Recent findings implied that the progression of hematologic malignancies, like that of solid tumors, is dependent on neovascularization. Recent studies on patients with acute myeloid leukemia (AML) showed increased levels of leukocyte-associated vascular endothelial growth factor (VEGF) and neovascularization of the bone marrow. Murine (32D, M1) and human (HEL, U937, and UKE-1) leukemic cell lines and freshly isolated leukemic cells were analyzed for the expression of VEGF and VEGF receptor mRNA. The expression of VEGF and VEGF receptors KDR and neuropilin-1 (NRP-1) was detected in these cells. In a murine chloroma model, delivery of VEGF165using microencapsulation technology resulted in enhanced tumor growth and vascularization, whereas treatment with a VEGF antagonist soluble NRP-1 (sNRP-1) inhibited tumor angiogenesis and growth. In a systemic leukemia model, survival of mice injected with adenovirus (Ad) encoding for Fc-sNRP-1 (sNRP-1 dimer) was significantly prolonged as compared with mice injected with Ad-LacZ. Further analyses showed a reduction in circulating leukemic cells and infiltration of liver and spleen as well as bone marrow neovascularization and cellularity. Taken together, these results demonstrate that angiogenic factors such as VEGF promote AML progression in vivo. The use of VEGF antagonists as an antiangiogenesis approach offers a potential treatment for AML. Finally, our novel in vivo drug delivery model may be useful for testing the activities of other peptide antiangiogenic factors.
Introduction
In recent years it has become evident that solid tumors beyond a given volume are dependent on the supply of oxygen and nutrients from the vascular system.1 Therefore, the vascular network has to grow concomitantly with the tumor, similar to embryonic development. The process of growing capillaries from pre-existing vessels has been termed angiogenesis.2 The importance of angiogenesis in tumor growth has been demonstrated in numerous studies.3-5 Several factors are being produced from tumor cells in hypoxic areas in order to enhance vascular formation. Among those, vascular endothelial growth factor (VEGF) plays a central role in promoting migration, proliferation, and differentiation of endothelial cells.6 There are 5 different VEGF subgroups which have been characterized, with VEGF-A being the most potent angiogenic factor.7 VEGF-A (hereafter VEGF) is a secreted homodimeric protein with various isoforms, consisting of 121, 165, and 189 amino acids, that are generated by alternative splicing.8 There are 4 receptors for VEGF which have been identified: Flt-1 (VEGFR-1), Flk-1/KDR (VEGFR-2), Flt-4 (VEGFR-3), and neuropilin-1 (NRP-1). The first 3 have a typical cytoplasmic tyrosine kinase domain, whereas NRP-1 has a short cytoplasmatic domain lacking enzymatic activity. NRP-1 binds specifically the VEGF165 isoform and enhances its binding to VEGFR-1, suggesting that it functions as a coreceptor.9
Expression levels of VEGF and its receptors as well as microvessel density as indicators of angiogenesis are accepted parameters in solid tumors and have been shown to correlate with clinical outcome in various cancers.10,11 Perez-Atayde et al12 were the first to describe increased microvessel density in the bone marrow of children with acute lymphatic leukemia (ALL) as an indicator of enhanced angiogenesis in hematologic malignancy. Fiedler et al13 demonstrated that acute myeloid leukemia (AML) blasts not only express VEGF but also its receptors. Endothelial cells responded to conditioned media of myeloblasts with increased production of hematopoetic growth factors, implying a paracrine loop. Autocrine stimulation of leukemic cells expressing KDR by VEGF in vitro was demonstrated by Dias et al in a recent report.14 They also showed inhibition of leukemia progression in vivo with KDR-blocking antibodies.14
We studied the role of VEGF in the progression of AML using murine and human leukemic cell lines that express VEGF and VEGF receptors. A model of chloroma, using M1 cells, was established in order to test the in vivo effects of VEGF. Delivery of VEGF165 and a VEGF antagonist, soluble NRP-1, was achieved using microencapsulation of cells overexpressing the recombinant proteins. VEGF supplementation significantly enhanced M1 tumor growth and tumor weights, whereas in sNRP-1–treated animals tumors grew slower, as compared with the control group. In a systemic leukemia model, administration of sNRP-1 inhibited AML progression, as determined by analyses of peripheral blood and bone marrow and liver infiltration, and resulted in prolonged survival. Our results demonstrate that angiogenic factors such as VEGF promote AML progression in vivo and that a soluble form of NRP-1 might be used for antiangiogenesis therapy.
Materials and methods
Cell culture
Leukemic cell lines M1, HEL, UKE-1,15 and U937 and WEHI (ATCC, Rockville, MD) were cultured in RPMI with 10% fetal bovine serum (FBS), penicillin (100 U/mL), and streptomycin (100 μg/mL). Cells of the murine line 32D (ATCC) were cultured in Dulbecco modified Eagle medium (DMEM) with 10% FBS and addition of 1% WEHI-conditioned medium. Normal murine mammary gland (NMuMG) epithelial cells were cultured in DMEM with high glucose, 10% FBS, and penicillin/streptomycin. Leukemic cells of patients with newly diagnosed AML were isolated from peripheral blood using Ficoll-Histopaque (Pharmacia, Uppsala, Sweden).
Reverse transcriptase–polymerase chain reaction
RNA from clinical specimens and cultured cells was isolated using the RNAzol reagent according to the manufacturer's protocol (Tel-Test, Friendswood, TX). RNA (1 μg) was processed for cDNA synthesis using Superscript II reverse transcriptase (RT) with random hexamers (Life Technologies, Rockville, MD). The cDNA (1 μl) was used for polymerase chain reaction (PCR) in a final volume of 30 μL with 200 nM dNTP, 15 pM of each primer, 0.3 U Taq DNA polymerase, and reaction buffer (Boehringer, Mannheim, Germany), in a PTC-100 cycler (MJ-Research, Watertown, MA). All primers were obtained from Life Technologies. The primers used for human VEGF (5′ ATGAACTTTCTGCTGTCTTGG and 3′ TCACCGCCTCGGCTTGTCACA, 30 cycles, annealing at 60°C) amplified fragments of 450 bp, 570 bp, and 650 bp for the 121, 165, and 189 isoforms of VEGF, respectively. Glyceraldehyde phosphate dehydrogenase (GAPDH) primers (5′ GTCTTCACCACCATGGAG, 3′ CCACCCTGTTGCTGTAGC, 28 cycles at 60°C) amplified a fragment of 673 bp. Murine Flk-1 primer (5′ GCATCACCAGCAGCCAGAG, 3′ GCAACACACCGAAAGACCAC, 35 cycles at 60°C) and human KDR primer (5′ TTACAGATCTCCATTTATTGC, 3′TTCATCTCACTCCCAGACT, 35 cycles at 60°C) yielded fragments of 390 bp and 479 bp, respectively. Murine (5′ GGTTTTAACTGCGAGTTTG, 3′ ATCATCCACAGCAATTCCACC, 30 cycles at 55°C) and human (5′ TTTCGCAACGATAAATGTGGCGAT, 3′ TATCACTCCACTAGGTGTTG, 30 cycles at 55°C) NRP-1 were detected as bands of 452 bp and 409 bp. PCR products were resolved on a 2% agarose gel and stained with ethidium bromide. The amplified DNA was visualized under UV light and images were obtained by the “Eagle Eye” digital camera (Stratagene, La Jolla, CA).
KDR phosphorylation studies
KDR phosphorylation was assessed as previously described.16 Leukemic (M1, HEL, U937), porcine aortic endothelial (PAE), and PAE-KDR17 cells were starved for 12 hours and then incubated in serum-free medium with 25 ng/mL VEGF for 30 minutes at 4°C. The cells were shifted to 37°C for 7 minutes and then lyzed. Cellular proteins were absorbed on Con A Sepharose, separated by 6% sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) and blotted onto a membrane, which was probed with antiphosphotyrosine-specific antibodies (4G-10, Upstate Biotechnology, Lake Placid, NY).
In vitro proliferation studies
After 12 hours incubation in serum-free medium, leukemic cells (M1, HEL) were counted and incubated in 48-well dishes (5000 cells per well) in serum-free medium in the presence or absence of VEGF (10 ng/mL). Cell numbers were obtained after 24 hours and 48 hours using a Coulter cell counter.
Transfection and encapsulation of cells releasing VEGF165 and sNRP-1
VEGF165 cDNA and the encoding sequence for the extracellular portion of human NRP-1 (amino acids 1-853) were cloned into the pcDNA3.1 expression vector (Invitrogen). NMuMG cells were transfected with the expression vector using the lipofectamine reagent (Life Technologies). Stable clones expressing large amounts of VEGF165 and sNRP-1 were isolated. NmuMG, NMuMG/VEGF, and NMuMG/sNRP-1 cells were trypsinyzed and resuspended in 1.2% sodium alginate. The cell suspension was passed through a nozzle and the resulting capsules were collected in 1.5% mM CaCl2/13 mM HEPES (N-2-hydroxyethylpiperazine-N'-2-ethanesulfonic acid) buffer and subsequently coated with poly-l-lysine, as described.18
Construction of adenovirus vectors
Adenovirus (Ad) vectors encoding for VEGF and β-galactozidase (LacZ) were kindly provided by the Harvard Gene Therapy Initiative core facility. Ad-Fc-sNRP-1 (a dimeric form of sNRP-1) was constructed as previously described.16
Murine chloroma experiments and immunohistochemistry
M1 cells (2 × 106 per mouse) were injected subcutaneously to severe combined immunodeficiency (SCID) mice. After 72 hours, encapsulated NMuMG, NMuMG/VEGF, and NMuMG/sNRP-1 cells (5 × 105 cells per mouse) were injected near the leukemic cells. Mice (10 per group) were followed for tumor growth and tumor size was measured. After 21 days tumors were harvested and their weight was measured. Tumor tissues were fixed in Histochoice (Amresco, Solon, OH) for 24 hours, processed, and embedded in paraffin. Tissue sections were stained with hematoxylin and eosin (H&E) or incubated overnight with monoclonal anti–mouse CD31 (1:100 dilution; BD Pharmingen, San Diego, CA) and polyclonal anti–human VEGF (1:250 dilution; R&D Systems, Minneapolis, MN) antibodies. The primary antibodies were detected using ABC and DAB substrate kits (Vector Laboratories, Burlingame, CA) and the sections were counterstained with “Gills” hematoxylin. Microvessel density (MVD) was determined by the counting of stained capillary structures in 10 high-power fields (HPF) of each tumor.
Systemic leukemia model
M1 cells (2 × 106 cells per mouse) were injected intravenously into SCID mice. After 7 days, adenoviral vectors (Ad) encoding for Fc-sNRP-1, VEGF, and LacZ were injected intravenously (Ad-Fc-sNRP-1 and Ad-LacZ, 2 × 108 pfu/mouse; Ad-VEGF, 5 × 107 pfu/mouse) and mice (10 mice per group) were followed for survival. On day 28, 2 mice from each group were analyzed by blood smears and histology of liver, spleen, and bones. Small pieces of the liver were used for a functional staining of LacZ, as previously described.19 Femoral bones were fixed in 3.7% formaldehyde, decalcified in 0.5 M EDTA (ethylenediaminetetraacetic acid), pH 8.0, and embedded in paraffin. Bone sections were stained with H&E and immunostained with monoclonal anti–mouse CD31 antibodies, as described for the chloroma model.
Results
Expression of VEGF and VEGF receptors by leukemic cells
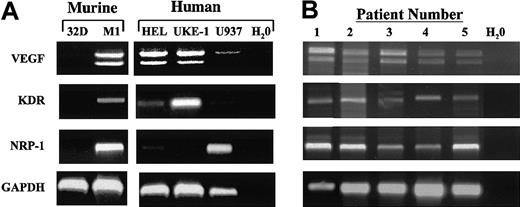
Leukemic cells were analyzed for expression of VEGF, VEGFR-2 (human KDR and murine Flk-1), and neuropilin-1 (NRP-1) using RT-PCR. The VEGF primers were designed to amplify all splicing forms of VEGF, and species-specific primers were used for KDR, Flk-1, and NRP-1. In order to ensure equal mRNA levels, the expression of GAPDH mRNA was monitored. Most cell lines (Figure 1A) and all of the freshly isolated human leukemic cells (Figure 1B) expressed VEGF mRNA with comparable levels of VEGF121 and VEGF165 isoforms. Expression of KDR was found in M1 and UKE-1 lines and in all of the freshly isolated human leukemic cells. Low levels were found in HEL cells. NRP-1 was expressed by M1 HEL and U937 lines and in all of the freshly isolated human leukemic cells. This result indicates that VEGF and its receptors KDR and NRP-1 are expressed by murine and human AML blasts but not in basophilic cells such as 32D.
Expression of VEGF and VEGF receptors in leukemic cells.
RNA was isolated from leukemic cell lines grown in culture (A) and freshly isolated human leukemia cells (B) as described in “Materials and methods.” RT-PCR was performed on the RNA, using primers corresponding for human VEGF, KDR, NRP-1, and GAPDH cDNA sequences and murine Flk-1 (KDR) and NRP-1 cDNA sequences, as indicated. PCR products were resolved on a 2% agarose gel, stained with ethidium bromide, and visualized under UV light.
Expression of VEGF and VEGF receptors in leukemic cells.
RNA was isolated from leukemic cell lines grown in culture (A) and freshly isolated human leukemia cells (B) as described in “Materials and methods.” RT-PCR was performed on the RNA, using primers corresponding for human VEGF, KDR, NRP-1, and GAPDH cDNA sequences and murine Flk-1 (KDR) and NRP-1 cDNA sequences, as indicated. PCR products were resolved on a 2% agarose gel, stained with ethidium bromide, and visualized under UV light.
VEGF autocrine stimulation of leukemic cells
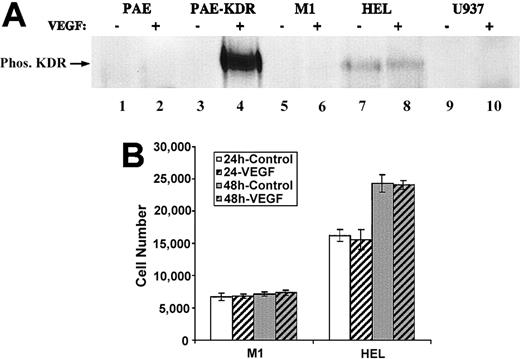
Since many leukemic cell lines express VEGF and VEGF receptors, we tested a possible VEGF autocrine stimulation in these cells. First, VEGF receptor phosphorylation was evaluated (Figure2A). PAE, PAE-KDR, and leukemic cell lines were transiently incubated with VEGF in order to induce receptor phosphorylation. VEGF induced abundant KDR phosphorylation in PAE-KDR, as previously shown.16 In contrast, no phosphorylated proteins corresponding to the size of the VEGF receptors could be detected in leukemic cell lines that do not express KDR (U937) or that express low levels of KDR (M1). Interestingly, HEL cells that express low levels of KDR showed an unchanged KDR phosphorylation, suggesting maximal autocrine stimulation by endogenous VEGF in these cells. Subsequently, we evaluated VEGF-induced proliferation of M1 and HEL cells, which showed different KDR phosphorylation patterns. M1 and HEL cells were starved for 12 hours and incubated in the presence of 10 ng/mL VEGF165 in serum-free medium. Cell numbers were obtained after 24 hours and 48 hours (Figure 2B). Although HEL showed a higher proliferation rate than M1, exogenously added VEGF did not induce an increase in cell numbers compared with untreated cells. These results suggest that M1 and HEL cells do not proliferate in response to VEGF and support the previous findings that VEGF treatment did not induce KDR phosphorylation in M1 cells and did not increase the phosphorylation of KDR in HEL cells over a constitutive level.
The leukemic cell lines M1 and HEL do not respond to exogenous VEGF.
(A) KDR phosphorylation. PAE, PAE-KDR, M1, HEL, and U937 were incubated in the presence (+) or absence (−) of VEGF165 (20 ng/mL) for 30 minutes on ice and then shifted to 37°C for 7 minutes, as described in “Materials and methods.” Cells were lysed and proteins were absorbed on ConA Sepharose and resolved by a 6% SDS-PAGE. Proteins were blotted onto polyvinylidenefluoride (PVDF) membrane, which was subsequently probed with antiphosphotyrosine antibodies. Phosphorylated KDR (Phos KDR) was detected as a band with a molecular weight of approximately 220 kd. (B) Cell proliferation. M1 and HEL cells were incubated in the presence (VEGF) or the absence (control) of VEGF (10 ng/mL) in serum-free medium, as described in “Materials and methods.” Cell numbers were obtained after 24 and 48 hours. Cell numbers represent the average of 3 individual wells.
The leukemic cell lines M1 and HEL do not respond to exogenous VEGF.
(A) KDR phosphorylation. PAE, PAE-KDR, M1, HEL, and U937 were incubated in the presence (+) or absence (−) of VEGF165 (20 ng/mL) for 30 minutes on ice and then shifted to 37°C for 7 minutes, as described in “Materials and methods.” Cells were lysed and proteins were absorbed on ConA Sepharose and resolved by a 6% SDS-PAGE. Proteins were blotted onto polyvinylidenefluoride (PVDF) membrane, which was subsequently probed with antiphosphotyrosine antibodies. Phosphorylated KDR (Phos KDR) was detected as a band with a molecular weight of approximately 220 kd. (B) Cell proliferation. M1 and HEL cells were incubated in the presence (VEGF) or the absence (control) of VEGF (10 ng/mL) in serum-free medium, as described in “Materials and methods.” Cell numbers were obtained after 24 and 48 hours. Cell numbers represent the average of 3 individual wells.
Effect of VEGF and the VEGF antagonist, sNRP-1, on chloroma growth in vivo
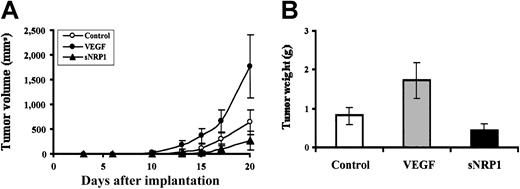
We have established an in vivo model of M1 cells in SCID mice. Upon subcutaneous injection, M1 cells (2 × 106 per mouse) form solid tumors, that is, chloromas. This model allows quantitative tumor volume measurements for the study of tumor growth in vivo. For constant delivery of VEGF and the VEGF antagonist, sNRP-1, we used the cell encapsulation technique,18 with NMuMG/VEGF and NMuMG/sNRP-1 cells that have been engineered to produce high levels of recombinant VEGF and sNRP-1, respectively. The encapsulated cells (5 × 105 per mouse) were injected next to the M1 cell injection site 3 days later, and the mice were followed for tumor growth for an additional 20 days. Mice injected with VEGF-producing cells showed an accelerated tumor growth compared with mice injected with control NMuMG cells, with tumor volumes of 1774 ± 633 mm3 and 646 ± 241 mm3 after 20 days, respectively (Figure 3A). In contrast, mice injected with sNRP-1–producing cells developed smaller tumors (278 ± 187 mm3). At the end of the experiment, tumors were harvested and weighed (Figure 3B). A significant weight difference was noted between the groups, with weights of 0.82 ± 0.22 g, 1.735 ± 0.48 g, and 0.43 ± 0.2 g for control, VEGF, and sNRP-1, respectively (VEGF vs control, P < .002; sNRP-1 vs control, P < .03).
The effects of VEGF and sNRP-1 supplementation on chloroma growth in vivo.
(A) Tumor volume curves. M1 cells (2 × 106 per mouse) were injected subcutaneously into SCID mice. NMuMG-encapsulated (○), NMuMG/VEGF-encapsulated (●), and NMuMG/sNRP-1–encapsulated (▴) cells (5 × 105 cells per mouse) were injected at the site of M1 cell injection, 72 hours later. The mice (10 per group) were followed for tumor volumes at the indicated times. (B) Final tumor weights. Tumors were harvested 21 days after M1 cell injection and weighed. The average weight and standard deviations were calculated for NMuMG (control), NMuMG/VEGF (VEGF), and NMuMG/sNRP-1 (sNRP-1) groups.
The effects of VEGF and sNRP-1 supplementation on chloroma growth in vivo.
(A) Tumor volume curves. M1 cells (2 × 106 per mouse) were injected subcutaneously into SCID mice. NMuMG-encapsulated (○), NMuMG/VEGF-encapsulated (●), and NMuMG/sNRP-1–encapsulated (▴) cells (5 × 105 cells per mouse) were injected at the site of M1 cell injection, 72 hours later. The mice (10 per group) were followed for tumor volumes at the indicated times. (B) Final tumor weights. Tumors were harvested 21 days after M1 cell injection and weighed. The average weight and standard deviations were calculated for NMuMG (control), NMuMG/VEGF (VEGF), and NMuMG/sNRP-1 (sNRP-1) groups.
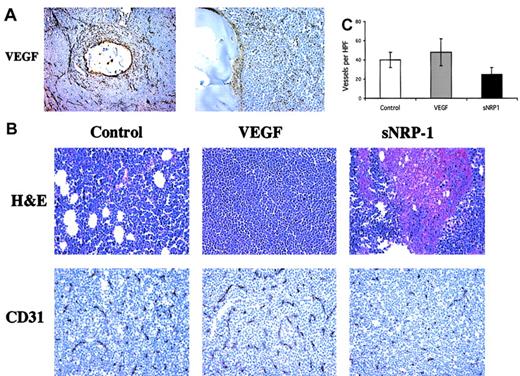
The tumors and surrounding tissue were retrieved for histologic analysis. Immunostaining for human VEGF revealed a concentric staining pattern around the capsules with high immunoreactivity at their borders (Figure 4A). Several VEGF-producing cells could be detected within the capsules and extracellular matrix-bound VEGF in the tissues surrounding the capsules. Tumor specimens were further analyzed by H&E staining, revealing healthy and packed cells in control and VEGF-treated tumors but large necrotic areas in the sNRP-1–injected group (Figure 4B, top panels). Staining of tumor sections with anti–CD31 antibodies (Figure 4B, bottom panels) revealed a dense capillary network in tumors retrieved from mice that were injected with VEGF-producing cells. A developed capillary network was also observed in tumors of mice that were injected with control NMuMG cells. In contrast, the tumors in mice that were injected with sNRP-1–producing cells showed fewer and smaller capillaries, consistent with the necrosis of the tumors observed in these mice. Quantification of microvessel density (MVD) revealed 40 ± 8 capillaries per high-power field in controls and 49.2 ± 14 capillaries in VEGF-treated mice, without a significant difference (Figure 4C). MVD was significantly lower (P < .05) in mice treated with sNRP-1, with 27.4 ± 7 capillaries per field. These results indicate that supplementation of VEGF at the site of chloroma tumors induces tumor vascularization and promotes tumor growth. On the other hand, the VEGF antagonist sNRP-1 repressed tumor vascularization and, subsequently, tumor growth.
Opposite effects of VEGF and sNRP-1 on chloroma neovascularization.
(A) In vivo secretion of VEGF. Tumors from mice injected with encapsulated NMuMG/VEGF cells were harvested after 21 days and processed for histologic examination. Tissue sections were stained with anti–human VEGF antibodies and photographed under × 100 (left) and × 400 (right) original magnification. A cross-sectioned capsule and surrounding tumor tissue is shown. Some VEGF-positive stained cells can be seen within the capsule. (B) Tumor neovascularization. Tumors from NMuMG (control), NMuMG/VEGF (VEGF), and NMuMG/sNRP-1 (sNRP-1) groups, as indicated, were harvested and processed for histologic examination. Tumor sections were stained with H&E (top panels) and with anti–mouse CD31 antibodies (bottom panels) and photographed under × 400. (C) Microvessel density (MVD) was determined by counting 10 high-power fields (HPFs) from the CD31 stained sections in panel B. The numbers represent the MVD average, and standard deviations were calculated.
Opposite effects of VEGF and sNRP-1 on chloroma neovascularization.
(A) In vivo secretion of VEGF. Tumors from mice injected with encapsulated NMuMG/VEGF cells were harvested after 21 days and processed for histologic examination. Tissue sections were stained with anti–human VEGF antibodies and photographed under × 100 (left) and × 400 (right) original magnification. A cross-sectioned capsule and surrounding tumor tissue is shown. Some VEGF-positive stained cells can be seen within the capsule. (B) Tumor neovascularization. Tumors from NMuMG (control), NMuMG/VEGF (VEGF), and NMuMG/sNRP-1 (sNRP-1) groups, as indicated, were harvested and processed for histologic examination. Tumor sections were stained with H&E (top panels) and with anti–mouse CD31 antibodies (bottom panels) and photographed under × 400. (C) Microvessel density (MVD) was determined by counting 10 high-power fields (HPFs) from the CD31 stained sections in panel B. The numbers represent the MVD average, and standard deviations were calculated.
The VEGF antagonist, sNRP-1, inhibits progression of systemic leukemia
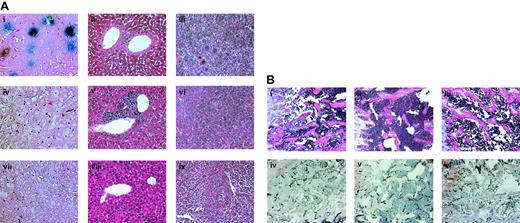
SCID mice injected intravenously with murine M1 cells develop leukemia with bone marrow infiltration and circulating blasts. In order to achieve maximal systemic levels of a VEGF antagonist, we used an adenoviral vector producing a dimeric form of sNRP-1, Fc-sNRP-1. Dimeric sNRP-1 has a higher binding affinity for VEGF than the monomer and is a more potent inhibitor of VEGF binding to endothelial cells (not shown). Since high concentrations of VEGF lead to early morbidity and mortality, VEGF treatment in this experiment was carried out using injection of a low titer of an adenoviral vector–producing VEGF. M1 cells were injected intravenously into SCID mice (2 × 106 cells per mouse) and after 7 days adenoviruses coding for Fc-sNRP-1 (Ad.Fc-sNRP-1), VEGF (Ad.VEGF), or lacZ (Ad.LacZ) as control, were injected intravenously. After 28 days, 2 mice from each group were killed and peripheral blood samples were analyzed for the proportion of leukemic cells. Blood samples from Ad.LacZ- and Ad.VEGF-injected mice had 49% and 60% leukemia blast cells, respectively. In contrast, mice injected with Ad.Fc-sNRP-1 had only 6.3% leukemia blast cells. Liver tissue specimens were tested for the expression of LacZ using a functional assay that stained the expressing cells blue (Figure 5Ai,iv,vii). Only mice injected with Ad.LacZ had blue cells within the liver, the preferred site of adenovirus infection, indicating efficient viral infection. Liver and spleen specimens were also analyzed for cell infiltration by H&E staining (Figures 5Aii,v,viii and iii,vi,ix, respectively). Massive leukemic cell infiltration was observed in the liver and spleen of mice injected with Ad.VEGF. In contrast, liver specimens of Ad.Fc-sNRP-1–injected mice had a normal abundance of lymphocytelike cells. Analyses of bone marrow sections by H&E staining (Figure 5Bi-iii) revealed higher cellularity in Ad.VEGF-injected mice than in control, Ad.LacZ mice. Mice injected with Ad.Fc-sNRP-1 had lower cellular content than control mice. Identification of M1 cells in the bone marrow was confirmed using Ad.LacZ-infected M1 cells and lacZ staining of bone sections (not shown). Immunostaining of bone sections using anti–CD31 antibodies (Figure 5Biv-vi) revealed many bone marrow capillaries in Ad.VEGF-injected mice. Far fewer capillaries were observed in Ad.LacZ- and Ad.Fc-sNRP-1–injected mice.
Soluble NRP-1 inhibits liver, spleen, and bone infiltration of leukemic cells.
M1 cells (2 × 106 cells per mouse) were injected intravenously into SCID mice. After 7 days, adenoviral vectors encoding for Fc-sNRP-1 (Avii-ix and Biii-iv), VEGF (Aiv,vi and Bii,v), and lacZ (Ai-iii and Bi,iv) were injected intravenously, as described in “Materials and methods.” On day 28, 2 mice from each group were killed and their bones, livers, and spleens were processed for histologic examination. (A) Liver tissues were processed for LacZ staining (Ai,iv,vii), as described in “Materials and methods.” Liver and spleen tissues were also stained with H&E (ii,v,viii and iii,vi,ix, respectively). Stained sections were examined microscopically and representative images were recorded (original magnification × 200). (B) Bone sections were stained with H&E (i-iii) and with anti–mouse CD31 antibodies (iv-vi). Stained sections were examined microscopically and representative images were recorded (original magnification × 200).
Soluble NRP-1 inhibits liver, spleen, and bone infiltration of leukemic cells.
M1 cells (2 × 106 cells per mouse) were injected intravenously into SCID mice. After 7 days, adenoviral vectors encoding for Fc-sNRP-1 (Avii-ix and Biii-iv), VEGF (Aiv,vi and Bii,v), and lacZ (Ai-iii and Bi,iv) were injected intravenously, as described in “Materials and methods.” On day 28, 2 mice from each group were killed and their bones, livers, and spleens were processed for histologic examination. (A) Liver tissues were processed for LacZ staining (Ai,iv,vii), as described in “Materials and methods.” Liver and spleen tissues were also stained with H&E (ii,v,viii and iii,vi,ix, respectively). Stained sections were examined microscopically and representative images were recorded (original magnification × 200). (B) Bone sections were stained with H&E (i-iii) and with anti–mouse CD31 antibodies (iv-vi). Stained sections were examined microscopically and representative images were recorded (original magnification × 200).
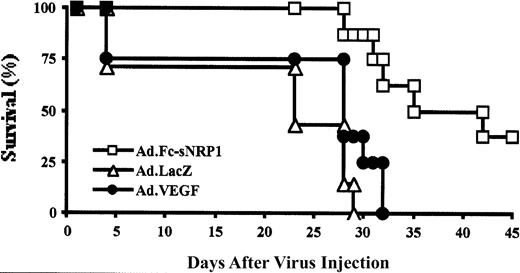
The mice were followed for 45 days in order to test the effect of the VEGF antagonist on leukemic mouse survival (Figure6). There was no significant difference in the median survival of mice treated with Ad.LacZ and Ad.VEGF (approximately 28 days), whereas leukemic mice injected with Ad.Fc-sNRP-1 had a significantly higher median survival rate (approximately 42 days; P < .001). These results indicate that inhibition of VEGF decreases leukemia-induced neoangiogenesis in bone marrow and liver and spleen infiltration and slowed mouse death due to leukemia.
Soluble NRP-1 prolonged the survival of leukemic mice.
SCID mice were injected with M1 cells followed by intravenous injection of adenoviral vectors encoding for Fc-sNRP-1 (Ad.Fc-sNRP-1; ■), VEGF (Ad.VEGF; ●), and lacZ (Ad.LacZ; ▵), as described in the Figure 5legend. Mice (10 per group) were followed for 45 days after viral injection and a survival curve was generated. Mice injected with Ad.LacZ or Ad.VEGF had a mean survival of 28 days and mice injected with Ad.Fc-sNRP-1 had a mean survival of 35 days.
Soluble NRP-1 prolonged the survival of leukemic mice.
SCID mice were injected with M1 cells followed by intravenous injection of adenoviral vectors encoding for Fc-sNRP-1 (Ad.Fc-sNRP-1; ■), VEGF (Ad.VEGF; ●), and lacZ (Ad.LacZ; ▵), as described in the Figure 5legend. Mice (10 per group) were followed for 45 days after viral injection and a survival curve was generated. Mice injected with Ad.LacZ or Ad.VEGF had a mean survival of 28 days and mice injected with Ad.Fc-sNRP-1 had a mean survival of 35 days.
Discussion
Acute myeloid leukemia is a rapid progressive disease characterized by high proliferation of leukemic blasts with consequent repression of normal hematopoiesis in the bone marrow.20Although the bone marrow space is filled mainly with hematopoietic cells, it contains other cells such as adipocytes, fibroblasts, osteogenic cells, and endothelial cells.21 The endothelium forms a network of vasculature spanning the whole cavity, thus providing a blood supply like in solid organs. Perez-Atayde et al showed a significantly higher density of bone marrow capillaries in children with acute lymphatic leukemia as compared with healthy volunteers.12 It was shown that leukemic cells secrete VEGF and thereby stimulate the microenvironment to produce cytokines, thus providing a paracrine loop.13,22,23 Angiogenesis seems to play a key role not only in AML but also in other hematologic malignancies like chronic lymphatic leukemia (CLL), non-Hodgkin lymphomas, and multiple myeloma.24-26
In this study we investigated how exogenously supplied VEGF affects the progression of AML in vivo and conversely, if VEGF antagonists repress this process. VEGF is a potent angiogenic factor in vivo and has been implicated in the growth of a wide variety of solid tumors.27 NRP-1 acts as a high-affinity receptor for VEGF on endothelial and tumor-derived cells9,28 but it does not have a kinase enzymatic activity and acts as a coreceptor for KDR by enhancing VEGF binding to KDR.16 A naturally occurring sNRP-1 protein is produced by tumor cells and it can inhibit VEGF receptor binding in vitro and tumor growth in vivo when overexpressed in prostate cancer cells.29
We tested several human and murine cell lines for the expression of VEGF, KDR, and NRP-1 by RT-PCR. We found VEGF expression in all lines tested except for 32D, which is a basophilic line, and in freshly isolated human leukemia cells. Additionally, most leukemic cells expressed mRNA for KDR and NRP-1. Recently, Flt-4 (VEGFR-3), a receptor for VEGF-C, was detected on leukemic cell lines and on primary leukemia cells.30 VEGF-C induced leukemia cell proliferation and provided protection from chemotherapy-induced apoptosis.30These results may point to hemangioblasts as a common origin of leukemia and endothelial cells.31 Several studies have shown that a high percentage of leukemic cells express VEGF and VEGF receptors,13,22,32 suggesting a possible autocrine stimulation of the malignant cells. VEGF was shown to stimulate proliferation of leukemic cells in vitro,14,33 as well as to act as an antiapoptotic factor.34 VEGF activation of KDR was postulated to lead to its angiogenic effects. To test this hypothesis, we have analyzed KDR phosphorylation in our leukemic cell system. KDR was constitutively phosphorylated in HEL cells, possibly through autocrine VEGF stimulation, and its phosphorylation did not increase with the addition of exogenous VEGF. On the other hand, M1 cells expressed VEGF receptors but did not induce KDR phosporylation. These results were supported by cell proliferation experiments, which showed no enhancement in HEL and M1 cell numbers upon addition of VEGF. This result suggests that M1 cells may not be affected by VEGF or VEGF antagonism. It is possible that leukemic cells express different amounts of VEGF or its receptors, which determines whether the receptors will be constitutively activated or they could be further activated by exogenous VEGF. Alternatively, VEGF expression may be up-regulated in leukemia cells as a result of relative hypoxia or cytokine induction.35
In order to examine the role of VEGF in AML progression in vivo, we established 2 mouse models with M1 cells forming chloromas when injected subcutaneously or leukemia when injected intravenously into SCID mice. M1 cells in a chloroma model formed solid tumors. VEGF and sNRP-1 were supplied in vivo from encapsulated cells producing high amounts of recombinant proteins. Analysis of VEGF secretion from encapsulated cells in vivo revealed a concentric pattern of VEGF release from the capsules into the surrounding tissue. This control release system allows continuous delivery of recombinant proteins avoiding in vivo DNA transfection.18 VEGF dramatically enhanced growth of M1 chloromas with earlier onset and formation of larger tumors, whereas release of sNRP-1 resulted in a significant inhibition of chloroma growth. Analysis of tumor sections showed tightly packed cells in the VEGF and control groups but areas with necrosis were present in sNRP-1–treated tumors. Accordingly, the microvessel density was significantly higher in the VEGF group and significantly lower in the sNRP-1 group as compared with controls. Blood capillaries in sNRP-1–treated chloromas were not only fewer but also smaller in size. Similar results were seen in human patients with AML following treatments with chemotherapy or with a KDR antagonist SU5416.36 37 Since VEGF is required for the growth and maintenance of blood capillaries, VEGF inhibition may lead to a reduction in the total number of capillaries as well as their size. These results indicate that chloroma progression is regulated by the availability of angiogenesis factors to the leukemia cells and suggest that this process is angiogenic dependent, like the growth of a solid tumor.
Since a chloroma does not involve the bone marrow environment and the systemic nature of acute leukemia, we tested VEGF inhibition in a leukemia model using M1 cells. To achieve systemic delivery of sNRP-1 we chose to use an adenovirus-mediated delivery of recombinant proteins. Coupling recombinant proteins to carriers, such as the immunoglobulin G (IgG) Fc domain, may enhance their stability and prolong their presence in the circulation. In this study we used an adenovirus vector encoding for the Fc-sNRP-1 fusion protein that produces dimers of sNRP-1. Although the exact molecular interaction between NRP-1 and VEGF is not clear, the affinity of VEGF165 to NRP-1 is dramatically increased with increasing NRP-1 density.38 We observed higher125I-VEGF165 binding capacity of sNRP-1 dimers compared with sNRP-1 monomers (M.N. and S.S., unpublished data, August 2001). Adenoviral delivery of Fc-sNRP-1 resulted in prolonged survival of the leukemic mice. Accordingly, massive M1 infiltration to liver and spleen was evident in VEGF-treated (Ad.VEGF-injected) mice compared with Fc-sNRP-1–treated (Ad.Fc-sNRP-1–injected) mice. Fc-sNRP-1–treated mice had significantly lower numbers of circulating blasts 4 weeks after M1 cell injection. Examination of bone marrow sections revealed enhanced neovascularization of control (Ad.LacZ-injected) and VEGF-treated mice. In contrast, mice treated with Fc-sNRP-1 exhibited only a few bone marrow capillaries, similar to the effects of sNRP-1 seen in the chloroma model. Bone marrow neovascularization was associated with increased bone marrow infiltration of M1 cells in VEGF-treated mice and conversely, decreased cellularity in Fc-sNRP-1–treated mice. Taken together, these results show the opposite effects of VEGF and sNRP-1 on leukemia progression in 2 different mouse models. These findings are supported by clinical data, which show increased microvessel density in the bone marrow of patients with AML and myelodysplastic syndrome.39-42Aguayo et al found that intracellular VEGF levels associated with the leukemic cells could serve to predict the outcome of patients with AML.43
We have engineered a VEGF antagonist, based on the sequence of the extracellular portion of NRP-1. This region contains the VEGF-binding site of NRP-138,44 and was shown to specifically block VEGF165 activity.27 Since many tumor cells express NRP-1 and bind VEGF via this receptor,9,17 sNRP-1 represents a novel VEGF antagonist that can bind circulating VEGF and prevent it from interacting with tumor endothelial cells and displace tumor cell NRP-1–bound VEGF. We have previously shown that rat prostate tumor cells expressing high levels of sNRP-1 formed tumors with large necrotic areas, probably due to insufficient blood supply caused by VEGF inhibition.29 In our chloroma model, sNRP-1 released from encapsulated cells had similar VEGF inhibition effects, as suggested by the many necrotic areas within the tumors. In the systemic leukemia model, inhibition of bone marrow vascularization by sNRP-1 may affect circulation and survival of the leukemic cells. Since we have not detected a direct effect of VEGF on M1 cells, a direct effect of sNRP-1 is not likely. However, we are currently testing sNRP-1–induced apoptosis of leukemia cell lines and freshly isolated leukemia cells.
Other VEGF antagonists have been used as antiangiogenic factors. Promising results have been observed using antibodies to VEGF, soluble receptors, or antibodies to receptors of VEGF.45-47Treatment of human leukemic cell lines expressing KDR with anti–KDR antibodies was shown to inhibit leukemia progression in nonobese diabetic (NOD)–SCID mice.14 Combination treatment of anti-KDR and anti–Flk-1 (murine KDR) completely inhibited tumor growth of these cell lines in mice. The authors concluded that VEGF may play a dual role in AML progression by inducing leukemic cell proliferation via an autocrine stimulation loop as well as induction of host endothelium–dependent angiogenesis via a paracrine loop. Our results suggest that in patients with AML, where the leukemic cells are not able to directly respond to VEGF, tumor progression might be promoted by induction of host-dependent angiogenesis. The use of sNRP-1 as a VEGF antagonist prevents VEGF-induced angiogenesis and AML tumor progression. Interestingly, VEGF was shown to promote AML progression via an alternative pathway. A positive correlation was shown between VEGF production and the engraftment of leukemic cells in NOD-SCID mice.48 Clinical trials using the antiangiogenesis approach to treat hematologic cancers are underway. KDR inhibitors such as SU5416 and PTK78749 or thalidomide50showed encouraging preliminary results.
Collectively, we have demonstrated that AML progression is induced by VEGF and that a VEGF antagonist, sNRP-1, significantly inhibited AML progression in vivo. Thus, our results suggest that AML progression is angiogenic dependent and that VEGF antagonist therapy with soluble receptor proteins and blocking antibodies might represent a promising alternative to the current therapy approaches.
The authors wish to thank Dr Michael Klagsbrun for helpful discussions and Dr Evelyn Flynn for her assistance in the immunostaining procedures.
Supported by a grant of Deutsche Krebshilfe (G.S.).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Shay Soker, Department of Urology, Children's Hospital, Harvard Medical School, 300 Longwood Ave, Boston, MA 02115; e-mail: shay.soker@tch.harvard.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal