Veno-occlusive disease (VOD) is the most common regimen-related toxicity accompanying stem cell transplantation (SCT). Severe VOD complicated by multisystem organ failure (MOF) remains almost uniformly fatal. Preliminary experience with defibrotide (DF), a single-stranded polydeoxyribonucleotide with fibrinolytic, antithrombotic, and anti-ischemic properties, in the treatment for severe VOD has suggested safety and activity. Eighty-eight patients who developed severe VOD after SCT were treated with DF under a defined treatment plan. At diagnosis, median bilirubin was 76.95 μM (4.5 mg/dL), median weight gain was 7%, ascites was present in 84%, and abnormal hepatic portal venous flow was present in 35%. At DF initiation, median bilirubin had increased to 215.46 μM (12.6 mg/dL), and MOF was present in 97%. DF was administered intravenously in doses ranging from 5 to 60 mg/kg per day for a median of 15 days. No severe hemorrhage or other serious toxicity related to DF was reported. Complete resolution of VOD was seen in 36%, with 35% survival at day +100. Predictors of survival included younger age, autologous SCT, and abnormal portal flow, whereas busulfan-based conditioning and encephalopathy predicted worse outcome. Decreases in mean creatinine and plasminogen activator inhibitor 1(PAI-1) levels during DF therapy predicted better survival. The complete response rate, survival to day +100, and absence of significant DF-associated toxicity in this largest patient cohort reported to date confirm the results of earlier studies. Certain features associated with successful outcome may correlate with DF-related treatment effects, and prospective evaluation of DF therapy for severe VOD should allow better definition of predictors of response or failure.
Introduction
Hepatic veno-occlusive disease (VOD) is recognized as one of the most common and important regimen-related toxicities experienced after allogeneic (allo) and autologous (auto) hematopoietic stem cell transplantation (SCT).1-4 VOD is a clinical syndrome characterized by painful hepatomegaly, jaundice, ascites, fluid retention, and weight gain. The onset is usually before day +35 after stem cell reinfusion, and other causes of these symptoms and signs are absent.1-4 VOD develops in 5% to 60% of patients after SCT and ranges in severity from mild, reversible disease to a severe syndrome associated with multiorgan failure (MOF) and death, with established severe VOD shown to have a mortality rate approaching 100% by day +100 after SCT.1-6
VOD is believed to be caused by primary injury to sinusoidal endothelial cells and hepatocytes with subsequent damage to the central veins in zone 3 of the hepatic acinus.7-9 Early changes include deposition of fibrinogen, factor VIII, and fibrin within venular walls and sinusoids. Subendothelial edema, collagen deposition, sclerosis, and fibrosis of the abluminal venular area follow, with stellate cell proliferation and collagenization contributing to matrix deposition.2,9,10 As the process of venular microthrombosis, fibrin deposition, ischemia, and fibrogenesis advances, widespread zonal disruption leads to portal hypertension, hepatorenal syndrome, MOF, and death.8 Despite therapeutic interventions, including the use of antithrombotic and thrombolytic agents such as prostaglandin E1 and tissue-plasminogen activator (t-PA) with or without concurrent heparin, little success has been achieved in the treatment of severe VOD.4,11-14 In aggregate, despite multiple interventions and intensive treatment, day +100 mortality for severe VOD has remained in excess of 90% (E. Carreras, personal communication, March 2002).4 6
The use of DF, a single-stranded polydeoxyribonucleotide that has specific aptameric binding sites on vascular endothelium, has shown promise in the treatment of VOD.15-19 DF up-regulates the release of prostacyclin (PG I2), prostaglandin E2, thrombomodulin (TM), and t-PA both in vitro and in vivo.20-24 Moreover, it has been shown to decrease thrombin generation, tissue factor expression, PAI-1 release, and endothelin activity.20,25,26 Preclinical studies have also demonstrated profibrinolytic effects and inhibition of fibrin deposition with selective activity on small vessels.27,28No significant effects on systemic coagulation have been shown in either preclinical studies or clinical trials of DF.29Initial clinical reports of DF used as treatment for severe VOD have recorded complete resolution in 36% to 42% of patients with most surviving past day +100.15,16 Furthermore, these reports and others have suggested both safety and activity in patients with VOD complicated by MOF, including patients who were intubated and dialysis dependent.15-19 However, the relatively small number of patients in these preliminary studies has precluded a more detailed analysis of treatment results. Herein, we analyze the largest patient cohort reported to date and attempt to define factors predictive of survival that may assist in the further study of DF in the treatment of severe VOD.
Patients, materials, and methods
Patient selection
From March 1995 to May 2001, 88 patients in the United States with severe VOD were treated with DF (Gentium SpA, Como, Italy) on an emergency use basis. As previously reported,15 19 patients were treated on individual, Investigational New Drug (IND) applications until August 1997. On the basis of this initial experience, 8 transplantation programs formed a working group that enrolled 69 further patients prospectively under IND 52668. Each program followed the same eligibility criteria and treatment protocol, with prior approval of the institutional review board obtained at each participating center.
Patients were eligible with a clinical diagnosis of VOD, based on jaundice (bilirubin ≥ 34.2μM [2 mg/dL]), hepatomegaly and/or right upper quadrant (RUQ) pain, and ≥ 5% weight gain from admission, with or without ascites. Patients who did not meet all the above criteria but met at least 2 criteria and had a diagnostic liver biopsy were also eligible. In addition, patients addressed by the Bearman model were required to have a predicted risk of 30% or more of severe VOD.30 If not addressed by the Bearman model (ie, onset of VOD beyond day +16 and/or not treated with one of the reported high-dose regimens, namely cyclophosphamide/total body irradiation [CyTBI], busulfan/cyclophosphamide [BuCy], or cyclophosphamide, carmustine, etoposide [CBV]), patients were eligible if VOD was considered their major clinical problem and organ failure was present in at least one other organ system. MOF was, therefore, defined as either an oxygen requirement with an oxygen saturation of 90% or less on room air and/or ventilator dependence; and/or renal dysfunction (defined as a doubling of baseline creatinine and/or dialysis dependence); and/or encephalopathy, in addition to liver failure. Patients with a concurrent, potential confounding cause of liver dysfunction such as graft-versus-host disease (GVHD) or inconsistent findings evident on ultrasound imaging were required to have biopsy-proven VOD to be considered eligible. Patients who had failed prior treatment with t-PA and heparin were eligible, but patients with significant uncontrolled bleeding or hemodynamic instability were excluded. Concurrent therapy with heparin, t-PA, warfarin, or nonsteroidal inflammatory drugs was prohibited. Patients or their parents/guardians or designated proxy gave voluntary informed consent and in each case approval by the institutional review board was obtained per the guidelines of each participating institution.
Laboratory and clinical evaluation
In addition to history and physical examination, each patient was evaluated and followed with abdominal ultrasound scans and serial laboratory studies. Time of onset of VOD was defined as the first day that the patients fulfilled the diagnostic criteria detailed above. Patients were said to have MOF if there was documentation of dysfunction of at least one other organ system in addition to the liver, as defined above.
Treatment design
DF was administered intravenously in crystalloid solution (either normal saline [NS] or 5% dextrose in water [D5W]), typically as 4 divided doses, each infused over 2 hours, starting at an initial total daily dose of 10 mg/kg. Where possible, doses were rounded to the nearest 10 or 100 mg in children and adults, respectively, to facilitate efficient drug administration. Drug was mixed to a maximum concentration of 4 mg/cc, and DF was increased incrementally by 10 mg/kg every 2 to 4 days to a maximum potential total daily dose of 60 mg/kg, depending on tolerance and response. For purposes of continuation of dose escalation, tolerance was defined as the ability to administer drug without adverse events attributable to DF. DF was discontinued or the dose was reduced if significant toxicity potentially attributable to the drug was encountered. The planned treatment course was for a minimum of 14 days. During therapy, wherever possible, transfusions were used to keep platelets greater than 20 × 109/L (20 000/μL), hematocrit (HCT) more than 30%, with factor replacement to keep prothrombin time (PT) less than 15 seconds and fibrinogen more than 150 mg/dL (1.5 g/L). Patients were followed prospectively for potential adverse events and response. Toxicities were graded according to the National Cancer Institute's common toxicity criteria. Doses of DF could be held and restarted with a dose reduction of 50% if toxicity occurred that was considered possibly DF related by the treating physician. Clinical improvement with fluid mobilization, decrease in bilirubin, reduction in hepatomegaly and/or RUQ pain, improvement in coagulopathy, and/or reduction in other end-organ dysfunction was used to measure response. Complete response (CR) was defined as evidence of improvement in VOD-related symptoms and concurrent MOF, and a concomitant or subsequent decrease in bilirubin to less than 34.2 μM (2 mg/dL). Any patient who failed to achieve CR was defined as having no response (NR).
Special laboratory studies
Analyses for PAI-1 were carried out from both pretreatment and sequential samples during treatment up to the conclusion of therapy. Samples were collected and centrifuged to provide platelet-poor plasma stored at −70°C. They were catalogued and batched for analysis by enzyme-linked immunosorbent assay (ELISA; American Diagnostics, Greenwich, CT).
Statistical methods
For comparing 2 rates or proportions, the exact confidence intervals and Fisher exact tests for their difference were constructed. For examining the associations between the CR rate, mortality at day +100 after SCT, and continuous baseline variables, the exact Wilcoxon tests were performed. Furthermore, univariate and multivariate logistic regression analyses were conducted for dichotomous responses (CR or survival at day +100) with baseline variables and the changes of certain biomarkers (bilirubin, creatinine, and PAI-1) during the study. The univariate and multivariate Cox proportional hazards model was used to analyze the time to death from DF initiation with the same set of explanatory variables. In multivariate regression analysis, a stepwise selection method was used with a significance value of less than or equal to 0.1 for inclusion, and more than 0.1 for deletion of a covariate. Several measures of liver injury and MOF were analyzed, and these measures are obviously correlated. No adjustments were made for multiple comparisons in the estimation of reported P values; for this reason, P values between .01 and .05 should be considered as suggestive or hypothesis generating.
Results
Patient characteristics
The median age of the patients treated was 35 years (range, 8 months to 62 years). Forty-seven patients were male and 41 were female. Sixty patients underwent allo-SCT and 28 underwent auto-SCT. Seventy-five percent (n = 66) received Cy-based chemotherapy, 53% (n = 47) received Bu-based therapy, and 33% (n = 29) received TBI. Most of the patients had hematologic malignancies (n = 63) consisting predominantly of acute myeloid leukemia (n = 22), non-Hodgkin lymphoma (n = 9), Hodgkin disease (n = 9), myelodysplastic syndrome (n = 8), and acute lymphoblastic leukemia (n = 6). A minority had solid tumors (n = 17), including breast cancer (n = 8) and neuroblastoma (n = 3). Eight patients had nonmalignant diagnoses, including thalassemia, severe combined immune deficiency syndrome, and various other congenital disorders. Fourteen patients had received prior therapy with t-PA and heparin subsequent to the diagnosis of VOD and prior to the administration of DF. None of these patients had evidence of clinical response to treatment with t-PA and heparin, nor did they respond by the 50% reduction of bilirubin criterion used in published trials of t-PA and heparin.14 31
Characterization of VOD
Median bilirubin at VOD diagnosis was 76.95 μM (range, 34.2-596.79 μM) (4.5 mg/dL [range, 2-34.9 mg/dL]). Median weight gain was 7% (range, −9%-36%). RUQ pain was documented in 80% (n = 70). Ascites was documented radiologically in 84% (n = 74) and hepatomegaly in 73% (n = 64) with abnormal or reversal of portal flow by doppler in 35% (n = 31). The median time from diagnosis of VOD to initiation of DF therapy was 3 days (range, 0-46 days). At initiation of DF, median bilirubin had increased to 215.46 μM (range, 34.2-930.24 μM) (12.6 mg/dL [range, 2.0-54.4 mg/dL]), with the median peak bilirubin reached during therapy being 352.26 μM (range, 111.15-1128.6 μM) (20.6 mg/dL [range, 6.5-66.0 mg/dL]). MOF was documented in 97% (n = 85) of patients, with 27 patients having organ dysfunction in 2 systems, 40 in 3 systems, and 18 in 4 systems. Eleven patients were ventilator dependent and 8 on dialysis at the start of DF therapy. Subsequently, an additional 21 patients went on to become ventilator dependent, and 19 patients required dialysis. Thus, in total, 32 (36%) patients were intubated and 27 (31%) were dialysis dependent during treatment.
DF administration, response, and survival
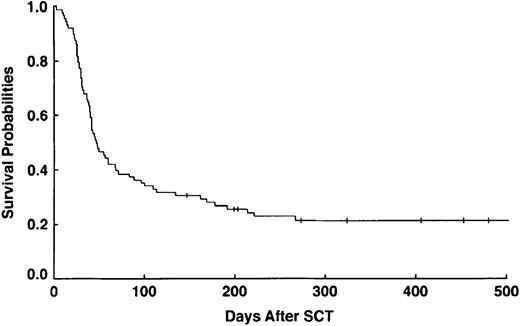
The median duration of DF therapy was 15 days (range, 1-139 days), with the patient who received 1 day of DF dying from uncal and cerebellar herniation as a result of prior conditioning regimen neurotoxicity. Most of the responses were seen at doses of DF between 20 and 40 mg/kg per day. However, evidence of response was reported in 1 patient at 10 mg/kg per day and in another patient at 60 mg/kg per day. CR was observed in 32 (36%) of 88 patients (95% confidence interval [CI]: 26%, 47%), and day +100 survival was observed in 31 (35%) of 88 (95% CI: 25%, 46%). Thirty-one of 32 patients achieving CR survived to day +100, and of those, 18 (60%) are alive as of October 2001. No mortality from VOD or other regimen-related toxicity was seen beyond day +134, with the most common cause of later death being relapse (Figure 1).
Survival probabilities for patients with severe VOD/MOF treated with DF (n = 88).
Survival probabilities for patients with severe VOD/MOF treated with DF (n = 88).
Toxicity
All patients were either thrombocytopenic (defined as a platelet count of ≤ 20 × 109/L [20 000/μL]), platelet transfusion and plasma product dependent, and/or uremic (ie, blood urea nitrogen [BUN] above the normal range) at the time of DF initiation. No worsening of clinical bleeding as measured by hemodynamic instability, acute transfusion requirement, or end-organ compromise was seen, and no other grade 3 or 4 toxicity attributable to DF administration was reported. Mild to moderate toxicity (grade 1-2) documented during the course of therapy included nausea, transient mild systolic hypotension, fever, abdominal cramping, and vasomotor symptoms such as hot flashes, all of which are recognized side effects of DF.29 Conversely, serious grade 3 or 4 adverse events that did occur during treatment were those commonly observed in such critically ill patients in the posttransplantation setting (eg, bacteremia, acute renal failure, and pulmonary edema) and were not attributed to DF by the treating physicians. Moreover, in patients in whom DF was withheld per treatment guidelines and subsequently restarted at the equivalent or decreased dose, a causative temporal relationship of any grade 3 or 4 toxicity to DF could not be demonstrated.
Pathology
Liver biopsies were carried out in 27 patients and in all but one case were performed by the transvenous route. Twenty-three (85%) patients were reported as positive for VOD. Of these 23 patients, 7 (30%) achieved CR. In the remaining 4, one patient had an initial biopsy that was nonconfirmatory, based on a limited tissue sample, and subsequent histology at autopsy confirmed VOD. In the other 3 patients biopsy was carried out as diagnostic uncertainty emerged; GVHD predominated as the alternate histologic diagnosis, and none of these patients responded. Twenty-nine patients underwent autopsy, and findings consistent with VOD were confirmed in 22 (76%). In the 7 patients who were reported as negative for VOD at autopsy, GVHD was the most common finding, with hepatic necrosis, cholestasis, and centrilobular congestion also reported, but no venular occlusion was seen. One of these patients died from complications related to acute renal failure on day +69 after demonstrating a clinical CR of her VOD. DF therapy had been discontinued 44 days previously after completion of a 14-day course. No evidence of VOD was found on autopsy with material limited to needle biopsy, and, in the view of her treating physicians, this finding was consistent with resolution of her syndrome as opposed to a different diagnosis.
Predictors of outcome
Eighteen pretreatment variables were examined by the Fisher exact test for association (Table 1) and univariate logistic regression analysis for day +100 survival. Age as a categorical variable (dichotomized by ≤ 18 years) and as a continuous variable attained statistical significance with P = .033 and P = .025, respectively. Patients receiving auto-SCT did better than patients receiving allo-SCT (P = .018). Patients with either a nonmalignant diagnosis or solid tumor were more likely to survive to day +100, versus those with hematologic malignancies (P = .001). Prior exposure to t-PA/heparin also attained significance (P = .029), but the numbers were small (n = 14). Conversely, worse survival was seen in patients who received busulfan-based conditioning (P = .015).
Variables before defibrotide (DF) treatment and day +100 survival (univariate analysis; N = 88)
| . | n . | Day + 100 survival, % . | P . |
|---|---|---|---|
| Patient characteristics | |||
| Age, y | |||
| Younger than 18 | 29 | 51.7 | .033* |
| 18 or older | 59 | 27.1 | |
| Sex | |||
| Female | 41 | 31.7 | .655 |
| Male | 47 | 38.3 | |
| Diagnosis | |||
| Nonmalignant | 8 | 50 | .001* |
| Hematologic malignancy | 63 | 23.8 | |
| Nonhematologic malignancy | 17 | 70.6 | |
| Graft | |||
| Auto | 28 | 53.6 | .018* |
| Allo | 60 | 26.7 | |
| No. BMT | |||
| More than 1 | 14 | 50 | .233 |
| 1 | 74 | 32.4 | |
| AST elevation preconditioning | |||
| No | 69 | 34.8 | 1.000 |
| Yes | 19 | 36.8 | |
| Conditioning regimen | |||
| Cy | |||
| No | 22 | 27.3 | .446 |
| Yes | 66 | 37.9 | |
| Bu | |||
| No | 41 | 48.8 | .015* |
| Yes | 47 | 23.4 | |
| TBI | |||
| No | 59 | 33.9 | .813 |
| Yes | 29 | 37.9 | |
| Characteristics of VOD | |||
| RUQ pain | |||
| No | 18 | 27.8 | .584 |
| Yes | 70 | 37.1 | |
| Ascites | |||
| No | 14 | 28.6 | .762 |
| Yes | 74 | 36.5 | |
| Hepatomegaly | |||
| No | 24 | 25 | .317 |
| Yes | 64 | 37.5 | |
| Portal flow | |||
| Normal | 57 | 28.1 | .066 |
| Abnormal | 31 | 48.4 | |
| Prior t-PA/heparin | |||
| No | 74 | 29.7 | .029* |
| Yes | 14 | 64.3 | |
| Abnormal creatinine | |||
| No | 23 | 39.1 | .800 |
| Yes | 65 | 33.8 | |
| Oxygen requiring | |||
| No | 24 | 50 | .086 |
| Yes | 64 | 29.7 | |
| Encephalopathy | |||
| No | 56 | 41.1 | .166 |
| Yes | 32 | 25 | |
| No. of systems as part of MOF (liver + renal + pulmonary + CNS) | |||
| 2 or fewer | 30 | 46.7 | .065 |
| More than 2 | 58 | 29.3 |
| . | n . | Day + 100 survival, % . | P . |
|---|---|---|---|
| Patient characteristics | |||
| Age, y | |||
| Younger than 18 | 29 | 51.7 | .033* |
| 18 or older | 59 | 27.1 | |
| Sex | |||
| Female | 41 | 31.7 | .655 |
| Male | 47 | 38.3 | |
| Diagnosis | |||
| Nonmalignant | 8 | 50 | .001* |
| Hematologic malignancy | 63 | 23.8 | |
| Nonhematologic malignancy | 17 | 70.6 | |
| Graft | |||
| Auto | 28 | 53.6 | .018* |
| Allo | 60 | 26.7 | |
| No. BMT | |||
| More than 1 | 14 | 50 | .233 |
| 1 | 74 | 32.4 | |
| AST elevation preconditioning | |||
| No | 69 | 34.8 | 1.000 |
| Yes | 19 | 36.8 | |
| Conditioning regimen | |||
| Cy | |||
| No | 22 | 27.3 | .446 |
| Yes | 66 | 37.9 | |
| Bu | |||
| No | 41 | 48.8 | .015* |
| Yes | 47 | 23.4 | |
| TBI | |||
| No | 59 | 33.9 | .813 |
| Yes | 29 | 37.9 | |
| Characteristics of VOD | |||
| RUQ pain | |||
| No | 18 | 27.8 | .584 |
| Yes | 70 | 37.1 | |
| Ascites | |||
| No | 14 | 28.6 | .762 |
| Yes | 74 | 36.5 | |
| Hepatomegaly | |||
| No | 24 | 25 | .317 |
| Yes | 64 | 37.5 | |
| Portal flow | |||
| Normal | 57 | 28.1 | .066 |
| Abnormal | 31 | 48.4 | |
| Prior t-PA/heparin | |||
| No | 74 | 29.7 | .029* |
| Yes | 14 | 64.3 | |
| Abnormal creatinine | |||
| No | 23 | 39.1 | .800 |
| Yes | 65 | 33.8 | |
| Oxygen requiring | |||
| No | 24 | 50 | .086 |
| Yes | 64 | 29.7 | |
| Encephalopathy | |||
| No | 56 | 41.1 | .166 |
| Yes | 32 | 25 | |
| No. of systems as part of MOF (liver + renal + pulmonary + CNS) | |||
| 2 or fewer | 30 | 46.7 | .065 |
| More than 2 | 58 | 29.3 |
BMT indicates bone marrow transplantation; AST: aspartate transferase; and CNS, central nervous system.
Significant at .05 level.
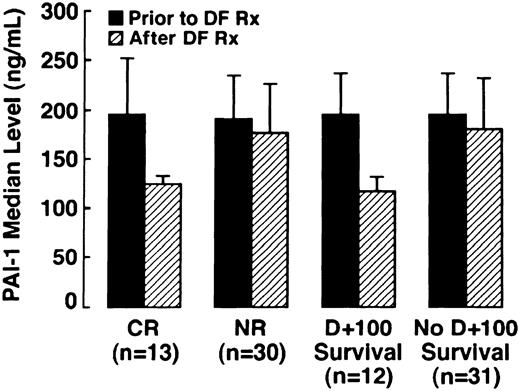
In a subset of patients in whom samples were serially collected (n = 43), PAI-1 levels were determined prior to, during, and at the conclusion of DF therapy. PAI-1 levels were analyzed in both patients with CR (n = 13) and NR (n = 30). The results are illustrated graphically in Figure 2, which compares patients with CR versus NR before and after DF therapy, and patients surviving to day +100 versus nonsurvivors. Median PAI-1 pre-DF in patients achieving CR was 196 ng/mL, (95% CI: 128, 260) and 204 ng/mL (95% CI: 182, 235) in patients with NR, with the normal range for this assay being less than 50 ng/mL. In all patients with CR, there was a marked reduction in median PAI-1 with the median post-DF PAI-1 level decreasing by 32% to 113 ng/mL (95% CI: 76, 133;P = .01). One half of the patients with NR had a median increase in median PAI-1 of 20%, and a decrease in median PAI-1 of 26% was seen in the other half. Therefore, the median PAI-1 level subsequent to DF for the 30 patients analyzed with NR did not change significantly from baseline at 186.8 ng/mL (95% CI: 158, 227;P = .564).
Median PAI-1 levels in patients with CR, median PAI-1 levels in patients surviving to day +100 versus NR across DF therapy (n = 43) versus nonsurvivors (n = 43).
Median PAI-1 levels in patients with CR, median PAI-1 levels in patients surviving to day +100 versus NR across DF therapy (n = 43) versus nonsurvivors (n = 43).
Four of the patients with NR did not have pathology confirmatory of VOD subsequent to DF treatment at autopsy. One patient had a low PAI-1 (67 ng/mL) prior to DF initiation and hepatic GVHD. High values were seen in the other 3 patients, at 500.0 ng/mL in 2 patients and 226.0 ng/mL in the third. In these patients, no venular occlusion was seen, but centrilobular congestion and hepatocellular necrosis were present in one and sinusoidal obstruction in the second; in the third patient a limited specimen revealed marked congestion and cholestasis with some early GVHD.
Bilirubin, creatinine, and PAI-1 were analyzed as continuous variables by using a multivariate logistic regression model; reduction of serum creatinine was significant in predicting survival at day +100 (P = .037), but actual changes in PAI-1 and bilirubin were not. On univariate Cox regression analysis using time to death from DF initiation as the end point (Table 2), older patients and patients who received an allo-SCT who had normal portal flow or encephalopathy had a higher risk with respect to early death (P ≤ .05). Other categorical variables, such as underlying diagnosis and exposure to tPA/heparin, did not achieve significance. With the use of this univariate model for the continuous variables analyzed in the 56 patients who had at least 10 days of DF treatment, the mean decrease in creatinine during treatment proved highly significant in predicting better outcome (P = .0003). Combining the above variables and the mean changes of bilirubin, PAI-1, and creatinine in a multivariate Cox model analysis, younger age, autograft (versus allograft), and the mean decrease in both creatinine and PAI-1 during the first 10 days of DF therapy were statistically significant at predicting better outcome (P < .05), but mean change in bilirubin was not (P = .07).
Variables before defibrotide (DF) treatment associated with shorter time to death (univariate Cox regression analysis; n = 88)
| . | P . | Hazard ratio . |
|---|---|---|
| Age | .003 | 1.020 |
| Allo-SCT | .03 | 1.835 |
| Normal portal flow | .01 | 1.946 |
| Encephalopathy | .02 | 1.757 |
| . | P . | Hazard ratio . |
|---|---|---|
| Age | .003 | 1.020 |
| Allo-SCT | .03 | 1.835 |
| Normal portal flow | .01 | 1.946 |
| Encephalopathy | .02 | 1.757 |
Time to death is defined as time from DF initiation to death: median, 47 days (range, 3-2422 days).
Discussion
In this report, we describe the largest experience to date of DF administration to patients with severe hepatic VOD and MOF occurring after SCT. Thirty-six percent (32 of 88) of patients achieved CR and 35% (31 of 88) survived beyond day +100, with longer term survival also confirmed. This result is both much better than expected given the extremely compromised condition of these patients and noteworthy in that neither primary liver dysfunction nor recurrent VOD was reported as part of long-term follow-up.
Although up to one half of the patients undergoing SCT meet one of the current definitions of VOD, there is a broad spectrum of clinical illness. All of the patients in this report had characteristics associated with severe disease and poor outcome, with an expected day +100 mortality in excess of 90% (E. Carreras, personal communication, March 2002).5,6 Independent of VOD, patients undergoing SCT with evidence of organ dysfunction in more than one system have been reported to have a much higher mortality rate during SCT.32,33 For example, dialysis dependence alone has been reported to be associated with an 84% mortality rate, whereas doubling of the serum creatinine has been associated with a 37% mortality rate.34 35 Thus, the very high proportion (97%) demonstrating MOF in our series, including ventilator dependence in 32 (36%) patients and 27 (31%) patients requiring dialysis reinforces the fact that these patients would be predicted to have a very low probability of survival.
DF was administered by IV infusion in doses ranging from 5 to 60 mg/kg per day with intrapatient dose escalation and was well tolerated for up to 139 days. Most notably, and consistent with prior reports in severe VOD, no severe adverse events were attributable to DF, and, in contrast to the published experience with t-PA, no life-threatening hemorrhage attributable to treatment was observed in this high-risk population.14,31 This finding is also consistent with the experience reported in treating patients with severe hemolytic uremic syndrome and thrombotic thrombocytopenic purpura in whom no serious side effects were seen and reflects the extensive experience with DF in aged individuals with peripheral vascular disease where its safety profile was well established in a placebo-controlled phase III trial.36 37
The use of DF as a novel approach in the treatment of severe VOD was originally based on its unique pharmacologic characteristics and its lack of systemic anticoagulant activity.29 Recent in vitro work has demonstrated DF's selective effects on small vessels compared with large vessels, with a reduction in PAI-1 release and a protective action seen in a lipopolysaccharide-stimulated, microvessel-derived endothelial cell model.27,28 It is intriguing to speculate that these observations might explain the activity of DF in VOD, given the unique microvascular basis of key initial events in VOD pathogenesis.8,10,38,39 Such a hypothesis would be consistent with the observation that patients with abnormal portal flow, presumably as a reflection of more extensive damage to hepatic sinusoids and venules, appear to have a higher likelihood of survival. It is also consistent with the notion that DF, as a modulator of sinusoidal endothelial cell injury, may interrupt the progression of the endothelial-based processes that contribute to the syndrome. The fact that younger patients appear to do better may reflect that younger patients are better able to tolerate MOF and thus live long enough for the drug to be effective. Alternatively, there is evidence that advancing age may result in an increased endothelial stress response with sequelae that are less easily reversible.40,41 It is, therefore, possible that pediatric patients may benefit as a result of a more robust repair process engendered at the level of the sinusoidal endothelium by DF. It is also important to recognize that, although prospective comparisons have not been done, no obvious differences in outcome between pediatric and adult SCT patients with VOD have been described.42
Conversely, it is noteworthy that the patients who received Bu-based conditioning appeared to derive less benefit. Given that Bu appears to mediate its toxicity primarily through hepatocyte injury and glutathione depletion, as opposed to sinusoidal endothelial damage, this finding supports the concept that the effect of DF on sinusoidal endothelium may be its primary site of action.43 44 It is possible that the association of encephalopathy with poor treatment response may also reflect relatively less activity of DF in patients with pronounced hepatocellular dysfunction.
Continuous variables associated with better outcome included mean decrease in creatinine and PAI-1 levels during DF therapy. This association is noteworthy given that the major function of PAI-1 is regulation of endogenous t-PA, fibrin deposition, and fibronectin release, which may be particularly important early events in this disease.45-48 Consistent with this concept is that major sources of PAI-1 include endothelial cells, hepatocytes, and stellate cells, and major inducers of PAI-1 release include various cytokines, including interleukin 1 (IL-1), IL-8, IL-6, and tumor necrosis factor α (TNF-α) that appear important in the pathogenesis of this syndrome.48-51 The marked elevation of PAI-1 noted in the subset of patients evaluated is consistent with the reports of Salat et al,46,47 but our analysis could not support the diagnostic utility of PAI-1 measurement as a means of discriminating this syndrome from other forms of liver injury. Specifically, in 3 patients with high PAI-1 levels (> 200 ng/mL) recorded prior to DF therapy, histopathologic material at autopsy revealed sinusoidal obstruction and centrilobular congestion but no venular occlusion, and early GVHD was present in one patient. It is possible that PAI-1 levels may be less reliable as a diagnostic discriminant in patients with MOF and systemically increased vascular stress, given that PAI-1 levels are markedly elevated in patients with shock.52,53Nonetheless, the mean reduction of PAI-1, which paralleled the mean reduction seen in creatinine in those patients with improved survival, suggests that PAI-1 may be a relevant laboratory surrogate in VOD and MOF. The observation that decreases in both actual and mean creatinine were especially important in predicting better outcome is of great interest and may have relevance to the favorable renovascular effects seen with DF treatment in other disease states.25,36 This finding is also consistent with the notion that hepatorenal dysfunction is fundamental to the morbidity and mortality associated with severe VOD.5,32 35
It is of note that in the 29 patients who did not respond and in whom autopsy was carried out, 7 did not have VOD, with GVHD predominating as the alternate diagnosis. Obviously, prospective trials of DF therapy in VOD will be greatly aided by increased diagnostic certainty, as GVHD is unlikely to benefit from what we currently understand as the mechanism of action of DF in this setting. Future trials will thus be enhanced by the exclusion of patients with hepatic GVHD to minimize the effects of this disease as a confounding diagnosis.
As a first step in further defining the role of DF, a randomized, prospective, multicenter, phase II study of DF in severe VOD is under way to determine the effective dose (25 versus 40 mg/kg per day) and should be completed soon.54 In that trial, the use of rigorous selection criteria and central review has been mandated to minimize the confounding effects of investigator variability in both the diagnosis of VOD and the assessment of its acuity. Patients with grade 2 or greater GVHD are excluded, and liver biopsy with wedged hepatic venous pressure gradient measurement is required before initiation of treatment in diagnostically complex cases. Further analysis of laboratory correlates, including PAI-1, is being pursued as part of this trial, and it is hoped that such prospective studies of surrogate variables will provide the basis for a risk model to predict the efficacy of DF therapy. This risk model could also provide a means of triage, providing a platform for additional treatments to be pursued in those patients who may not benefit from DF alone. For example, to affect more favorably on the course of VOD in those patients receiving Bu and not responding to DF, the addition of other agents such asN-acetyl cysteine to support hepatic glutathione may warrant study.55
We gratefully acknowledge the support of Dr Massimo Iacobelli (Medical Director, Gentium SpA) for the provision of defibrotide (Gentium SpA, Como, Italy). We also acknowledge the expert biostatistical advice of Prof L. J. Wei (Harvard School of Public Health) and Prof Jawed Fareed (Loyola University Medical Center) for guidance regarding PAI-1 analysis. We thank Nancy Kinchla and Peggy Koval for their assistance in data management, as well as our research pharmacy team led by Caroline Harvey and the medical and nursing staff members who cared for these patients and facilitated their treatment. Most importantly, we express our deepest appreciation to the patients and their families.
Prepublished online as Blood First Edition Paper, August 1, 2002; DOI 10.1182/blood-2002-04-1216.
Correspondence:Paul G. Richardson, Dana-Farber Cancer Institute, Hematologic Oncology, 44 Binney St, Boston, MA 02115; e-mail: paul_richardson@dfci.harvard.edu.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal