Hypercoagulability and thrombotic tendency are frequently induced by a variety of stressors. Clinically, aged subjects and obese patients are more susceptible to thrombotic diseases associated with stress, but the underlying mechanisms are unknown. We investigated the expression of a procoagulant gene, tissue factor (TF), in a mouse model of restraint stress. Twenty hours of restraint stress to mice caused a substantial induction of TF mRNA in several tissues. Importantly, the magnitude of induction of TF mRNA by restraint stress was larger in aged mice compared with young mice. In situ hybridization analysis of the stressed aged mice revealed that strong signals for TF mRNA were localized to renal epithelial cells, smooth muscle cells, adventitial cells, and adipocytes but not to vascular endothelial cells. These observations suggest that restraint stress induces the TF expression in a tissue-specific and cell type–specific manner. Genetically obese mice were also hyperresponsive to restraint stress in the induction of TF gene, especially in their livers and adipose tissues. Stress-induced microthrombi formation was pronounced in renal glomeruli and within the vasculature in adipose tissues of aged mice. Tumor necrosis factor-α (TNF-α) antigen in plasma was elevated by stress in aged mice and obese mice, and pretreatment of mice with anti–TNF-α antibody partially attenuated the stress-mediated induction of TF gene in adipose tissues in these mice. These results suggest that the induction of TF gene may increase the risk of stress-associated thrombosis in older and obese subjects and that TNF-α may be involved.
Introduction
The stress response is thought to be required for the maintenance of homeostasis and is characterized by rapid changes in the gene expression of stress proteins.1 This response is best demonstrated in the neuro-endocrine system (eg, the hypothalamic-pituitary-adrenal axis)2 and, in this setting, may be mediated by the activation of the glucocorticoid cascade3 and of the sympathetic nervous system.4,5 The restraint stress model often has been used to investigate the stress response experimentally in terms of pharmacologic, physiologic, or pathologic phenomena in vivo.6 For example, restraint stress induces the expression of heat shock protein, a typical stress protein, in the rat,2,4 and this induction may be mediated by the sympathetic nervous system.4,5 The induction of stress proteins may contribute to the development of a number of clinically relevant phenomena, including tissue and organ damage, and the immune response.7 Hypercoagulability in the blood and thrombotic tendency also may be induced by physical8,9 and mental stress.10 In this context, aged animals2-5and/or obese mice11,12 may have lower tolerance to stress insults. Clinically, older and obese individuals are susceptible to the stress-mediated pathologic changes, including thrombotic complications,13 14 possibly because of the stress-induced imbalance in the coagulation and fibrinolytic system.
Tissue factor (TF) is the primary cellular initiator of the coagulation protease cascade and serves as a specific cofactor for plasma factors VII/VIIa.15 TF is constitutively expressed by several extravascular cell types (eg, epithelial cells, adventitial cells, adipocytes) and is inducibly expressed by monocytes and endothelial cells within the vasculature. Cis-acting regulatory elements within the human TF promoter would control constitutive and inducible expression in various cell types.16 TF gene expression appears to be regulated by a variety of transcription factors (eg, nuclear factor-κB [NF-κB], AP-1, and Egr-1)16,17 and is activated by external signals, such as inflammatory cytokines (eg, tumor necrosis factor-α [TNF-α], interleukins), growth factors, or bacterial lipopolysaccharide. Aberrant expression of TF may be responsible for thrombotic episodes in patients with a variety of clinical disorders, including atherosclerosis,18sepsis,19 and cancer.20 A couple of studies have shown an increase in TF-mediated coagulation and/or factor VII activity in obese patients.21 Aging is also associated with increased plasma level of factor VII, which is an independent risk factor for thrombotic disease.22 Thus, TF/factor VII may contribute to the hypercoagulable state under a variety of pathologic conditions.
We previously reported that plasminogen activator inhibitor-1 (PAI-1) and/or antithrombin was involved in the development of renal glomerular thrombosis induced by restraint stress.23 24 In the present study, we analyzed the gene expression of another key molecule for thrombosis, TF, in a murine model of restraint stress in vivo. Furthermore, we investigated the effects of aging and obesity on the stress-induced TF expression and tissue thrombosis by using extremely aged (12- and 24-month-old) mice and genetically obese (C57BL/6J ob/ob) mice. Overnight restraint stress to mice substantially induced TF mRNA expression in some tissues. More importantly, the stress-induced TF expression and/or microvascular thrombosis were pronounced in aged mice and in obese mice. Finally, endogenous TNF-α, which was also increased by stress, may be a primary mediator for the induction of TF gene in this restraint model. Thus, elevated TF expression by stress may, in part, contribute to the stress-associated thrombosis, and this response of TF gene is exacerbated by aging and obesity.
Materials and methods
Restraint stress
Male C57BL/6J mice aged 2, 12, and 24 months, were obtained from SLC Japan (Shizuoka, Japan) and through the National Institute of Aging. Mice were placed into 50-mL conical centrifuge tubes fitted with multiple punctures so as to allow ventilation. The tubes were placed in horizontal holders, and the animals thus were maintained for a continuous period of restraint.5 During this time, the animals were provided with water only. After 2 or 20 hours of restraint, the mice were killed by overdose inhalation anesthesia with methoxyflurane (Pitman-Moore, Mundelein, MD). Tissues were rapidly removed by standard dissection techniques and were either minced and immediately frozen in liquid nitrogen for preparation of total RNA or fixed in chilled 4% paraformaldehyde and embedded in paraffin for in situ hybridization and for staining with periodic acid Schiff (PAS) or hematoxylin/eosin. This experimental protocol was approved by the animal resource committee of our university.
In separate experiments, 8-week- and 24-month-old male C57BL/6J mice were pretreated intraperitoneally either with control (nonimmune) hamster immunoglobulin G (IgG; 25 μg/mouse; Genzyme, Cambridge, MA) or with the IgG fraction of a neutralizing hamster monoclonal antibody (mAb) to mouse TNF-α (25 μg/mouse; Genzyme)25 before 20 hours of continuous restraint stress. The blood was collected into 20 mM EDTA (ethylenediaminetetraacetic acid) (final concentration) and centrifuged at 3000g for 5 minutes, and then the plasma was removed and stored at −70°C. The tissues were collected and prepared as described above. Meanwhile, 6-week-old male obese mice (C57BL/6J ob/ob) and their lean counterparts (C57BL/6J +/?), both of which were obtained from The Jackson Laboratories (Bar Harbor, ME), were put into restraint tubes for 20 hours and then killed. The tissues were removed and prepared for subsequent analysis as described earlier. They were also pretreated intraperitoneally either with control hamster IgG (1 μg/g) or with hamster antimouse TNF-α antibody (1 μg/g) before 20 hours of continuous restraint stress, and the tissues were collected.
RNA extraction and quantitative RT-PCR assay
Total RNA was prepared from unfixed tissues by using the ULTRASPEC RNA ISOLATION SYSTEM (Biotecx Laboratories, Houston, TX) and then quantitated by measuring absorption at 260 nm. The content of TF mRNA in murine tissues was determined by quantitative reverse transcription–polymerase chain reaction (RT-PCR) assay by using a competitor RNA (cRNA) containing sequences of upstream and downstream primers for mouse TF and β-actin, as described previously.26 After reverse transcription using the cRNA, ranging from 0.1 to 10 pg, and PCR amplification of 30 cycles (95°C for 1 minute, 60°C for 1 minute, and 72°C for 1 minute) using32P-end-labeled sense primer (5 × 105 cpm), 20-μL aliquots of the PCR products were electrophoresed on a 2.5% agarose gel. The appropriate bands corresponding to the cRNA product and the target mRNA product were excised from the gel, and the incorporated radioactivity in each was determined with the use of a scintillation counter. Finally, the content of TF mRNA in each tissue was determined by extrapolation using the cRNA standard curve, and the data were represented as picogram TF mRNA per microgram total RNA. All of the RT-PCR experiments were performed in triplicate. Variations in sample loading were assessed by comparison with β-actin mRNA.
Statistical analysis
Statistical analysis of all quantitative RT-PCR results was performed by using the unpaired Student t test. Differences were not considered significant when P > .05 (group size, n = 6 or 8).
Determination of TNF-α antigen in mouse plasma
Total TNF-α antigen in plasma (picogram per milliliter was measured using the ELISA-view kit (BioSource International, Camarillo, CA).
In situ hybridization
In situ hybridization was performed using35S-labeled antisense riboprobes, as described previously.26 After hybridization, the slides were dehydrated by immersion in a graded alcohol series containing 0.3 M NH4Ac and dried. Then the slides were coated with NTB2 emulsion (Kodak, Rochester, NY; 1:2 in water), and exposed in the dark at 4°C for 8 to 12 weeks. The slides were developed for 2 minutes in D19 developer (Kodak), fixed, washed in water, and counterstained with hematoxylin/eosin. No specific hybridization signal could be detected in parallel sections using 35S-labeled sense probes in each experiment (not shown).
Preparation of antimouse TF antibody and Western blot analysis
Polyclonal rabbit antiserum specific for mouse TF (2 mg/mL, 1:2000 dilution in Tris (tris(hydroxymethyl)aminomethane)–buffered saline (TBS) containing 0.1% Tween-20 (TBS-T) was kindly provided by Drs K. Enjyoji and H. Kato, National Cardiovascular Center and Research Institute, Osaka, Japan. This specific antibody was raised in rabbits by the direct introduction of the encoding cDNA of mouse TF cloned into the pcDNA3 plasmid as described previously.27 28 Serum titers and the specificity of the antibody were determined by standard Western blot analysis using protein extracts from COS-7 cells expressing mouse TF and recombinant mouse TF fused to glutathione-S-transferase protein (Amersham Pharmacia Biotech, Tokyo, Japan) (not shown). TF antigen in the lysates of adipose tissues obtained from obese and lean mice after 0, 2, and 20 hours of restraint stress was determined by sodium dodecylsulfate–polyacrylamide gel electrophoresis (SDS-PAGE) and Western blot analysis using the enhanced chemiluminescent (ECL) detection system (Amersham International, Buckinghamshire, United Kingdom). Briefly, each 2 μg of the tissue lysates was electrophoresed under reduced conditions on an 8% SDS-PAGE and transferred to polyvinylidene diflouride (PDVF) membranes (Bio-Rad Laboratories, Hercules, CA). The membranes were soaked in TBS containing 5% nonfat milk and 0.1% Tween-20 for 1 hour at room temperature to block additional protein binding sites and washed 3 times (15 minutes/wash) in TBS-T. The membranes were then incubated with antimouse TF, washed 4 times in TBS-T, and incubated for 1 hour with peroxidase-linked donkey antirabbit antibody (Amersham). After 3 washes in TBS-T, the membranes were developed with the ECL detection kit according to manufacturer's instructions.
Results
Induction of TF mRNA in tissues of the restraint-stressed mice
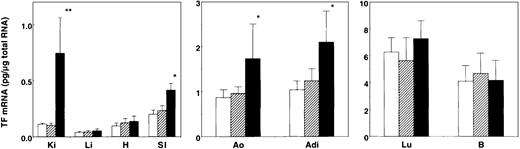
Initially, the effects of short and long exposure to restraint stress on the expression of TF mRNA in tissues were investigated in 8-week-old male C57BL/6J mice by using quantitative RT-PCR technique (Figure 1). Short duration (2 hours) of stress to mice did not substantially increase TF mRNA in all tissues examined. In contrast, a substantial induction of TF mRNA was detected in kidneys (6-fold), small intestines (2-fold), aortas (2-fold), and adipose tissues (2-fold) after 20 hours of restraint stress. Unexpectedly, little or no responses of TF gene to restraint stress were observed in livers, lungs, hearts, and brains. These results suggest that the induction of TF gene by restraint stress occurred in a tissue-specific manner.
Changes in the expression level of TF mRNA in mouse tissues by restraint stress.
Eight-week-old C57BL/6J mice were placed into restraint tubes for 2 or 20 hours, and then the tissues were removed. Total tissue RNA was prepared and analyzed for TF mRNA expression level by quantitative RT-PCR assay as described in “Materials and methods.” For each tissue type, ■ indicates level before stress; ▨, after 2 hours of restraint stress; and ▪, after 20 hours of restraint stress. The data are represented as the means and SD (n = 8) in each amount of time under stress, and the error bars represent interanimal variation. *P < .05; **P < .02. Ki indicates kidney; Li, liver; H, heart; SI, small intestine; Ao, aorta; Adi, adipose (epididymal fat); Lu, lung; B, brain.
Changes in the expression level of TF mRNA in mouse tissues by restraint stress.
Eight-week-old C57BL/6J mice were placed into restraint tubes for 2 or 20 hours, and then the tissues were removed. Total tissue RNA was prepared and analyzed for TF mRNA expression level by quantitative RT-PCR assay as described in “Materials and methods.” For each tissue type, ■ indicates level before stress; ▨, after 2 hours of restraint stress; and ▪, after 20 hours of restraint stress. The data are represented as the means and SD (n = 8) in each amount of time under stress, and the error bars represent interanimal variation. *P < .05; **P < .02. Ki indicates kidney; Li, liver; H, heart; SI, small intestine; Ao, aorta; Adi, adipose (epididymal fat); Lu, lung; B, brain.
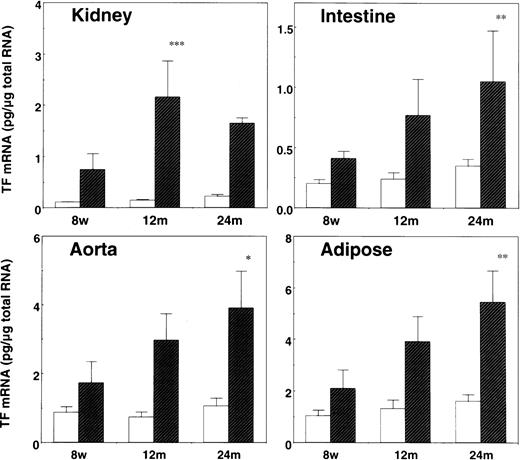
Stress-induced TF mRNA expression in young and aged mice
Experiments were performed to investigate the effect of aging on the induction of TF expression by restraint stress, using young (8-week-old) and extremely aged (12- and 24-month-old) mice (Figure2). Basal (before stress) levels of TF mRNA expression in the tissues were slightly elevated in aged mice, but the differences were not substantial. Importantly, the magnitude of induction of TF mRNA expression in kidneys, small intestines, aortas, and adipose tissues substantially increased as animals age (Figure 2). Only in kidneys, TF mRNA expression was more increased by stress in 12-month-old mice than in 24-month-old mice. Again, no substantial induction of TF mRNA by 20 hours of restraint stress was observed in livers, lungs, hearts, and brains both in young and aged mice (not shown).
Induction of TF mRNA by restraint stress in the tissues of young and aged mice.
The indicated tissues were removed from 8-week- (8w), 12-month- (12m), and 24-month-old (24m) C57BL/6J mice before (open bars) and after (hatched bars) 20 hours of restraint stress. Total tissue RNA was prepared and analyzed for TF mRNA expression level by quantitative RT-PCR. The data are represented as the means and SD (n = 8) in each age group, and the error bars represent interanimal variation. *P < .05 in 24m versus 8w mice; **P < .04 in 24m versus 8w mice; ***P < .02 in 12m versus 8w mice.
Induction of TF mRNA by restraint stress in the tissues of young and aged mice.
The indicated tissues were removed from 8-week- (8w), 12-month- (12m), and 24-month-old (24m) C57BL/6J mice before (open bars) and after (hatched bars) 20 hours of restraint stress. Total tissue RNA was prepared and analyzed for TF mRNA expression level by quantitative RT-PCR. The data are represented as the means and SD (n = 8) in each age group, and the error bars represent interanimal variation. *P < .05 in 24m versus 8w mice; **P < .04 in 24m versus 8w mice; ***P < .02 in 12m versus 8w mice.
Cellular localization of TF mRNA in tissues of the restraint-stressed mice
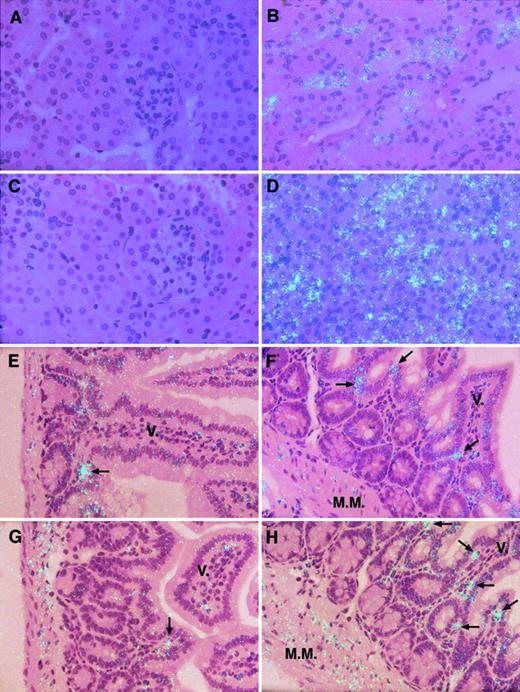
To localize the TF mRNA induced by restraint stress in each tissue, in situ hybridization analysis was performed by using tissue sections from the control and stressed mice. Although there was no detectable signal for TF mRNA in kidneys of the unstressed aged mice (Figure 3 A,C), the epithelial cells of proximal and distal tubules (Figure 3B,D) in the stressed mice expressed abundant TF mRNA. Moreover, signals for TF mRNA in renal tubular epithelial cells were dramatic in the stressed aged mice compared with young mice (Figure 3B,D). In small intestines of the stressed mice, several cell types, including smooth muscle cells and inflammatory cells, in the villous core, in lamina propria, and in muscularis mucosae, showed strong signals for TF mRNA (Figure 3F,H) although these cells occasionally expressed TF mRNA in the unstressed mice (Figure 3E,G). Again, the increased signals for TF mRNA by stress in the intestinal cells were dramatic in aged mice (compare Figure 3F with 3H). These results are consistent with the data obtained by quantitative RT-PCR assay (Figure 2).
In situ hybridization analysis of TF mRNA in the kidneys and small intestines of control and stressed mice.
Kidneys were harvested from 8-week-old and 12-month-old mice before and after 20 hours of restraint stress. Small intestines were also harvested from 8-week-old and 24-month-old mice before and after 20 hours of restraint stress. Both tissues were analyzed by in situ hybridization with the use of 35S-labeled cRNA probes as described in “Materials and methods.” The hybridization signal for TF mRNA corresponds to the light blue dots in panels B,D-H. (A-D) Kidneys of the unstressed (A, 8 weeks old; C, 12 months old) and stressed (B, 8 weeks old; D, 12 months old) mice. (E-H) Small intestines of the unstressed (E, 8 weeks old; G, 24 months old) and stressed (F, 8 weeks old; H, 24 months old) mice. Arrows indicate cells that are strongly positive for TF mRNA. M.M. indicates muscularis mucosae; V., villous core. All slides were exposed for 10 weeks at 4°C. Original magnification × 400.
In situ hybridization analysis of TF mRNA in the kidneys and small intestines of control and stressed mice.
Kidneys were harvested from 8-week-old and 12-month-old mice before and after 20 hours of restraint stress. Small intestines were also harvested from 8-week-old and 24-month-old mice before and after 20 hours of restraint stress. Both tissues were analyzed by in situ hybridization with the use of 35S-labeled cRNA probes as described in “Materials and methods.” The hybridization signal for TF mRNA corresponds to the light blue dots in panels B,D-H. (A-D) Kidneys of the unstressed (A, 8 weeks old; C, 12 months old) and stressed (B, 8 weeks old; D, 12 months old) mice. (E-H) Small intestines of the unstressed (E, 8 weeks old; G, 24 months old) and stressed (F, 8 weeks old; H, 24 months old) mice. Arrows indicate cells that are strongly positive for TF mRNA. M.M. indicates muscularis mucosae; V., villous core. All slides were exposed for 10 weeks at 4°C. Original magnification × 400.
In aortas, only focal signals for TF mRNA were detected in the unstressed young and aged mice (Figure 4A,C). However, increased signals for TF mRNA were observed in adventitial cells of the vascular wall and in surrounding adipose tissues after restraint stress, and the signals were stronger in aged mice than young mice (Figure 4B,D). In control epididymal fat tissues, a few adipocytes were positive for TF mRNA both in young and aged mice (Figure 4E,G). In epididymal fat tissues of the stressed mice, more numbers of adipocytes specifically expressed considerable amounts of TF mRNA, and, again, the adipocyte-specific signals for TF mRNA were stronger in aged mice than those in young mice (Figure 4F,H). We observed no specific hybridized signals for TF mRNA in vascular endothelial cells in any organs examined in the stressed mice (data not shown).
In situ hybridization analysis of TF mRNA in the aortas and adipose tissues of control and stressed mice.
Aortas and epididymal fat tissues were harvested from 8-week-old and 24-month-old mice before and after 20 hours of continuous restraint stress and then analyzed by in situ hybridization as described in “Materials and methods.” The hybridization signal for TF mRNA corresponds to the light blue dots in all panels. (A-D) Aortas of the unstressed (A, 8 weeks old; C, 12 months old) and stressed (B, 8 weeks old; D, 12 months old) mice. Arrows indicate cells that are strongly positive for TF mRNA in the adventitia of aorta. S.M. indicates vascular smooth muscle layer; Ad, adipose tissue around the vessel wall of aorta. (E-H) Epididymal fat tissues of the unstressed (E, 8 weeks old; G, 24 months old) and stressed (F, 8 weeks old; H, 24 months old) mice. All slides were exposed for 10 weeks at 4°C. Original magnification for all panels × 400.
In situ hybridization analysis of TF mRNA in the aortas and adipose tissues of control and stressed mice.
Aortas and epididymal fat tissues were harvested from 8-week-old and 24-month-old mice before and after 20 hours of continuous restraint stress and then analyzed by in situ hybridization as described in “Materials and methods.” The hybridization signal for TF mRNA corresponds to the light blue dots in all panels. (A-D) Aortas of the unstressed (A, 8 weeks old; C, 12 months old) and stressed (B, 8 weeks old; D, 12 months old) mice. Arrows indicate cells that are strongly positive for TF mRNA in the adventitia of aorta. S.M. indicates vascular smooth muscle layer; Ad, adipose tissue around the vessel wall of aorta. (E-H) Epididymal fat tissues of the unstressed (E, 8 weeks old; G, 24 months old) and stressed (F, 8 weeks old; H, 24 months old) mice. All slides were exposed for 10 weeks at 4°C. Original magnification for all panels × 400.
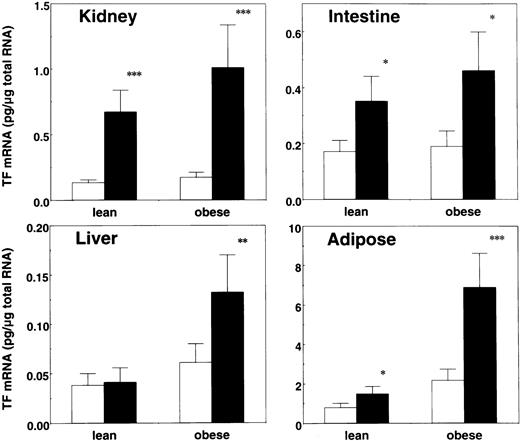
Induction of TF mRNA and antigen by restraint stress in obese mice
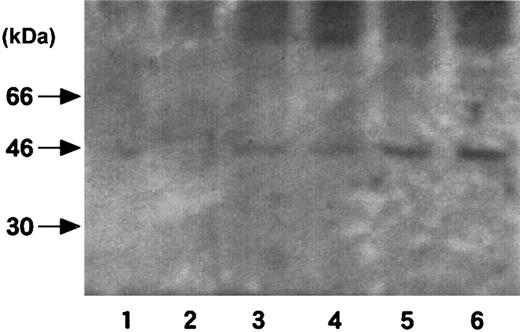
To investigate the effect of obesity on the stress-induced TF expression, we performed restraint experiments by using genetically obese mice and their lean counterparts and then analyzed TF mRNA expression in the tissues (Figure 5). Interestingly, 20 hours of restraint stress caused a substantial increase in TF mRNA level in livers of obese mice, which revealed degeneration to fatty liver but not in livers of lean mice. In kidneys and guts, differences in the induction of TF mRNA by stress between obese and lean mice were not so dramatic. In adipose tissues, the basal expression level of TF mRNA was 2-fold higher in obese mice than in lean mice. More importantly, the magnitude of induction of TF mRNA by stress was larger in adipose tissues of obese mice than in those of lean mice. The stress-induced TF expression in adipose tissues was analyzed at antigen level as well as by Western blotting using polyclonal rabbit antimouse TF antibody (Figure6). Although TF antigen in the lysates of adipose tissues from lean mice was at an undetectable level, a specific band of 46 kDa corresponding to TF was detected in those of obese mice. The amount of TF antigen produced in adipose tissues dramatically increased after 20 hours of restraint stress in obese mice (Figure 6, lane 6), which was consistent with the data at mRNA level (Figure 5). This increase in the adipose-derived TF production may result in the higher procoagulant potential in obese mice than in lean mice because obese mice carry abundant adipose mass.
Induction of TF mRNA by restraint stress in obese and lean mice.
Six-week-old male obese mice and their lean counterparts were stressed in restraint tubes for 20 hours. Mice were killed and the indicated tissues were removed. Total tissue RNA was prepared and analyzed for TF mRNA expression level by quantitative RT-PCR. The data are expressed as the means and SD (n = 8) in each phenotype. ■ represents level before stress; ▪, after 20 hours of restraint stress. *P < .05; **P < .04; ***P < .02.
Induction of TF mRNA by restraint stress in obese and lean mice.
Six-week-old male obese mice and their lean counterparts were stressed in restraint tubes for 20 hours. Mice were killed and the indicated tissues were removed. Total tissue RNA was prepared and analyzed for TF mRNA expression level by quantitative RT-PCR. The data are expressed as the means and SD (n = 8) in each phenotype. ■ represents level before stress; ▪, after 20 hours of restraint stress. *P < .05; **P < .04; ***P < .02.
Western blot analysis of TF antigen in adipose tissues of the stressed obese mice.
Six-week-old male obese mice and their lean counterparts were stressed in restraint tubes for 2 or 20 hours. Mice were killed and epididymal fat tissues were removed and their lysates prepared. Two micrograms of each lysate was loaded on an 8% polyacrylamide gel, and TF antigen was analyzed by Western blotting with the use of polyclonal rabbit antimouse TF antibody. Lanes 1-3, lean mice (1, no stress; 2, 2 hours of stress; 3, 20 hours of stress); lanes 4-6, obese mice (4, no stress; 5, 2 hours of stress; 6, 20 hours of stress). The numbers to the left of the blots indicate molecular weight.
Western blot analysis of TF antigen in adipose tissues of the stressed obese mice.
Six-week-old male obese mice and their lean counterparts were stressed in restraint tubes for 2 or 20 hours. Mice were killed and epididymal fat tissues were removed and their lysates prepared. Two micrograms of each lysate was loaded on an 8% polyacrylamide gel, and TF antigen was analyzed by Western blotting with the use of polyclonal rabbit antimouse TF antibody. Lanes 1-3, lean mice (1, no stress; 2, 2 hours of stress; 3, 20 hours of stress); lanes 4-6, obese mice (4, no stress; 5, 2 hours of stress; 6, 20 hours of stress). The numbers to the left of the blots indicate molecular weight.
Stress-induced thrombosis in aged mice and in obese mice
Microscopic examination of tissue sections revealed that renal glomerular thrombus developed in the 20-hour restraint aged (24-month-old) mice (Figure 7B) but not in the stressed young (8-week-old) mice (Figure 7A). Stress-induced thrombi formation was also observed in the microvasculature in epididymal adipose tissues in aged mice (Figure 7E) but not in adipose tissues of the stressed young mice (Figure 7D). Quantitative analysis of the stress-induced thrombi was performed by counting positive glomeruli for thrombi and the number of thrombi within the microvasculature in epididymal adipose tissues. Although renal glomerular thrombi were detected in less than 5% glomeruli in only 1 of 8 restraint mice of 8 weeks old, all (n = 8) of the stressed aged mice showed glomerular thrombi. The percentage of positive glomeruli for thrombi increased 10% to 27% (Figure 7C).23Similarly, we observed occasional thrombi formation in adipose tissues in 6 of 8 restraint mice 24 months old, although no thrombi were detected in adipose tissues of the stressed young mice (n = 8) (Figure 7F). Meanwhile, rare microthrombi were observed within the vasculature in adipose tissues in only 2 of 8 stressed obese mice, and none of the stressed lean mice showed thrombi formation in their adipose tissues (not shown). No stress-induced thrombi were detected in livers, hearts, intestines, and aorta of the aged mice and obese mice (not shown).
Stress-induced thrombi formation in tissues of young and aged mice.
Renal and adipose tissues were removed from young (8-week-old) and aged (24-month-old) mice after 20 hours of restraint stress (n = 8 in each age group). Tissue sections were stained with PAS (A-B) or with hematoxylin/eosin (D-E) and examined microscopically. (A-B) Renal glomerulus of the stressed young (A) and aged (B) mice; original magnification, × 1000. An arrow denotes glomerular thrombus in aged mice. (D-E) Epididymal adipose tissues of the stressed young (D) and aged (E) mice; original magnification, × 400. An arrowhead indicates thrombi in the microvessels between adipocytes. Quantitation of stress-induced glomerular thrombi was achieved by counting the positive glomeruli for thrombi of at least 100 glomeruli in each kidney section from young (8w) and aged mice (24m), and the percentage of positive glomeruli for thrombi in each mouse is shown in panel C. Panel F shows each number of thrombi detected in the vasculature within the same size of microscopic area (90 mm2) in epididymal fat tissues of the stressed young (8w) and aged (24m) mice.
Stress-induced thrombi formation in tissues of young and aged mice.
Renal and adipose tissues were removed from young (8-week-old) and aged (24-month-old) mice after 20 hours of restraint stress (n = 8 in each age group). Tissue sections were stained with PAS (A-B) or with hematoxylin/eosin (D-E) and examined microscopically. (A-B) Renal glomerulus of the stressed young (A) and aged (B) mice; original magnification, × 1000. An arrow denotes glomerular thrombus in aged mice. (D-E) Epididymal adipose tissues of the stressed young (D) and aged (E) mice; original magnification, × 400. An arrowhead indicates thrombi in the microvessels between adipocytes. Quantitation of stress-induced glomerular thrombi was achieved by counting the positive glomeruli for thrombi of at least 100 glomeruli in each kidney section from young (8w) and aged mice (24m), and the percentage of positive glomeruli for thrombi in each mouse is shown in panel C. Panel F shows each number of thrombi detected in the vasculature within the same size of microscopic area (90 mm2) in epididymal fat tissues of the stressed young (8w) and aged (24m) mice.
Stress-mediated changes in plasma TNF-α level and effects of anti–TNF-α antibody on the stress-induced TF mRNA
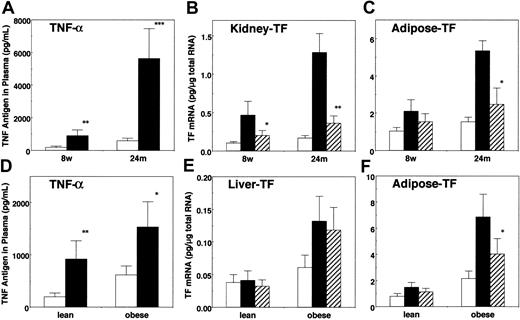
Changes in total TNF-α antigen in plasma of young and aged mice, and of obese and lean mice, by restraint stress were analyzed (Figure 8 A,D). The basal level of plasma TNF-α was already higher in aged mice versus young mice and in obese mice versus their lean counterparts. After 20 hours of restraint stress, a substantial elevation of TNF-α antigen in plasma was detected both in young and aged mice, and the magnitude of this stress-mediated induction of TNF-α was larger in aged mice (10-fold) versus young mice (4-fold). Similarly, substantial increases in plasma TNF-α level were observed in stressed obese (2.5-fold) and lean mice (4-fold).
Changes in TNF-α antigen in plasma by stress and effects of anti–TNF-α antibody on the stress-induced TF mRNA expression.
TNF-α antigen levels in plasma of 8-week-old (8w) and 24-month-old (24m) mice (A) and of 6-week-old obese and lean mice (D) before (■) and after (▪) 20 hours of restraint stress were determined by enzyme-linked immunosorbent assay (*P < .04; **P < .01; ***P < .005). In separate experiments, the mice were pretreated either with control or with anti–TNF-α antibody (25 μg/mouse or 1 μg/g) and then subjected to 20 hours of restraint stress. Kidneys, adipose tissues, or livers were harvested and analyzed for TF mRNA by quantitative RT-PCR (young and aged mice, B and C; obese and lean mice, E and F). ■ indicates no stress; ▪, pretreated with control antibody; ▨, pretreated with anti–TNF-α antibody. The data are expressed as the means and SD (n = 6). Statistical significances were analyzed between the groups of pretreatment with control antibody and with anti–TNF-α antibody in each tissue (*P < .05; **P < .02).
Changes in TNF-α antigen in plasma by stress and effects of anti–TNF-α antibody on the stress-induced TF mRNA expression.
TNF-α antigen levels in plasma of 8-week-old (8w) and 24-month-old (24m) mice (A) and of 6-week-old obese and lean mice (D) before (■) and after (▪) 20 hours of restraint stress were determined by enzyme-linked immunosorbent assay (*P < .04; **P < .01; ***P < .005). In separate experiments, the mice were pretreated either with control or with anti–TNF-α antibody (25 μg/mouse or 1 μg/g) and then subjected to 20 hours of restraint stress. Kidneys, adipose tissues, or livers were harvested and analyzed for TF mRNA by quantitative RT-PCR (young and aged mice, B and C; obese and lean mice, E and F). ■ indicates no stress; ▪, pretreated with control antibody; ▨, pretreated with anti–TNF-α antibody. The data are expressed as the means and SD (n = 6). Statistical significances were analyzed between the groups of pretreatment with control antibody and with anti–TNF-α antibody in each tissue (*P < .05; **P < .02).
To investigate the role of TNF-α in the induction of TF gene by stress, the mice were pretreated either with control IgG or with anti–TNF-α antibody and then subjected to restraint stress. Pretreatment of mice with anti–TNF-α antibody before restraint stress substantially attenuated the stress-induced TF mRNA expression in kidneys (65%) and adipose tissues (50%), and this effect was pronounced in aged mice (Figure 8B-C). Similarly, a substantial effect of anti–TNF-α antibody on the stress-induced TF expression was observed in adipose tissues of obese mice (45%) but not in livers (Figure 8E-F). These results suggest that the induction of TF gene in adipose tissues by stress is, at least in part, mediated by endogenous TNF-α in aged mice and obese mice.
Discussion
Although hypercoagulability and thrombotic diseases appear to be induced by a variety of stress,8-10 little information is available about underlying mechanisms. In this report, we observed that restraint stress to mice substantially induced the expression of TF gene (Figure 1). The stress-induced TF mRNA was localized primarily to renal tubular epithelial cells, adventitial cells, smooth muscle cells, and adipocytes but not to vascular endothelial cells (Figures 3-4), indicating that the induction of TF gene by stress occurs in a cell type–specific manner. It has been reported that these cells are a principal source of TF in vivo under physiologic29 and/or pathologic conditions.26,30 The constitutive production of TF in these cell populations suggests that TF forms a hemostatic envelope that activates the coagulation system when vascular integrity is disrupted by a variety of stressors. Another important source of TF in vivo is the adipose tissue/adipocytes.30 The adipose tissue is a highly vascularized organ, and the adipocytes appear to be in intimate contact with vascular beds. Under stressful conditions, the integrity of the circulation is important for the lipid utilization, and TF may help to maintain this integrity in the adipose tissue.
The incidence of thrombotic diseases is increasing in aged individuals. Aged subjects may have lower tolerance to stress2-5 and be more susceptible to thrombotic diseases caused by a variety of stressors than the young.13,14 We previously showed that the expression of PAI-1 was also induced by restraint stress and that this response was exacerbated by aging.23 In this study, we demonstrated the induction of TF mRNA in several tissues by restraint stress was substantially higher in aged mice than in young mice (Figures 2-4). Renal glomerular thrombosis and microthrombi formation in adipose tissues were markedly induced by stress in aged mice (Figure 7),23 suggesting that the stress-mediated TF induction contributes to the elevation of regional procoagulant activity and to the development of thrombosis. Thus, the increased expression of TF gene in response to stress may lead to a prothrombotic state in elderly individuals who are exposed to stress.
Obesity is associated with increased incidence of thrombotic diseases and accelerated atherosclerosis. We investigated the effect of obesity on the stress-induced TF expression and observed that TF mRNA was substantially increased by restraint stress in livers and adipose tissues of obese mice (Figures 5-6). This observation leads to a speculation that obese adipocytes and degenerative hepatocytes containing abundant lipids may show an increased response to stress in the expression of TF gene. Adipocytes/adipose tissues are the principal sites of TF production in obesity30; thus, obese animals may have a large potential of TF synthesis in response to stress, leading to an increase in the systemic and/or regional procoagulant activity. However, the induction of TF expression by stress in livers and adipose tissues of obese mice did not directly contribute to regional thrombi formation, implying that the process of obesity-linked thrombosis includes multifactorial and complex possibilities.
The expression of inflammatory cytokines could be altered by stress, as shown in the regulation of interleukins.31,32 We observed that the basal level of TNF-α in plasma was markedly elevated in aged mice and obese mice,33 as well as substantial increases in the plasma level of TNF-α antigen, which may be inducibly produced by adipose tissues,33,34 after 20 hours of restraint stress in these mice (Figure 8). TNF-α promotes a hypercoagulable state because this cytokine was shown experimentally to increase TF expression35 by activation of AP-1 and NF-κB sites.16,36 Especially, adipose-derived TNF-α could play a pathologic role in obesity and insulin resistance.33 The attenuation of the stress-induced TF expression in adipose tissues by pretreatment with anti–TNF-α antibody in aged mice and obese mice (Figure 8) suggests that this cytokine plays a key role in the stress-mediated induction of TF expression in adipose tissues. Partial effects of anti–TNF-α antibody on the stress-mediated TF induction suggest that other stress-induced neurologic substances, hormones (eg, angiotensin II),37 and growth factors (eg, transforming growth factor-β)38 may also contribute to the induction of TF expression by stress.
In conclusion, we demonstrate that restraint stress induces the TF expression in a tissue- and cell type–specific manner in the mouse and that aging and/or obesity enhance the stress-mediated induction of TF gene. The stress-induced endogenous TNF-α may, in part, mediate the induction of TF expression, especially in aged mice. In obese mice, TF derived from adipose tissues may contribute to an increase in regional procoagulant potential, resulting in the development of microvascular thrombi. This study presents a novel finding regarding the molecular process of the stress-induced hypercoagulability and suggests that 3 factors, including stress, aging, and obesity, may be responsible for the increased risk of thrombosis as a result of the induction of TF gene.
We thank T. Thinnes, E. Yamafuji, K. Kaneko, and K. Sakakura for their expert technical assistance; and Drs K. Enjyoji and H. Kato (National Cardiovascular Center and Research Institute, Osaka, Japan) for providing rabbit antimouse TF antibody.
Prepublished online as Blood First Edition Paper, July 25, 2002; DOI 10.1182/blood-2002-03-0945.
Supported by grants-in-aid from the Ministry of Education, Science, Sports and Culture from the Ministry of Health and Welfare, by Funds for Comprehensive Research on Aging and Health, Japan, and by grant HL-47819 (D.J.L.) from the National Institutes of Health.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Koji Yamamoto, First Department of Internal Medicine, Nagoya University School of Medicine, 65 Tsurumai, Showa, Nagoya 466-8550, Japan; e-mail:kojiy@med.nagoya-u.ac.jp.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal