A minor population of blood cells deficient of glycosylphosphatidylinositol (GPI)–anchored membrane proteins is often detected in patients with aplastic anemia (AA), though the clinical significance of such paroxysmal nocturnal hemoglobinuria (PNH)–type cells remains unclear. To clarify this issue, we studied 164 patients with myelodysplastic syndrome (MDS) for the presence of CD55−CD59− granulocytes and red blood cells using sensitive flow cytometry. Among the different subgroups of MDS, a significant increase (ie, at least 0.003%) of PNH-type cells was detected in 21 of 119 patients with refractory anemia (RA); this frequency (17.6%) of RA patients with increased PNH-type cells (PNH+ patients) was much lower than what we previously reported (52.0%) for AA patients. PNH+ RA patients had distinct clinical features compared with RA patients without increased PNH-type cells (PNH− patients), such as less pronounced morphologic abnormality of blood cells, more severe thrombocytopenia, lower rates of karyotypic abnormality (4.8% vs 32.8%) and of progression to acute leukemia (0% vs 6.2%), higher probability of response to cyclosporine therapy (77.8% vs 0%), and higher incidence of HLA-DR15 (90.5% vs 18.5%). These data indicate that the presence of a minor population of PNH-type cells suggests a benign type of bone marrow failure, probably caused by an immunologic mechanism. To choose an appropriate therapy, peripheral blood should be tested using sensitive flow cytometry for the presence of PNH-type cells in all patients with bone marrow failure before treatment.
Introduction
An increase of blood cells with a paroxysmal nocturnal hemoglobinuria (PNH) phenotype is often detected in patients with acquired aplastic anemia (AA).1-6 We previously demonstrated that a minor (less than 1%) population of CD55−CD59− granulocytes could be detected in the peripheral blood of patients with AA at a much higher frequency than previously reported.7 Given that the proportion of PNH-type granulocytes decreased in most patients in association with hematologic recovery after successful immunosuppressive therapy, such PNH-type cells appeared to be derived from PNH-type progenitor cells that evaded immune system attack against hematopoietic progenitor cells. However, among patients with newly diagnosed AA, the small number who did not show an increase of PNH-type cells (PNH− patients) made it difficult to compare clinical features between patients with increased PNH-type cells (PNH+ patients) and PNH− patients. Therefore, the clinical significance of the increase in PNH-type cells in the management of bone marrow failure remains unclear.
Myelodysplastic syndrome (MDS) is a clonal disorder of hematopoiesis characterized by cytopenia and bone marrow dysplasia.8Because the syndrome is diagnosed on the basis of morphologic abnormalities of blood cells, its pathophysiology is more heterogeneous than that of AA. Among the subtypes of MDS, the diagnosis of refractory anemia (RA) is particularly ambiguous because of the low percentage of immature blasts.9 10 Some patients with bone marrow failure may receive diagnoses of RA because of the presence of slight morphologic abnormalities, despite the fact that their immune pathophysiology is similar to that of typical AA patients. There is no good marker for distinguishing such a benign subset of RA from other RA occurrences that are potentially malignant.
Dunn et al11 demonstrated that MDS patients with increases in the proportion of CD15+CD16−CD66b− granulocytes are more likely to respond to antithymocyte globulin (ATG) therapy than those without increases in PNH-type granulocytes. Although their data suggested that PNH-type cells might be useful as a marker for a certain type of immune mechanism underlying bone marrow failure, such use of PNH-type cells could not be established because of the small number of patients studied. Moreover, the flow cytometry these authors used could determine that a patient had PNH+ only when 1% or more of CD15+CD16−CD66b− granulocytes were present. Thus, examining a large number of MDS patients for the presence of PNH-type cells using more sensitive flow cytometry and studying the correlation between the findings and clinical features may be useful in clarifying the significance of PNH-type cells in bone marrow failure.
Therefore, using sensitive flow cytometry that could detect as few as 0.002% PNH-type cells, we examined the peripheral blood of MDS patients for the presence of PNH-type granulocytes and red blood cells (RBCs).7 In contrast to previous findings in MDS patients11 and our own findings in AA patients,7 increased PNH cells were detected only in a small subset of RA patients. The PNH+ RA patients showed benign clinical features distinct from those of PNH− RA patients.
Patients, materials, and methods
Study subjects
We studied 164 patients with MDS (age range, 17-91 years; median, 67 years) with a male-female ratio of 1:1.1. MDS was diagnosed at Kanazawa University Hospital and other hospitals that took part in this cooperative study in the bone marrow failure study group led by the Ministry of Health, Labor and Welfare of Japan. The study group consisted of 119 patients with RA, 4 with RA with ring sideroblasts (RARS), 33 with RA with excess blasts (RAEB), and 8 with RAEB in transformation (RAEB-t).8 Patients with positive findings on Ham test were excluded from the study. All patients provided informed consent before sampling. Sixty-eight healthy control subjects, aged 21 to 77 years, were also studied. MDS was diagnosed on the basis of cytopenia in the peripheral blood and the presence of dysplasia in at least 2 lineages of bone marrow cells.8 Bone marrow smears were reviewed by 3 independent hematologists. Only patients who had more than 1% neutrophils with the Pseudo-Pelger-Hüet anomaly and who had micromegakaryocytes, in addition to signs of erythroid dysplasia such as megaloblastic changes and multinuclearity, were included in this retrospective study. The study was approved by the institutional human research committee.
Detection of PNH-type cells
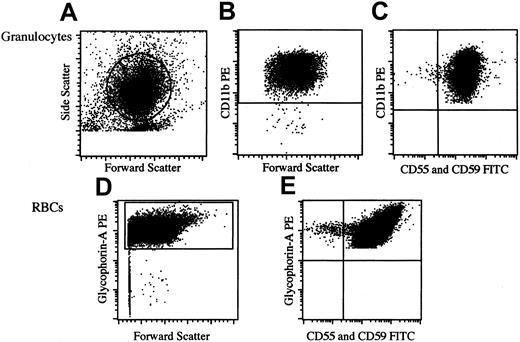
For the detection of PNH-type granulocytes, phycoerythrin (PE)–labeled anti-CD11b monoclonal antibodies (mAbs; Becton Dickinson, Mountain View, CA), fluorescein-isothiocyanate (FITC)–labeled anti-CD55 (clone IA10, mouse IgG2a; PharMingen, San Diego, CA), and FITC-labeled anti-CD59 (clone p282, mouse IgG2a; PharMingen) were used in combination with isotype-matched control mAbs as described in our previous report.7 For analysis of PNH-type RBCs, we used PE-labeled anti–glycophorin A mAb (clone JC159; DAKO, Glostrup, Denmark) instead of anti-CD11b mAb. Fresh peripheral blood was diluted to 3% with phosphate-buffered saline, and 50 μL diluted blood was incubated with PE-labeled anti–glycophorin A mAb, FITC-labeled anti-CD55, and anti-CD59 mAb on ice for 25 minutes. At least 105 CD11b+ granulocytes and glycophorin A+ RBCs within each corresponding gate (Figure1) were analyzed using FACScan flow cytometry (Becton Dickinson).
Protocol for identification of PNH-type granulocytes and RBCs.
(A) Forward and side scatter gates used to define granulocytes. (B) Expression of CD11b by gated cells from panel A. (C) Expression of CD55 and CD59 by CD11b+ granulocytes (defined in panel B) in patient 11. Percentage of PNH-type granulocytes (CD11b+CD55−CD59−granulocytes/total CD11b+ granulocytes) was 0.1%. (D) Glycophorin-A+ cell gate to define RBCs. (E) Expression of CD55 and CD59 by glycophorin-A+ RBCs (defined in panel D) in patient 11. Percentage of PNH-type RBCs (glycophorin-A+CD55−CD59−RBCs/total glycophorin-A+ RBCs) was 0.2%.
Protocol for identification of PNH-type granulocytes and RBCs.
(A) Forward and side scatter gates used to define granulocytes. (B) Expression of CD11b by gated cells from panel A. (C) Expression of CD55 and CD59 by CD11b+ granulocytes (defined in panel B) in patient 11. Percentage of PNH-type granulocytes (CD11b+CD55−CD59−granulocytes/total CD11b+ granulocytes) was 0.1%. (D) Glycophorin-A+ cell gate to define RBCs. (E) Expression of CD55 and CD59 by glycophorin-A+ RBCs (defined in panel D) in patient 11. Percentage of PNH-type RBCs (glycophorin-A+CD55−CD59−RBCs/total glycophorin-A+ RBCs) was 0.2%.
PIG-A gene analysis
Peripheral blood granulocytes of RA patients with 0.56 to 2.41% PNH-type granulocytes were isolated from heparinized blood using 6% dextran and Lymphoprep (Axis-shield PoC AS, Oslo, Norway) followed by RBC lysis by adding ammonium chloride buffer as previously described.12 To enrich granulocytes deficient in glycosylphosphatidylinositol-linked proteins, 1 million granulocytes were incubated in RPMI 1640 medium containing aerolysin (Protox Biotech, Victoria, BC, Canada)13(1.5 × 10−8 M) at 37°C for 90 minutes as described.14 CD55−CD59−granulocytes were enriched to more than 50% by aerolysin treatment. Genomic DNA was extracted from the granulocytes, and 5 exons of thePIG-A gene were amplified using 5 different primer pairs.15 Amplified products were denatured at 94°C for 3 minutes and were cooled slowly (1°C/min) to 37°C to allow heteroduplex formation. The products were electrophoresed in a mutation detection enhancement gel (BioWhittaker Molecular Applications, Rockland, ME) for 8 hours at 400 V. Polymerase chain reaction (PCR) products that showed extra bands from heteroduplex formation were ligated into pGEM using the pGEM-T Vector System (Promega, Madison, WI), and the ligation mixture was used to transform Escherichia coli JM109 competent cells (TaKaRa Biomedicals, Kyoto, Japan). The cloned DNA was sequenced using an ABI Prism BigDye Terminator Cycle Sequencing Ready Reaction Kit and a model 310 Genetic Analyzer (Applied Biosystems, Foster City, CA).
Typing of HLA-DRB1 alleles
HLA-DR serotypes were determined using a lymphocyte toxicity assay (class 2 lambda monoclonal trays; One Lambda, Canoga Park, CA). For patients with DR15, DRB1 alleles were determined by PCR with sequence-specific primers (Dynal classic SSP DRB1*15/16; Dynal, Bromborough Wirral, United Kingdom). For the other patients whose DR serotypes were unknown, only the presence or absence of DRB1*1501 and DRB1*1502 was determined using the same kit.
Cyclosporine therapy
Cyclosporin A (6 mg/kg per day; Novartis, Basel, Switzerland) was administered to 17 patients after an analysis of PNH-type cells. Responses were evaluated 6 months after therapy. Response criteria included the resolution of a requirement for transfusion and a 2 g/dL or greater increase in hemoglobin level.
Statistical analysis
Differences of data between PNH+ and PNH− patients were assessed using the Fisher exact test and the Mann-Whitney U test. P < .05 was considered statistically significant.
Results
Detection of PNH-type granulocytes and RBCs
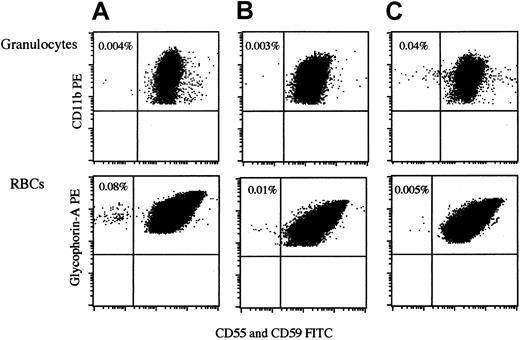
The flow cytometry we used could reliably detect as little as 0.002% CD11b+CD55−CD59− cells within the granulocytes gate, as we previously reported (Figure1).7 When granulocytes and RBCs were examined, the percentage of PNH-type cells detected was generally different for granulocytes and RBCs. In patients with low (0.01%) percentages of PNH-type cells in 2 lineages of cells, PNH-type RBCs were more easily recognized than PNH-type granulocytes because of a distinct cluster of glycophorin A+CD55−CD59− cells (Figure 2). Thus, the detection of PNH-type RBCs in addition to PNH-type granulocytes appeared to substantiate a diagnosis of bone marrow failure with a minor PNH-type cell population. Among 68 healthy controls, 26.5% exhibited 1 or 2 CD55−CD59− cells per 100 000 granulocytes, and 50.0% exhibited 1 or 2 CD55−CD59− cells per 100 000 RBCs. However, there was no healthy control who exhibited 3 or more CD55−CD59− cells per 100 000 granulocytes and 100 000 RBCs. To avoid false-positive results, the presence of more than 0.003% CD55−CD59−cells in granulocytes and RBCs was arbitrarily defined as an increase in PNH-type cells.
Detection of PNH-type granulocytes and RBCs in peripheral blood of RA patients.
Histograms of 3 RA patients exhibiting less than 0.1% CD55−CD59− cells are shown. A indicates patient 7; B, patient 12; C, patient 16. Each number represents a percentage of PNH-type cells.
Detection of PNH-type granulocytes and RBCs in peripheral blood of RA patients.
Histograms of 3 RA patients exhibiting less than 0.1% CD55−CD59− cells are shown. A indicates patient 7; B, patient 12; C, patient 16. Each number represents a percentage of PNH-type cells.
PIG-A gene abnormalities in a minor population of PNH-type granulocytes
A minor population of PNH-type granulocytes was enriched from 5 RA patients by aerolysin treatment, and all exons of the PIG-Agene in these granulocytes were examined using heteroduplex analysis followed by subcloning and sequencing. Table1 summarizes abnormalities of thePIG-A gene in each patient. Although the proportions of PNH-type cells in these patients were low (0.56 to 2.41%), various abnormalities were detected in all patients.
PIG-A mutations in PNH+ RA patients
| Patient . | Age, y/sex . | Exon . | Mutations . | Position . | Consequence . |
|---|---|---|---|---|---|
| 1 | 40/M | 4 | 1-bp deletion (T) | 998 | Frameshift, stop codon in 1002 |
| 2 | 48/M | 5 | 1-bp insertion (T) | 1219 | Frameshift, stop codon in 1244 |
| 4 | Point mutation (A to T) | 962 | Stop codon in 962 | ||
| 4 | 1-bp deletion (T) | 952 | Frameshift, stop codon in 953 | ||
| 3 | 68/F | 5 | 1-bp insertion (T) | 1219 | Frameshift, stop codon in 1244 |
| 4 | 39/F | 4 | 1-bp deletion (C) | 961 | Frameshift, stop codon in 981 |
| 5 | 40/F | 5 | 1-bp deletion (G) | 1393 | Frameshift, stop codon in 1392 |
| Patient . | Age, y/sex . | Exon . | Mutations . | Position . | Consequence . |
|---|---|---|---|---|---|
| 1 | 40/M | 4 | 1-bp deletion (T) | 998 | Frameshift, stop codon in 1002 |
| 2 | 48/M | 5 | 1-bp insertion (T) | 1219 | Frameshift, stop codon in 1244 |
| 4 | Point mutation (A to T) | 962 | Stop codon in 962 | ||
| 4 | 1-bp deletion (T) | 952 | Frameshift, stop codon in 953 | ||
| 3 | 68/F | 5 | 1-bp insertion (T) | 1219 | Frameshift, stop codon in 1244 |
| 4 | 39/F | 4 | 1-bp deletion (C) | 961 | Frameshift, stop codon in 981 |
| 5 | 40/F | 5 | 1-bp deletion (G) | 1393 | Frameshift, stop codon in 1392 |
Prevalence of patients with increased PNH-type blood cells among patients with MDS
A significant increase of PNH-type cells was detected in 21 of 119 (17.6%) RA patients. In contrast, increased PNH-type cells were not detected in any of the 4 RARS, 33 RAEB, or 8 RAEB-t patients. Table2 summarizes the clinical data on the 21 PNH+ RA patients. Bone marrow aspirates from the sternum were hypercellular or normocellular in most patients, though bone marrow biopsy from the iliac bone marrow showed hypocellularity in 10 of 14 patients tested. The percentage of PNH-type granulocytes varied from 0.003% to 2.41% and was less than 1.0% in 17 of 21 (81.0%) PNH+ patients. These low percentages would have been considered insignificant in previous studies. All samples of PNH+ RA patients who exhibited less than 0.01% PNH-type cells were reexamined within 1 month and gave similar results. Nine patients did not require treatment because their pancytopenia remained stable or improved spontaneously. The other 12 patients were treated with cyclosporine or anabolic steroids, and all of them, except patients 3 and 9, improved.
Characteristics of PNH+ RA patients
| Patient . | Age, y/sex . | Time from diagnosis, mo . | WBC, 109/L . | Hb, g/dL . | Platelets, 109/L . | NCC in the sternum, 109/L . | Iliac bone biopsy . | PNH-type granulocytes/ RBCs, % . | Therapy . | Response to therapy . | Presentation of HLA-DR15 (DRB1 allele) . |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 40/M | 58 | 2.6 | 10.0 | 30 | 68 | Hypercellular | 1.62/2.83 | Oxymetholone | (+) | – |
| 2 | 48/M | 14 | 2 | 11.9 | 88 | 50 | NT | 0.56/NT | (−) | NE | 1502 |
| 3 | 68/F | 26 | 2.4 | 7.9 | 23 | 207 | Normocellular | 1.1/4.65 | Cyclosporine | (−) | 1502 |
| 4 | 39/F | 2 | 2.6 | 7.1 | 40 | 73 | NT | 1.24/1.19 | (−) | NE | − |
| 5 | 40/F | 12 | 2.5 | 8.1 | 45 | 197 | NT | 2.41/10.01 | Cyclosporine | (+) | 1502 |
| 6 | 77/F | 50 | 3 | 7.7 | 31 | 186 | Hypocellular | 0.09/0.47 | (−) | NE | 1501/1502 |
| 7 | 73/F | 15 | 2.9 | 8.7 | 73 | 272 | Hypocellular | 0.004/0.08 | (−) | NE | 1502 |
| 8 | 60/F | 122 | 2.9 | 7.3 | 23 | 110 | NT | 0.01/0.1 | (−) | NE | 1501 |
| 9 | 68/M | 52 | 3.2 | 9.3 | 21 | 135 | Hypocellular | 0.08/0.3 | Cyclosporine | (−) | 1502 |
| 10 | 58/F | 78 | 2.9 | 7.9 | 67 | 188 | Normocellular | 0.12/0.57 | (−) | NE | 1502 |
| 11 | 79/F | 36 | 3.4 | 7.4 | 85 | 207 | Normocellular | 0.1/0.2 | (−) | NE | 1501 |
| 12 | 69/F | 85 | 3.2 | 7.9 | 21 | 139 | Hypocellular | 0.003/0.01 | Danazol | (+) | 1502 |
| 13 | 17/F | 6 | 2.1 | 9.1 | 19 | 139 | NT | 0.26/0.09 | Cyclosporine | (+) | 1501 |
| 14 | 59/M | 23 | 2.9 | 8.5 | 6 | 150 | Hypocellular | 0.16/0.13 | Cyclosporine | (+) | 1501 |
| 15 | 54/F | 1 | 2.4 | 8.2 | 38 | 222 | Hypocellular | 0.02/0.1 | Cyclosporine | (+) | 1502 |
| 16 | 51/M | 190 | 3.6 | 11.5 | 121 | 106 | Hypocellular | 0.04/0.005 | (−) | NE | 1501/1502 |
| 17 | 60/F | 1 | 4.7 | 5.9 | 15 | 53 | Hypocellular | 0.03/0.37 | Methenolone | (+) | 1501 |
| 18 | 67/F | 30 | 2.5 | 8.9 | 40 | 477 | NT | 0.45/0.36 | (−) | NE | 1502 |
| 19 | 56/M | 41 | 3.0 | 8.6 | 6 | 399 | Normocellular | 0.01/0.006 | Cyclosporine | (+) | 1501 |
| 20 | 68/F | 3 | 3.4 | 7.0 | 58 | 240 | Hypocellular | 0.02/0.01 | Cyclosporine | (+) | 1501 |
| 21 | 58/M | 47 | 2.3 | 8.0 | 4 | 358 | NT | 0.003/0.16 | Cyclosporine | (+) | 1501 |
| Patient . | Age, y/sex . | Time from diagnosis, mo . | WBC, 109/L . | Hb, g/dL . | Platelets, 109/L . | NCC in the sternum, 109/L . | Iliac bone biopsy . | PNH-type granulocytes/ RBCs, % . | Therapy . | Response to therapy . | Presentation of HLA-DR15 (DRB1 allele) . |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 40/M | 58 | 2.6 | 10.0 | 30 | 68 | Hypercellular | 1.62/2.83 | Oxymetholone | (+) | – |
| 2 | 48/M | 14 | 2 | 11.9 | 88 | 50 | NT | 0.56/NT | (−) | NE | 1502 |
| 3 | 68/F | 26 | 2.4 | 7.9 | 23 | 207 | Normocellular | 1.1/4.65 | Cyclosporine | (−) | 1502 |
| 4 | 39/F | 2 | 2.6 | 7.1 | 40 | 73 | NT | 1.24/1.19 | (−) | NE | − |
| 5 | 40/F | 12 | 2.5 | 8.1 | 45 | 197 | NT | 2.41/10.01 | Cyclosporine | (+) | 1502 |
| 6 | 77/F | 50 | 3 | 7.7 | 31 | 186 | Hypocellular | 0.09/0.47 | (−) | NE | 1501/1502 |
| 7 | 73/F | 15 | 2.9 | 8.7 | 73 | 272 | Hypocellular | 0.004/0.08 | (−) | NE | 1502 |
| 8 | 60/F | 122 | 2.9 | 7.3 | 23 | 110 | NT | 0.01/0.1 | (−) | NE | 1501 |
| 9 | 68/M | 52 | 3.2 | 9.3 | 21 | 135 | Hypocellular | 0.08/0.3 | Cyclosporine | (−) | 1502 |
| 10 | 58/F | 78 | 2.9 | 7.9 | 67 | 188 | Normocellular | 0.12/0.57 | (−) | NE | 1502 |
| 11 | 79/F | 36 | 3.4 | 7.4 | 85 | 207 | Normocellular | 0.1/0.2 | (−) | NE | 1501 |
| 12 | 69/F | 85 | 3.2 | 7.9 | 21 | 139 | Hypocellular | 0.003/0.01 | Danazol | (+) | 1502 |
| 13 | 17/F | 6 | 2.1 | 9.1 | 19 | 139 | NT | 0.26/0.09 | Cyclosporine | (+) | 1501 |
| 14 | 59/M | 23 | 2.9 | 8.5 | 6 | 150 | Hypocellular | 0.16/0.13 | Cyclosporine | (+) | 1501 |
| 15 | 54/F | 1 | 2.4 | 8.2 | 38 | 222 | Hypocellular | 0.02/0.1 | Cyclosporine | (+) | 1502 |
| 16 | 51/M | 190 | 3.6 | 11.5 | 121 | 106 | Hypocellular | 0.04/0.005 | (−) | NE | 1501/1502 |
| 17 | 60/F | 1 | 4.7 | 5.9 | 15 | 53 | Hypocellular | 0.03/0.37 | Methenolone | (+) | 1501 |
| 18 | 67/F | 30 | 2.5 | 8.9 | 40 | 477 | NT | 0.45/0.36 | (−) | NE | 1502 |
| 19 | 56/M | 41 | 3.0 | 8.6 | 6 | 399 | Normocellular | 0.01/0.006 | Cyclosporine | (+) | 1501 |
| 20 | 68/F | 3 | 3.4 | 7.0 | 58 | 240 | Hypocellular | 0.02/0.01 | Cyclosporine | (+) | 1501 |
| 21 | 58/M | 47 | 2.3 | 8.0 | 4 | 358 | NT | 0.003/0.16 | Cyclosporine | (+) | 1501 |
White blood cell, hemoglobin, and platelet counts were determined at the time of diagnosis.
NCC indicates nucleated cell count; NT, not tested; and NE, not evaluable.
Clinical features of PNH+ RA patients compared with PNH− RA patients
Laboratory data and treatment outcomes were compared between PNH+ RA patients and PNH− RA patients to analyze the clinical significance of the minor population of PNH-type cells. Table 3 summarizes the results of the comparison. Thirty-three percent of PNH− RA patients had various karyotypic abnormalities, such as monosomy 7 and trisomy 8, whereas only 1 of 21 PNH+ RA patients had a karyotypic abnormality of 46,XX,t(6;8)(q15;q22) in 11 of 20 dividing cells. When the degree of dysplasia was compared using the percentage of neutrophils with the Pseudo-Pelger-Hüet anomaly in the bone marrow as a marker, PNH+ RA patients showed significantly lower percentages of dysplastic neutrophils than PNH− RA patients. The median platelet count (31 × 109/L) in PNH+ RA patients was significantly lower than that in PNH− RA patients (91 × 109/L;P = .01).
Clinical features of PNH+ and PNH−RA patients
| . | PNH+ RA . | PNH− RA . | P . |
|---|---|---|---|
| Incidence of karyotypic abnormalities (%) | 1 of 21 (4.8) | 21 of 64 (32.8) | .013-150 |
| Neutrophils with the Pseudo-Pelger-Hüet anomaly, % (range) | 2.0 (0-35.0) | 6.0 (0.5-45.2) | .023-151 |
| Platelet count, 109/L (range) | 31 (4-121) | 91 (9-126) | .013-151 |
| Incidence of HLA-DR15 (DRB13-1501501 and DRB13-1501502) (%) | 19 of 21 (90.5) | 5 of 27 (18.5) | <.0013-150 |
| Response to cyclosporine therapy (%) | 7 of 9 (77.8) | 0 of 8 (0) | .0023-150 |
| Progression to advanced MDS or AML (%) | 0 of 21 (0) | 4 of 65 (6.2) | .573-150 |
| . | PNH+ RA . | PNH− RA . | P . |
|---|---|---|---|
| Incidence of karyotypic abnormalities (%) | 1 of 21 (4.8) | 21 of 64 (32.8) | .013-150 |
| Neutrophils with the Pseudo-Pelger-Hüet anomaly, % (range) | 2.0 (0-35.0) | 6.0 (0.5-45.2) | .023-151 |
| Platelet count, 109/L (range) | 31 (4-121) | 91 (9-126) | .013-151 |
| Incidence of HLA-DR15 (DRB13-1501501 and DRB13-1501502) (%) | 19 of 21 (90.5) | 5 of 27 (18.5) | <.0013-150 |
| Response to cyclosporine therapy (%) | 7 of 9 (77.8) | 0 of 8 (0) | .0023-150 |
| Progression to advanced MDS or AML (%) | 0 of 21 (0) | 4 of 65 (6.2) | .573-150 |
Progression to advanced disease was observed for 2.5 years.
Fisher exact test;
Mann-Whitney U test.
The most remarkable difference between the 2 groups was the frequency of HLA-DR15, a split antigen of HLA-DR2. Nineteen of 21 (90.5%) PNH+ RA patients had HLA-DRB1*1501 or HLA-DRB1*1502, whereas only 5 of 27 (18.5%) PNH− RA patients who were tested for HLA-DRB1 alleles had this DR antigen. The frequencies of HLA-DRB1*1501 (47.6%) and HLA-DRB1*1502 (52.4%) in PNH+RA patients were much higher than in the general Japanese population (6.1% and 8.7%).16
Seventeen RA patients were treated with cyclosporine for more than 3 months after examination of the levels of PNH-type cells. None of the 8 PNH− RA patients improved, whereas 7 of 9 PNH+RA patients responded to the therapy. During the observation period of 2.5 years, 4 RA patients progressed to advanced MDS or acute myeloid leukemia (AML). All 4 patients had been PNH−, and none of the 21 PNH+ RA patients underwent such progression.
Discussion
This study on a large number of MDS patients revealed that increased PNH-type cells were detected in a limited number of patients with RA resembling AA. The flow cytometric assay that we used could reveal the presence of increased PNH-type granulocytes with variousPIG-A gene mutations when their percentages were less than 1% of CD11b+ granulocytes. Even with this sensitive flow cytometry, increased PNH-type cells were detected only among RA patients at a much lower prevalence (17.6%) than among the AA patients (52.0%) we reported previously.7 The prevalence of PNH+ patients among all our MDS patients was 12.8%. This lower prevalence, compared with AA patients, was in sharp contrast to the results of a previous report that showed similar prevalence between AA and MDS patients.11,17 This was probably because of the differences in the study population and the specificity of flow cytometric assays used. In contrast to the recent report by Maciejewski et al,17 none of our 68 healthy controls exhibited more than 0.003% PNH-type cells in granulocytes and RBCs. Our failure to detect a single patient with increased PNH-type cells among RARS, RAEB, and RAEB-t patients indicates that the presence of increased PNH-type cells in MDS patients has little to do with the preleukemic nature and that, except for part of RA, MDS is a bone marrow failure distinct from AA in view of the prevalence of PNH-type cells. The absence of karyotypic abnormalities and the progression to advanced MDS or AML in PNH+ RA patients support these hypotheses.
In addition to the study by Dunn et al11 that detected more than 1% CD15+CD16−CD66b−granulocytes in 9 of 39 (23.1%) MDS patients, Iwanaga et al18 demonstrated that 4 of 40 (10%) MDS patients had more than 1% of CD55− or CD59− cells; the frequency in RA patients was 12.9%. The percentage of PNH-type blood cells in these 4 patients exceeded 10%, and all 4 patients had positive findings on Ham test. The frequency of PNH+patients in those previous studies cannot be compared to that (17.6%) in our study because the percentage of PNH-type cells in our PNH+ patients was less than 1% in 81.0% of the patients. It is possible that previous studies underestimated the frequency of PNH+ patients because of the low sensitivity of flow cytometry tests. In the present study, the low percentage of patients with more than 1% PNH-type cells may be attributed to the exclusion, by physicians who participated in the study, of patients with bone marrow failure and overt signs of hemolysis.
All the mutations we found in the PNH+ RA patients were in exons 4 and 5. This may be surprising because many of the mutations reported previously were in exons 2 and 6. Iwanaga et al18demonstrated that 2 of 4 RA patients with increased PNH cells showed a mutation in exon 4 or a deletion of exon 5. It is possible thatPIG-A mutations in a minor population of PNH cells from RA patients may be biased toward exons 4 and 5 rather than exons 2 and 6. However, mutations in exons 4 and 5 are not rare, according to a recent paper19 reviewing PIG-A gene analysis on 80 PNH patients. Thus, this possibility must be tested through the study of a larger number of RA patients with increased numbers of PNH- type cells.
The most striking feature of our RA patients who had a minor population of PNH-type cells was the high frequency of HLA-DR15. This DR antigen has been repeatedly reported to be associated with susceptibility to AA.20,21 The frequency of HLA-DR15 in PNH+ RA patients (90.5%) in the present study was higher than the frequency of HLA-DR2 (74%) in the recent study by Maciejewski et al.22This is probably because of the difference in the definition of PNH+ patients. RA patients with a minor population of PNH-type cells may be more likely to have HLA-DR15 than RA patients with 1% or more PNH-type cells. Among DRB1 alleles of HLA-DR15, the presentation of DRB1*1501 by AA patients21,23,24 and MDS patients25 has been linked to a good response to cyclosporine therapy. In keeping with this well-known association, 5 of 5 PNH+ RA patients in the present study who had DRB1*1501 responded to cyclosporine. These findings further support the hypothesis that the minor population of PNH-type cells detected in RA patients reflects the involvement of immune mechanisms in the development of bone marrow failure.
One might be concerned about the diagnosis of RA in our PNH+ patients because the extent of dysplasia in the blood cells of PNH+ patients was not as severe as that in PNH− patients, and bone marrow biopsy specimens from the iliac bone in 10 of 14 patients showed hypocellularity. It is possible that some hematologists may originally diagnose AA rather than RA in PNH+ patients. However, this cannot be resolved as long as there are no definitive criteria for discriminating between AA with moderate dysplasia and RA with less severe dysplasia.10There is a limit to the usefulness of morphology in discriminating benign from malignant bone marrow failure because some AA patients with no dysplastic signs have monosomy 7, and AML rapidly develops.26,27 Therefore, it seems more important to recognize patients showing pancytopenia with a minor population of PNH cells as having a benign type of bone marrow failure than to be particular about the differential diagnosis. Several reports have documented successful immunosuppressive therapy of RA patients in whom dysplastic signs of bone marrow cells were observed primarily in erythroblasts.28,29 Such RA patients seem to be similar to PNH+ patients in the present study. Orazi et al30 reported that hypoplastic MDS can be distinguished from aplastic anemia with normal or near-normal CD34+ cell counts on the bone marrow. Demonstrating low CD34+ cell counts in the bone marrow of PNH+ RA patients may be useful in substantiating our hypothesis that PNH+ RA patients have a pathophysiology similar to that of patients with AA.
The results of the present study may have important implications for clinical practice. Although several studies have shown that considerable proportions of MDS patients experience restoration of hematopoietic function with immunosuppressive therapy,31-34 there has been no marker for a good response to the therapy. The presence of a minor population of PNH+cells may serve as such a marker for immune-mediated bone marrow failure. PNH+ RA patients have probably been overlooked because of difficulties in detecting a minor population of PNH-type cells with conventional flow cytometry. They may have been treated with toxic therapy, such as bone marrow transplantation from unrelated donors and low-dose cytosine arabinoside. A prospective study in a larger group of patients is needed to establish the clinical significance of PNH-type cells in the management of bone marrow failure.
We thank the following physicians for providing us with patient samples and clinical information: K. Ohyashiki, Tokyo Medical University; H. Yamauchi, Kurobe City Hospital; H. Yamazaki, Toyama City Hospital; K. Kyoda, Fukuiken Saiseikai Hospital; K. Masuda, Okayama University Hospital; M. Saito, NTT Kanazawa Hospital; M. Teramura, Tokyo Women's Medical College; M. Ueda, Ishikawa Prefectural Central Hospital; N. Ichikawa, Nagano Red Cross Hospital; S. Fujii, Teraoka Memorial Hospital; S. Matano, Tonami General Hospital; T. Nakamoto, Tokyo University Hospital; and T. Yoshida, Toyama Prefectural Central Hospital.
Prepublished online as Blood First Edition Paper, August 8, 2002; DOI 10.1182/blood-2002-03-0799.
Supported in part by a Grant-in-Aid for Immunologic Research for Intractable Diseases from the Ministry of Health, Labor and Welfare of Japan.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Shinji Nakao, Cellular Transplantation Biology, Kanazawa University Graduate School of Medical Science, 13-1 Takaramachi, Kanazawa, Ishikawa, Japan 920-8641; e-mail:snakao@med3.m.kanazawa-u.ac.jp.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal