45.6) | — | — |
| Male | 20 | (57.1) | 92 | (54.4) | — | — |
| Age, y | <.001 | .02 | ||||
| <1 | 4 | (11.4) | 3 | (1.8) | — | — |
| 1-10 | 23 | (65.7) | 136 | (80.5) | — | — |
| >10 | 8 | (22.9) | 30 | (17.8) | — | — |
| WBC, ×109/L | .2 | .02 | ||||
| <50 | 23 | (71.9) | 140 | (82.8) | — | — |
| >50 | 9 | (28.1) | 29 | (17.2) | — | — |
| Immunophenotype | .2 | .1 | ||||
| T | 2 | (5.7) | 20 | (12.0) | — | — |
| B | 33 | (94.3) | 146 | (88.0) | — | — |
| DNA index‡ | .5 | .5 | ||||
| <1.16 | 23 | (82.1) | 132 | (87.4) | — | — |
| >1.16 | 5 | (17.9) | 19 | (12.6) | — | — |
| Risk groups | <.01 | .05 | ||||
| Standard | 14 | (40.0) | 65 | (38.5) | — | — |
| High | 15 | (42.9) | 98 | (58.0) | — | — |
| Very high | 6 | (17.1) | 6 | (3.6) | — | — |
| Treatment protocol | .4 | .04 | ||||
| 87–01 | 6 | (17.1) | 12 | (7.1) | — | — |
| 91–01 | 15 | (42.9) | 55 | (32.5) | — | — |
| 95–01 | 14 | (40.0) | 102 | (60.4) | — | — |
| . | No. subjects and frequency (%) . | |||||
|---|---|---|---|---|---|---|
| Event . | Nonevent . | P* . | P† . | |||
| Sex | .7 | .2 | ||||
| Female | 15 | (42.9) | 77 | (45.6) | — | — |
| Male | 20 | (57.1) | 92 | (54.4) | — | — |
| Age, y | <.001 | .02 | ||||
| <1 | 4 | (11.4) | 3 | (1.8) | — | — |
| 1-10 | 23 | (65.7) | 136 | (80.5) | — | — |
| >10 | 8 | (22.9) | 30 | (17.8) | — | — |
| WBC, ×109/L | .2 | .02 | ||||
| <50 | 23 | (71.9) | 140 | (82.8) | — | — |
| >50 | 9 | (28.1) | 29 | (17.2) | — | — |
| Immunophenotype | .2 | .1 | ||||
| T | 2 | (5.7) | 20 | (12.0) | — | — |
| B | 33 | (94.3) | 146 | (88.0) | — | — |
| DNA index‡ | .5 | .5 | ||||
| <1.16 | 23 | (82.1) | 132 | (87.4) | — | — |
| >1.16 | 5 | (17.9) | 19 | (12.6) | — | — |
| Risk groups | <.01 | .05 | ||||
| Standard | 14 | (40.0) | 65 | (38.5) | — | — |
| High | 15 | (42.9) | 98 | (58.0) | — | — |
| Very high | 6 | (17.1) | 6 | (3.6) | — | — |
| Treatment protocol | .4 | .04 | ||||
| 87–01 | 6 | (17.1) | 12 | (7.1) | — | — |
| 91–01 | 15 | (42.9) | 55 | (32.5) | — | — |
| 95–01 | 14 | (40.0) | 102 | (60.4) | — | — |
An event is described as a relapse or a fatal outcome.
†Tests of significance were derived from Kaplan-Meier and Cox regression analysis, respectively.
Ratio of DNA content of leukemia to normal cells. Factors shown here to influence risk of event were considered as confounding variables in a multivariate model built to assess the role of RFC1 on ALL outcome. In the analysis, categories associated with lower risk for relapse were considered as references for the analysis (age range between 1 and 10 years, WBC count lower than 50 × 109/L, B-cell type, standard risk group, and most recent treatment protocol).
Genotyping and MTX plasma levels
RFC1 amplification of patient DNA isolated from buccal epithelial cells, peripheral blood, or bone marrow in remission was obtained as described elsewhere.9 Polymerase chain reaction–restriction fragment-length polymorphisms (PCR-RFLP;HaeII digestion) was used to optimize allele-specific oligonucleotide (ASO) hybridization assay (Figure1A) subsequently used for the screening of patient DNA. Standard conditions were applied,16 except that ASOs specific for the G80 (5′gcacacgaggCgccg) or the A80 (5′gcacacgaggTgccg) RFC1 variant were designed and hybridized at 45°C with PCR products immobilized on a membrane. Measurement of MTX plasma levels was performed by fluorescence polarization immunoassay (TDx Abbott Laboratories, Chicago, IL) according to the manufacturer's instructions.
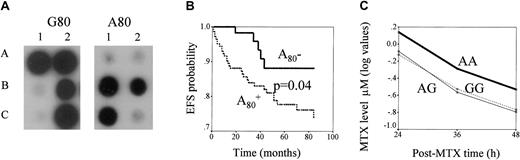
RFC, G80A polymorphism: genotyping and its impact on ALL outcome and MTX levels.
(A) ASO hybridization assay of RFC1 G80A polymorphism. PCR products of RFC1 gene obtained from the DNA of each patient are blotted in duplicate. The membranes are hybridized with ASO specific for G80 (left panel) or A80 (right panel). Hybridization signal in spots A1, A2, and C2 indicate homozygous patients with the GG genotype, in B2 a heterozygous patient with the AG genotype, and in B1 and C1 patients with the AA genotype. (B) Kaplan-Meier estimates of EFS for patients with and without the RFC1 A80 variant. EFS curves for patients positive (lower line) or negative (upper line) for the RFC1A80 variant are presented. The P value for the differences of survival between these patients groups is indicated. (C) Relationship between RFC1 G80A polymorphism and MTX plasma level. Lines represent log values of MTX levels (μM, measured at 3 time points) in GG, GA, and AA genotypes. MTX levels differ across RFC1 genotype groups (P = .02). When MTX levels in patients with the AA genotype are compared with those of the rest of patients, the difference is .004.
RFC, G80A polymorphism: genotyping and its impact on ALL outcome and MTX levels.
(A) ASO hybridization assay of RFC1 G80A polymorphism. PCR products of RFC1 gene obtained from the DNA of each patient are blotted in duplicate. The membranes are hybridized with ASO specific for G80 (left panel) or A80 (right panel). Hybridization signal in spots A1, A2, and C2 indicate homozygous patients with the GG genotype, in B2 a heterozygous patient with the AG genotype, and in B1 and C1 patients with the AA genotype. (B) Kaplan-Meier estimates of EFS for patients with and without the RFC1 A80 variant. EFS curves for patients positive (lower line) or negative (upper line) for the RFC1A80 variant are presented. The P value for the differences of survival between these patients groups is indicated. (C) Relationship between RFC1 G80A polymorphism and MTX plasma level. Lines represent log values of MTX levels (μM, measured at 3 time points) in GG, GA, and AA genotypes. MTX levels differ across RFC1 genotype groups (P = .02). When MTX levels in patients with the AA genotype are compared with those of the rest of patients, the difference is .004.
Statistics
Differences in the frequencies of genotypes dichotomized as carriers of RFC1 A80 variant versus noncarriers between children with and without an event, and those with or without the prognostic factors listed in Table 1, were assessed by χ2analysis. For event-free survival (EFS) analysis, survival time corresponded to the time between diagnosis and the event (n = 35). For patients without an event (censored cases, n = 169), it corresponded to the end of follow-up (5 years after treatment) or to February 2002 (patients who received treatment or who entered the follow-up period). Overall time to the event and of follow-up ranged from 1 to 84 months (interquartile range, 26-84 months); the median was 49.5 months. Differences between EFS obtained by Kaplan-Meier for the patients with and without the RFC1 A80variant were determined using a log-rank test. The impact of the RFC1 A80 variant on the EFS probabilities was estimated by Cox regression analysis with the enclosure of prognostic factors that influenced ALL outcomes in this group of patients and by applying forced entry of all variables or stepwise routine. The influence of the patients' characteristics on EFS was assessed by Kaplan-Meier and Cox regression analyses.
Repeated measures analysis of variance (ANOVA) was used to compare MTX levels (μM) based on the 3 time-points between children with and without event and among patients with different RFC1 genotype categories. For that purpose, log-transformed values of MTX level were used because of its skewed distribution. All analyses were performed by SPSS version 10.00.
Results and discussion
A significant difference in the frequency of RFC1 genotypes was observed between children with and without an event (Table 2). Carriers of the RFC1 A80 variant had a higher risk for events than were those with the GG genotype (odds ratio [OR] = 3.0; 95% CI, 1.1-8.1;P = .03). Similarly, Kaplan-Meier analysis showed that carriers of the A80 variant had the worst ALL outcomes (P = .04; Figure 1B). In Cox regression analysis, hazard ratio (HR) estimates for patients with RFC1 A80 retained their significance (HR = 2.8; 95% CI, 1.0-8.1; P = .05) in the presence of other prognostic factors, which also influenced ALL outcome (age, WBC, type of protocol, and risk classes; Table 1). When the initial Cox regression model was applied further to stepwise analysis, RFC1 genotype and age appeared to have the highest predictive value for an event (P = .05 and P = .03 respectively). We did not find any correlation between RFC1 genotypes and patient characteristics listed in Table 1.
Distribution of RFC1 G80A polymorphism among ALL patients with and without event
| RFC1 . | Event no. (%) . | Nonevent no. (%) . | OR (95% CI) . | P . |
|---|---|---|---|---|
| GG | 5 (14.3) | 56 (33.1) | 1 | — |
| GA | 20 (57.1) | 78 (46.2) | 2.9 (1.0-8.1) | .05 |
| AA | 10 (28.6) | 35 (20.7) | 3.2 (1.0-10.1) | .05 |
| RFC1 . | Event no. (%) . | Nonevent no. (%) . | OR (95% CI) . | P . |
|---|---|---|---|---|
| GG | 5 (14.3) | 56 (33.1) | 1 | — |
| GA | 20 (57.1) | 78 (46.2) | 2.9 (1.0-8.1) | .05 |
| AA | 10 (28.6) | 35 (20.7) | 3.2 (1.0-10.1) | .05 |
OR indicates odds ratio; CI, confidence interval.
For carriers of the A80 variant, OR = 3.0; 95% CI, 1.1-8.1; P = .03.
We next analyzed the influence of RFC1 polymorphism on MTX plasma levels (Figure 1C) and found a significant association (P = .02), which was mainly caused by higher MTX plasma levels in the patients with AA than in those with other genotypes (P = .004). However, we did not observe the association between MTX levels and disease outcome (P = .6, data not shown).
Although modest, the association between RFC1 polymorphism and ALL outcome suggests that this variant might contribute to the estimation of ALL prognosis. The finding of this study is in agreement with the reports of others who suggest the functional impact of this polymorphism: G80A influenced folate/homocysteine levels9,10 and correlated with neural tube defects,10 especially among children whose mothers reported low folate intake.11 The amino acid change (strong to weak basic amino acid) in a first transmembrane domain (TMD1) caused by G80A substitution was expected to alter RFC1 transport properties.17 Several alterations in TMD1 in cell lines selected for MTX resistance were shown to change the ratio of RFC1 affinities of MTX versus other folate substrates.18,19 Likewise, the carriers of A might have lower MTX affinity (and, presumably, higher MTX levels, as shown in this study) and higher affinity for other folate substrates (and higher level of folate cofactors, as shown in other studies).9-11On the other hand, MTX level, which shows high pharmacokinetic variability,13,20 may be influenced by different factors, such as hepatic or renal function, and is not a reliable indicator of the transport function. In addition recent in vitro studies in erythroleukemia cell lines showed no difference in MTX transport between the G and the A RFC180 variant, whereas only a minor (2-fold) difference in transport of 5′ formyl tetrahydrofolate cofactor was found.17 Therefore, additional studies are needed to explain the underlying mechanism linking RFC1 polymorphism and ALL outcome. A prospective study assessing intracellular MTX levels and RFC1 substrate binding affinities in patients with and without RFC1 A80 variant is under way in our laboratory. It would also be important to assess the relative impact of RFC1 polymorphism on ALL outcome with regard to other variants relevant for MTX response.
We thank our patients and their parents for their collaboration, our colleagues Daniel Sinnett and Damian Labuda for discussions and contributions of biologic material, and Mark Bernstein for facilitating access to clinical data.
Supported by the Canadian Institutes of Health Research, Leukemia Research Fund of Canada, and Centre de recherche, Hôpital Ste-Justine. M. K. is a scholar of the Fonds de la Recherche en Santé du Québec.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Maja Krajinovic, Centre de recherche, Hôpital Sainte-Justine, 3175 Côte Ste-Catherine, Montréal, QC, H3T 1C5, Canada; e-mail:maja.krajinovic@umontreal.ca.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal