Iron accumulation in the liver in hereditary hemochromatosis (HH) has been shown to be highly variable. Some studies point to the importance of major histocompatibility complex (MHC) class I (MHC-I) and CD8+ cells as modifiers of iron overload. In this report, using mice knockout for H2Kb−/−and H2Db−/− genes, it is demonstrated that lack of classical MHC-I molecules results in a spontaneous increase of nonheme iron content in the liver (mainly located in the hepatocytes) when compared to wild-type mice. In CD8−/−and Rag2−/− mice, no spontaneous hepatic iron accumulation was observed. These results demonstrate for the first time that classical MHC-I molecules could be involved in the regulation of iron metabolism and contribute to the established genotype/phenotype discrepancies seen in HH.
Introduction
Since the discovery of the HFEgene,1 several molecules implicated in heritable defects of iron metabolism have been identified, giving new insights into the molecular control of cellular pathways of iron balance.2Despite these advances, a considerable unexplained variability in the amount of iron loading in HFE hemochromatosis still persists.3,4 Thus, the finding of new molecular regulators to further improve our understanding of iron homeodynamics continues to be pertinent. Recently, Andrews and collaborators made an important contribution in this area by characterizing genes that modify the hemochromatosis phenotype in mice. They reported that mice double knockout for Hfe and β2-microglobulin (β2m) accumulate more tissue iron than mice lackingHfe only.4 This finding suggests that other(s) β2m-interacting protein(s), such as classical major histocompatibility complex (MHC) class I molecule(s) (MHC-I), may be involved in iron regulation. Alternatively, MHC-dependent cells such as CD8+ T lymphocytes or others could play a role in iron metabolism.5 To further explore other candidate molecular and cellular regulators of iron balance, we investigated the spontaneous iron status of mice with disrupted classicalMhc-I genes and of mice knockout for CD8 andRag2 genes.
Study design
C57BL/6J (B6), H2Kb−/−,H2Db−/− single-knockout andH2Kb−/−Db−/− double-knockout mice were raised at the Pasteur Institute animal facilities and are reported elsewhere.6 All H2 knockout mice were backcrossed onto the B6 background for 12 generations. TheCD8 knockout mice (CD8α−/−)7 that had been backcrossed to B6 for 13 generations were purchased from The Jackson Laboratory (Bar Harbor, ME) at 8 to 9 weeks and aged at the Institute for Molecular and Cell Biology (IBMC) animal facility. Rag2−/−8 were backcrossed 9 times onto the B6 background; additional B6 mice were bred in IBMC. All mice used in this study were male, aged 4 to 5 months, and were maintained on standard mouse diet. Iron status was evaluated as previously described.9 Iron staining in the liver was performed by using the Perls Prussian blue method, and evaluation of ferritin accumulation was done by electron microscopy in liver samples processed as described.10 The blood samples were obtained by cardiac puncture in mice under anesthesia or by retro-orbital bleeding. Serum transferrin saturation was calculated by dividing serum iron by total iron-binding capacity (TIBC) and multiplying by 100. For the hematocrit, fresh peripheral blood was harvested in EDTA (ethylenediaminetetraacetic acid) tubes and assessed on a Pentra 120 counter (ABX, Montpellier, France). Flow cytometry analysis of CD4+ and CD8+ T cells was performed on splenocytes by direct immunofluorescence. Red blood cells were removed by osmotic lysis, and splenic cells were stained with anti–T-cell receptor αβ (anti-TCRαβ)–CyChrome (H57-597; PharMingen Europe, Heidelberg, Germany), CD8β-fluorescein isothiocyanate (FITC), and CD4-phycoerythrin (PE) (Caltag Laboratories, Burlingame, CA) monoclonal antibodies. Cells were passed on a FACSCalibur flow cytometer, and data were analyzed by using CellQuest (Becton Dickinson, Aalst, Belgium).
Results and discussion
To investigate whether classical MHC-I molecules are involved in iron regulation, the iron status of mice totally devoid of classical MHC-I molecules(H2Kb−/−Db−/−)6 was evaluated. These mice are more adequate than other models that have been used as MHC-I deficient, such as mice knockout forβ2m, or for the transporter associated with antigen processing (TAP), in which low levels of classical MHC-I may still be expressed on the cell surface.11
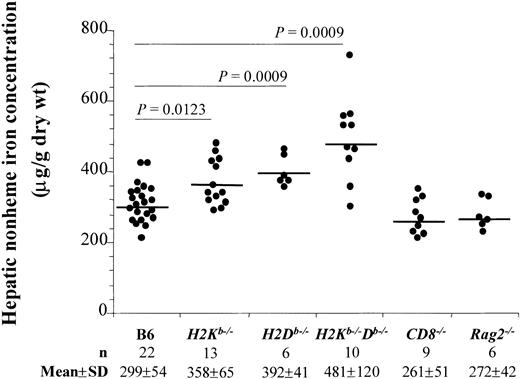
Interestingly, H2Kb−/−Db−/− mice had significantly higher (P = .0009) hepatic nonheme iron content (mean ± SD, 481 ± 120 μg/g dry wt) than the B6 control mice (299 ± 54 μg/g dry wt) (Figure1). Single H2Kband H2Db knockout mice had a phenotype intermediate between the double-knockout and the wild-type (mean ± SD, 358 ± 65 and 392 ± 41 μg/g dry wt, respectively, Figure 1).
Increased hepatic nonheme iron concentration in mice lacking classical MHC class I molecules.
Liver samples from B6, H2Kb−/−,H2Db−/−,H2Kb−/−Db−/−,CD8−/−, and Rag2−/−male mice aged 4 to 5 months were analyzed for nonheme iron concentration. Individual values are represented. n indicates the number of mice analyzed; the mean and the standard deviation (SD) values are indicated. P values were calculated by the unpaired Student 2-tailed t test; values ofP < .05 were considered significant. As indicated, mean values from H2Kb−/−,H2Db−/−, andH2Kb−/−Db−/− mice were significantly different from B6 control mice; not indicated, but also significantly different (P < .05), were the mean values for these groups: H2Kb−/− versusH2Kb−/−Db−/−;H2Kb−/− versusCD8−/−; H2Kb−/−versus Rag2−/−;H2Db−/− versusCD8−/−; H2Db−/−versus Rag2−/−;H2Kb−/−Db−/− versusCD8−/−; andH2Kb−/−Db−/− versusRag2−/−.
Increased hepatic nonheme iron concentration in mice lacking classical MHC class I molecules.
Liver samples from B6, H2Kb−/−,H2Db−/−,H2Kb−/−Db−/−,CD8−/−, and Rag2−/−male mice aged 4 to 5 months were analyzed for nonheme iron concentration. Individual values are represented. n indicates the number of mice analyzed; the mean and the standard deviation (SD) values are indicated. P values were calculated by the unpaired Student 2-tailed t test; values ofP < .05 were considered significant. As indicated, mean values from H2Kb−/−,H2Db−/−, andH2Kb−/−Db−/− mice were significantly different from B6 control mice; not indicated, but also significantly different (P < .05), were the mean values for these groups: H2Kb−/− versusH2Kb−/−Db−/−;H2Kb−/− versusCD8−/−; H2Kb−/−versus Rag2−/−;H2Db−/− versusCD8−/−; H2Db−/−versus Rag2−/−;H2Kb−/−Db−/− versusCD8−/−; andH2Kb−/−Db−/− versusRag2−/−.
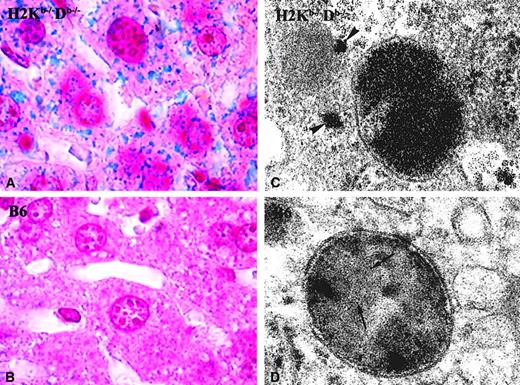
In agreement with the higher quantitative hepatic iron concentration of the H2Kb−/−Db−/− mice, positive Perls Prussian blue iron staining in a vast majority of hepatocytes was seen (Figure 2A). Occasional Kupffer cells also were positive. On the contrary, no stainable iron was observed in B6 mice (Figure 2B). In addition, numerous ferritin lysosomes with abundant iron-containing ferritin molecules were observed by electron microscopy in hepatocytes of theH2Kb−/−Db−/− mice. Moreover, in these mice, frequent cytosolic ferritin molecules also were observed (Figure 2C). Conversely, in wild-type mice (B6) lysosomal ferritin was scarce, and no ferritin accumulation was observed in the cytoplasm (Figure 2D).
Iron ferritin in the liver.
Representative sections from the liver of a double-knockout mouse for classical MHC-I molecules(H2Kb−/−Db−/−) (A,C) and from a control B6 mouse (B,D). (A-B) Histological sections stained for ferric iron (Perls); (C-D) ultrathin section of the same liver sample, contrasted with lead citrate. TheH2Kb−/−Db−/− and the B6 mice shown had a hepatic iron concentration of 517 μg/g dry wt and 252 μg/g dry wt, respectively. In the picture representative of the results seen in H2Kb−/−Db−/−mice, the iron was detected in hepatocytes (A), while in B6 mice no stainable iron could be found (B). Although in B6 mice, lysosomes containing some iron ferritin could be visualized (arrows, D),H2Kb−/−Db−/− mice had many lysosomes, with abundant iron ferritin, as well as iron ferritin in the cytoplasm (arrowheads, C). Original magnification × 1000 (A-B); EM magnification × 90 000 (C-D).
Iron ferritin in the liver.
Representative sections from the liver of a double-knockout mouse for classical MHC-I molecules(H2Kb−/−Db−/−) (A,C) and from a control B6 mouse (B,D). (A-B) Histological sections stained for ferric iron (Perls); (C-D) ultrathin section of the same liver sample, contrasted with lead citrate. TheH2Kb−/−Db−/− and the B6 mice shown had a hepatic iron concentration of 517 μg/g dry wt and 252 μg/g dry wt, respectively. In the picture representative of the results seen in H2Kb−/−Db−/−mice, the iron was detected in hepatocytes (A), while in B6 mice no stainable iron could be found (B). Although in B6 mice, lysosomes containing some iron ferritin could be visualized (arrows, D),H2Kb−/−Db−/− mice had many lysosomes, with abundant iron ferritin, as well as iron ferritin in the cytoplasm (arrowheads, C). Original magnification × 1000 (A-B); EM magnification × 90 000 (C-D).
The increased hepatic iron content observed could be associated with the reduction in CD8+ T-lymphocyte numbers seen in these mice. The mice with the H2Kb,H2Db, or both genes disrupted had a significantly lower percentage of CD8+ T lymphocytes in the spleen (mean ± SD, 13% ± 2%, 20% ± 5%, and 3% ± 1%, respectively, P < .05) compared to the B6 control mice (34% ± 4%). To control for this possibility, additional controls were examined. As shown in Figure 1, livers ofCD8−/− and Rag2−/−mice (both with less than 0.5% of splenic CD8+ T lymphocytes) had a hepatic iron concentration comparable to B6 mice (261 ± 51 μg/g dry wt and 272 ± 42 μg/g dry wt, respectively). Similar results were reported previously.12 13
The increased iron deposits in the liver were observed without a corresponding increase in serum transferrin saturation (32% to 40%) or in the hematocrit (44% to 47%). No significant differences were found between groups. The finding of hepatic iron overload, without changes in the transferrin saturation or in the hematocrit, may indicate that MHC class I molecules are important for the regulation of iron import/export by hepatocytes. Iron accumulation before the circulating transferrin is fully saturated has been seen by others in both humans14 and mice.15 Studies of iron absorption are needed to define further the mechanism leading to hepatic iron load presented in this first report. However, in comparison with published reports of liver iron overload inβ2m−/− andHfe−/− mice, the values found inH2Kb−/−Db−/− mice are similar or slightly higher than those seen in heterozygousβ2m+/−16 andHfe+/−.17
The present data indicate that the reported spontaneous increase in hepatic iron content is specific of MHC class I genotype. Earlier studies have shown that hepatic magnesium and zinc content in mice were associated with the H2 genotype.18,19 Even though many of these associations could be due to genes within the MHC locus, there also is evidence indicating that classical MHC antigens themselves may regulate physiological processes by interacting physically on the cell surface with different ligands.20-22 HFE itself, a nonclassical MHC-I protein, was shown to interact with the transferrin receptor.23Recently, a study in β2m−/− mice demonstrated that MHC-I molecules are functionally required for the development and plasticity of the central nervous system.24 Thus, besides the well-characterized role of MHC-I molecules in immune responses, they may play other regulatory functions. Classical MHC-I molecules could associate with molecules critical for iron transport within or on the cell surface. In conclusion, the present results indicate that MHC class I expression may be an additional contributory factor to the clinical heterogeneity found in iron overload.
We thank Luisa Mariano (Molecular Immunology and Pathology, ICBAS) and Ana do Vale (Immunobiology, IBMC) for assistance during the preparation of samples for electron microscopy, and Júlia Carvalho (Clinical Chemistry, Santo António General Hospital) for serum iron analysis. We thank Jean Luc Decourt, Marie Françoise Hurtaud (Hematologie, Hopital Robert Debré, Paris), F. Arosa (IBMC, Oporto) and C. S. Cardoso for their valuable help.
Prepublished online as Blood First Edition Paper, August 8, 2002; DOI 10.1182/blood-2002-05-1565.
Supported by the EU (QLG1-CT-1999-00665) and the American Portuguese Biomedical Research Fund.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Maria de Sousa, Molecular Immunology, Institute for Molecular and Cell Biology (IBMC), Campo Alegre 823, 4150-180 Oporto, Portugal; e-mail: mdesousa@ibmc.up.pt andecardoso@ibmc.up.pt.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal