Abstract
T lymphocytes have been found to harbor P-glycoprotein (Pgp) and to demonstrate modulation of its ion channel transporter function according to the state of activation of T lymphocytes. We hypothesized that cytotoxic chemicals that are extruded by Pgp could be used to specifically eliminate immunoreactive T-cell populations. In this study, we evaluated the capacity of 4,5-dibromorhodamine methyl ester (TH9402), a photosensitizer structurally similar to rhodamine, a dye transported by Pgp, and which becomes highly cytotoxic on activation with visible light to selectively deplete alloreactive T lymphocytes. Stimulation of T cells with mitogens or allogeneic major histocompatibility complex–mismatched cells resulted in the preferential retention of the TH9402 rhodamine-derivative in activated T cells, both CD4+ and CD8+. Photodynamic cell therapy of TH9402-exposed T cells led to the selective elimination of immunoreactive T-cell populations. In addition, this treatment preserved resting T cells and their capacity to respond to third-party cells. Inhibition of Pgp enhanced cellular trapping of the dye in nonactivated T cells and resulted in their depletion after exposure to light. Targeting of Pgp-deficient cells may therefore represent an appealing strategy for the prevention and treatment of graft-versus-host disease and other alloimmune or autoimmune disorders.
Introduction
Graft-versus-host disease (GVHD) is the principal cause of mortality and the primary limitation to the early and widespread use of allogeneic stem cell transplantation (SCT), a treatment that often represents the only curative option for numerous patients with malignant diseases and hereditary metabolic disorders. Depletion of T cells capable of recognizing and mounting an immune response toward host cells from stem cell grafts abrogates GVHD.1-4 However, the elimination of T cells also results in delayed T-cell reconstitution and, thus, an increased rate of infection, particularly with viral agents such as cytomegalovirus, herpes zoster, and Epstein-Barr virus.5-7 In addition, the eradication of mature T cells is associated with an increased risk of graft rejection and an increased incidence of relapse of malignant disease.1,5,8-10 Thus, T cells are required early after allogeneic transplantation and depleting the graft of its T-cell content is not an ideal approach to prevention of complications after transplantation. Although new immunosuppressive agents offer options to decrease the incidence and severity of GVHD, most of the time these strategies are only partially effective and may also increase the incidence of viral and fungal infections and other adverse effects of profound immunosuppression. To provide a solution to this conundrum, selective inactivation or elimination of alloreactive donor T lymphocytes could allow early immune recovery and response toward infectious agents, and potentially preserve graft-versus-leukemia (GVL) activity.11-13 In addition, a strategy to selectively eliminate immunoreactive T cells could represent an important advance for the treatment of a large number of patients with autoimmune disorders.
Recently, there have been significant efforts to try to identify and eliminate T-cell subsets capable of mounting an immune response toward host cells and mediating GVHD. Strategies targeting CD6+ or CD8+ T cells demonstrated convincing potential for the prevention of GVHD in HLA-matched transplants using related and even unrelated donors.14-16 Interestingly, the elimination of donor T-cell subsets did not translate into a greater incidence of graft rejection.17 Moreover, the partial loss of GVL activity that occurs with T-cell depletion was able to be restored through the administration of donor lymphocyte infusions at a time when patients are at lower risk for GVHD.18 Delaying the infusion of donor T cells until the early posttransplantation surge in proinflammatory cytokines has abated may contribute to limiting the development of GVHD.19 However, in the context of HLA-mismatched transplantation, antigenic differences between donor and host cells are particularly immunogenic. In addition, it is extremely difficult to identify a priori T-cell antigens that will be unique markers for cells capable of targeting both major histocompatibility complex (MHC)–disparate antigens and the numerous distinguishing peptide sequences expressed by host tissues.20 In contrast, when donor cells are exposed to host cells ex vivo, cell subsets capable of recognizing host MHC antigens become activated and thus display the peculiar antigenic and biochemical properties rendering these cells “visible.” Importantly, a few monoclonal antibody–based strategies have been developed to specifically eliminate such alloreactive cells.21-25
Rhodamine enters all cells and is extruded from the intracellular milieu through P-glycoprotein (Pgp) active transport.26Pgp, the product of the multidrug-resistance-1 (MDR1) gene, is a protein expressed not only in normal stem cells, but also in T lymphocytes.27-30 Investigators have proposed that T-cell activation may actually lead to the inactivation of Pgp.29Thus, activated T cells should fail to extrude rhodamine. Although rhodamine is not cytotoxic, 4,5-dibromorhodamine methyl ester (TH9402), a rhodamine derivative, was found to harbor important photosensitizing potential.31-33 Its phototoxicity is mediated primarily by singlet oxygen production, with oxidative damage concentrated to mitochondria by the virtue of drug localization.31,34 The structural similarity between rhodamine and TH9402 prompted us to evaluate the capacity of the latter photosensitizing agent to be preferentially retained in Pgp-deficient activated T cells and, thus, lead to their selective elimination after exposure to visible light (514 nm).35
In the present study, we found that photodynamic cell therapy (PDCT) with TH9402 was highly toxic against CD4+ and CD8+ T cells activated in response to mitogens or MHC-mismatched antigens. This PDCT selectively preserved resting T lymphocytes and their ability to proliferate and to demonstrate cytotoxicity toward third-party antigens. PDCT may therefore have clinical utility for the selection of non–MHC-reactive T cells to prevent GVHD and accelerate immune reconstitution after transplantation or for the treatment of immunoreactive disorders. Moreover, our findings identify targeting of MDR1 inhibition as a unique physiologic approach to specifically eliminate activated T cells.
Materials and methods
Human cells
Blood samples were obtained with the informed consent of healthy donors under clinical protocols approved by the Human Subjects Protection Committee of the Maisonneuve-Rosemont Hospital. Peripheral blood (PB) samples were collected in preservative-free heparin and mononuclear cells separated by Ficoll-Hypaque density gradient centrifugation (Ficoll-Paque; Pharmacia, Piscataway, NJ). The T-lymphoblastic cell line CEM and the Pgp-expressing KG1a cells were obtained from the American Type Culture Collection (ATCC, Rockville, MD).
Phytohemagglutinin stimulation
Peripheral blood mononuclear cells (PBMCs) were cultured for 72 hours at a concentration of 3 × 106 cells/mL in flasks (Nunclon, Nunc, Roskilde, Denmark) with 2 μg/mL phytohemagglutinin (PHA; Sigma, St Louis, MO) in X-Vivo 15 medium (Bio-Whittaker, Walkersville, MD) supplemented with 15% human AB serum (HAB; Sigma), 2 mM l-glutamine, 1 mM sodium pyruvate, 100 U/mL penicillin, and 100 μg/mL streptomycin (all from Gibco, Grand Island, NY).
Allogeneic T-cell activation
Activation of responder (A) T lymphocytes against stimulator (B) cells was conducted in a one-way mixed lymphocyte reaction (MLR).36 A and B individuals presented 3 major HLA mismatches. Briefly, responder cells from subject A were cultured for 4 days at 37°C with the same number of irradiated PBMCs from subject B (AB*) (50 Gy; 137Cs; Gamma Cell, Atomic Energy of Canada, Ottawa, ON, Canada) in medium supplemented with 50 U/mL recombinant human interleukin 2 (rhIL-2; R & D Systems, Minneapolis, MN).
Photodynamic cell therapy with TH9402
After in vitro activation, cells were harvested, washed, resuspended at a final concentration of 1 × 106cells/mL, and incubated at 37°C with 10 μM TH9402 (Theratechnologies, Montreal, QC, Canada) in X-Vivo 15 medium with 2.5% HAB. After a 40-minute incubation, cells were centrifuged and dye efflux favored by resuspending cells in TH9402-free medium for 90 minutes. At the end of the latter dye efflux period, cells were exposed to a fluorescent light-scanning device (PDCT-Xerox Series 4, Theratechnologies) delivering 5 J/cm2 at a wavelength of 514 nm.
T-cell proliferation assay
Proliferative activity of responder cells exposed to photodynamic therapy and nontreated controls was assessed on day 5 of an MLR in a standard 3H-thymidine labeling assay. The total number of cells (responder [A] and irradiated stimulator [B] cells) present before PDCT was not adjusted after PDCT. These cells were restimulated with a fixed number (1 × 105) of irradiated stimulator (B*) or third-party (C*) cells at different responder/stimulator cell ratios (2:1, 1:1, 1:2, and 1:4) in 96-well U-bottomed microtiter plates (Nunc). Cultures in triplicate were labeled with 1 μCi (0.037 MBq) 3H-thymidine (Perkin Elmer, Woodbridge, ON, Canada) per well for 18 hours and harvested onto glass fiber filter mats; 3H-thymidine incorporation was measured using a liquid scintillation counter (Wallac, Gaithersburg, MD).
Cytotoxic T-lymphocyte precursor and limiting dilution assays
Limiting dilution assays (LDAs) were used to calculate the frequencies of responding cytotoxic T-lymphocyte precursor (CTLp) cells and clonogenic CEM and KG1a cells in treated and untreated conditions using previously described methods.37 38 Briefly, to determine CTLp frequency, 24 replicates of graded numbers of treated or untreated responder (A) cells (3 × 106 to 450 cells/well) were seeded in 96-well microtiter plates in the presence of 1 × 105 irradiated (50 Gy) fresh PBMC stimulator cells (B or C). Control wells consisted of stimulator cells only. After 9 days of culture in medium supplemented with 50 U/mL rhIL-2, each well was tested for cytolytic activity against 5 × 103initial stimulator (B) and third-party (C) cells using a standard51Cr-release assay. The supernatant (100 μL) was harvested from each well and counted in a γ counter. Spontaneous release was less than 15%. Results for individual wells were expressed as a percentage of specific lysis calculated as follows: percent specific lysis = 100 × (experimental release − spontaneous release [medium only])/(maximum release [1% Triton X-100] − spontaneous release). To measure CEM and KG1a, clonogenic cell frequencies, cells were grown in a similar LDA (from 5 × 105 to 0.5 cells/well) in RPMI 1640 medium (Life Technologies, Gaithersburg, MD) supplemented with 10% fetal bovine serum, fed every 4 days, and scored for growth under an inverted phase microscope.
Immunophenotypic analysis
Expression of cell surface T-cell antigens was evaluated by direct immunofluorescence using standard techniques.39Monoclonal antibodies (mAbs) used in this study were anti-CD3–fluorescein isothiocyanate (UCHT1), anti-CD25–phycoerythrin (B1.49.9), anti-CD3–allophycocyanin (APC; UCHT1; Coulter Immunology, Hialeah, FL), anti-CD4–APC (RPA-T4), anti-CD8–APC (RPA-T8; Pharmigen, San Diego, CA). Nonspecific binding was determined using appropriate isotypic controls. Immunofluorescence reactivity was determined by automated multiparameter flow cytometry analyzing at least 104 cells in each sample (FACSCalibur; Becton Dickinson, Mountain View, CA) and processed using Cell Quest software (Becton Dickinson).
Hematopoietic progenitor cell assay
Peripheral blood cells from healthy donors mobilized with granulocyte colony-stimulating factor (G-CSF; Amgen, Thousand Oaks, CA) underwent PDCT and were plated in methylcellulose medium (MethoCult H4434; Stem Cell Technologies, Vancouver, BC, Canada) on 35-mm plastic culture dishes, according to the manufacturer's instructions. Colony-forming units–granulocyte-macrophage (CFU-GMs) and colony-forming units–mix (CFU-mix) were scored after 14 days of culture at 37°C in a fully humidified 5% CO2atmosphere.38
Functional evaluation of Pgp-170
Pgp substrate efflux modulation by cyclosporin A (CSA) and verapamil was determined in an accumulation assay using TH9402. Cells were stained with 10 μM TH9402 for 40 minutes, washed, and resuspended in either medium alone, with 1 μg/mL CSA (Novartis Pharma, Dorval, QC, Canada) or 5 μg/mL verapamil (Sabex, Boucherville, QC, Canada). Cellular retention of the dye was assessed by flow cytometry (FACSCalibur, Becton Dickinson).40 41Calibration beads were used in all experiments to ensure stable energy delivery (Calibrate3 and -APC, Becton Dickinson). Positive controls for functional Pgp expression consisted of KG1a cells.
Statistical analysis
To determine CTLp frequency, experimental wells were scored positive if the percent specific lysis of a well exceeded the mean + 3 SD of the wells where only stimulator cells were present. Cytotoxicity and clonogenicity at each serial cell concentration were assessed in an “all-or-nothing” (positive or negative) fashion, and frequency within the test population was estimated by use of χ2 minimization.38 42
Results
Photodynamic elimination of T cells
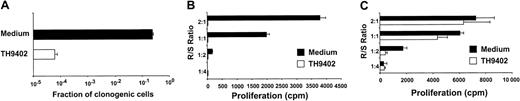
To assess the sensitivity of proliferating T cells to TH9402 PDCT, the lymphoblastic T-cell line CEM was used as target. Cells were incubated for 40 minutes with 10 μM TH9402, washed, and incubated with TH9402-free medium for 90 minutes and then exposed to light (5 J/cm2). These parameters were previously found to eliminate more than 99.9% of malignant K562 cells, while preserving more than 50% of hematopoietic progenitor cells.32 TH9402 PDCT when compared to untreated controls depleted more than 99.97% of CEM T cells measured by LDA (Figure1A).
TH9402 eliminates proliferating T cells.
(A) CEM T cells were incubated with 10 μM TH 9402 for 40 minutes, washed, and exposed to 5 J/cm2 light after a dye efflux period of 90 minutes. Survival of clonogenic cells for treated (white) and untreated (black) cells was evaluated using a limiting dilution assay. (B) PBMCs exposed to PHA or (C) medium only for 72 hours underwent PDCT. Proliferation to MHC-incompatible cells was evaluated in an MLR at different responder-stimulator (R/S) ratios. Results are expressed as mean ± SEM of experiments performed in triplicate.
TH9402 eliminates proliferating T cells.
(A) CEM T cells were incubated with 10 μM TH 9402 for 40 minutes, washed, and exposed to 5 J/cm2 light after a dye efflux period of 90 minutes. Survival of clonogenic cells for treated (white) and untreated (black) cells was evaluated using a limiting dilution assay. (B) PBMCs exposed to PHA or (C) medium only for 72 hours underwent PDCT. Proliferation to MHC-incompatible cells was evaluated in an MLR at different responder-stimulator (R/S) ratios. Results are expressed as mean ± SEM of experiments performed in triplicate.
The potential of this TH9402-based PDCT method to eliminate activated T lymphocytes was evaluated by comparing proliferative responses of treated (PDCT) versus untreated PHA-activated normal PBMCs toward MHC-mismatched stimulator cells. In untreated controls, PHA-stimulated cells were able to proliferate when subsequently exposed to MHC-disparate stimulatory cells in an MLR (Figure 1B). In contrast, TH9402 PDCT completely abrogated the response of PHA-stimulated cells to MHC-mismatched cells. The specificity of PDCT for activated cells was evaluated by treating resting PBMCs, incubated in IL-2 containing medium only, and then measuring proliferative response in an allogeneic mismatch MLR (Figure 1C). Interestingly, PDCT did not affect the response of these resting cells, a finding that indicates a higher level of sensitivity to PDCT for primed versus resting T cells.
Depletion of alloreactive T-lymphocyte subsets
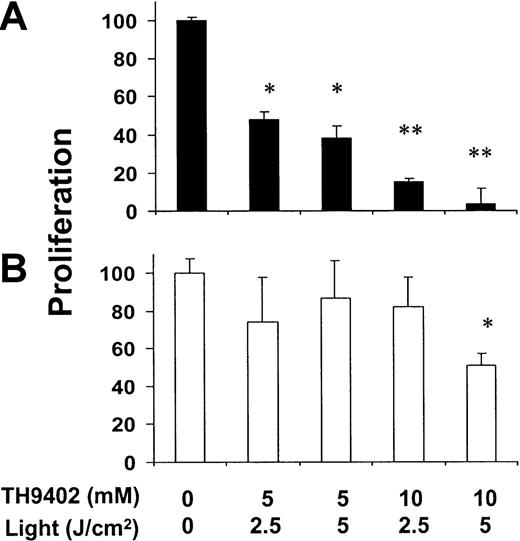
The clinical application of PDCT in the context of allogeneic transplantation must rely on both specific elimination of T cells that are reactive toward host cells and preservation of T cells capable of subsequent response to infectious or other foreign antigens. To clarify this issue, PBMCs (individual A) were first exposed for 4 days to allogeneic stimulator (individual B) cells mismatched at 3 MHC loci (A, B, and DR). After this activation process, cells were exposed to PDCT and then presented with either the same stimulator (B) cells for a second time or with third-party (C) cells in a conventional3H-thymidine incorporation assay (Figure2). Increasing concentrations of TH9402 and light intensity induced gradually decreasing proliferation toward stimulator B cells. In contrast, the capacity of residual cells to proliferate when exposed to third-party C cells was preserved except at the highest treatment intensity. Moreover, to discriminate between the effect of TH9402, light, and PDCT, A cells were treated with either TH9402 alone, light alone, or TH9402 PDCT. Proliferative responses were preserved after exposure to either TH9402 without light or to light alone (data not shown; P = NS). The highest TH9402 concentration (10 μM) and light intensity (5 J/cm2) were selected for all subsequent experiments because these achieved maximum elimination of specific alloreactivity and only slightly affected response to third-party C cells.
Effect of TH9402 concentration and light intensity on the depletion of host- and third-party–reactive T cells.
Donor cells were first primed against host cells in a one-way, 4-day MLR, and then treated with increasing concentrations of TH9402 and light intensity. (A) After treatment, cells were cocultured with irradiated stimulator cells from the same host or (B) third-party cells for 5 days and proliferation was measured after addition of3H-thymidine (*P < .05; **P < .01). Results are expressed as mean ± SEM of the percentage of proliferation of the untreated control at an R/S ratio of 2:1; experiments were performed in triplicate.
Effect of TH9402 concentration and light intensity on the depletion of host- and third-party–reactive T cells.
Donor cells were first primed against host cells in a one-way, 4-day MLR, and then treated with increasing concentrations of TH9402 and light intensity. (A) After treatment, cells were cocultured with irradiated stimulator cells from the same host or (B) third-party cells for 5 days and proliferation was measured after addition of3H-thymidine (*P < .05; **P < .01). Results are expressed as mean ± SEM of the percentage of proliferation of the untreated control at an R/S ratio of 2:1; experiments were performed in triplicate.
Immunophenotypic analysis
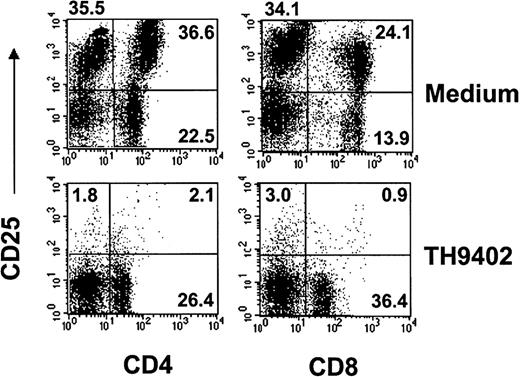
To evaluate the specificity of PDCT for activated T lymphocytes, the expression of the inducible α chain of the IL-2 receptor (IL-2R; CD25) was measured on CD4+ and CD8+ T-cell populations from treated samples and untreated controls (Figure3). At the end of 4-day MLR, cells were exposed to TH9402 PDCT or medium and immunophenotypic analysis performed after a 3-day culture in medium containing IL-2. At least 98% of CD8+CD25+ cells and 96% of CD4+CD25+ cells were eliminated by PDCT when measured by flow cytometry (Table 1). In contrast, most unactivated (CD25−) T cells were spared; their increased proportion after PDCT confirms the selectivity of PDCT for activated lymphocytes. In addition, CD25− T cells also stained negatively for propidium iodide, a finding that indicates preservation of T-cell integrity (data not shown).
TH9402 PDCT eliminates activated CD4+ and CD8+ cells.
MLR-activated cells underwent TH9402 PDCT and after 72 hours, T-cell populations were assessed for CD25 expression using flow cytometry. Numbers indicate the percentage of cells, and dot plots are representative of 3 experiments.
TH9402 PDCT eliminates activated CD4+ and CD8+ cells.
MLR-activated cells underwent TH9402 PDCT and after 72 hours, T-cell populations were assessed for CD25 expression using flow cytometry. Numbers indicate the percentage of cells, and dot plots are representative of 3 experiments.
Impact of TH9402 PDCT on activated and nonactivated CD4+ and CD8+ cells evaluated using flow cytometry
| Treatment† . | CD4+cells (×106 cells) . | CD8+ cells (×106 cells) . | ||
|---|---|---|---|---|
| CD25+ . | CD25− . | CD25+ . | CD25− . | |
| Medium | 1.68 ± 0.61 | 0.96 ± 0.69 | 1.11 ± 0.40 | 0.50 ± 0.34 |
| TH9402 | 0.060 ± 0.011 | 0.21 ± 0.09 | 0.026 ± 0.012 | 0.26 ± 0.09 |
| Treatment† . | CD4+cells (×106 cells) . | CD8+ cells (×106 cells) . | ||
|---|---|---|---|---|
| CD25+ . | CD25− . | CD25+ . | CD25− . | |
| Medium | 1.68 ± 0.61 | 0.96 ± 0.69 | 1.11 ± 0.40 | 0.50 ± 0.34 |
| TH9402 | 0.060 ± 0.011 | 0.21 ± 0.09 | 0.026 ± 0.012 | 0.26 ± 0.09 |
After exposure to TH9402 PDCT or medium, AB
cells were cultured for 3 days in medium supplemented with IL-2. Absolute cell numbers are expressed in million cells (mean ± SEM of 3 experiments).
CTLp frequency after PDCT
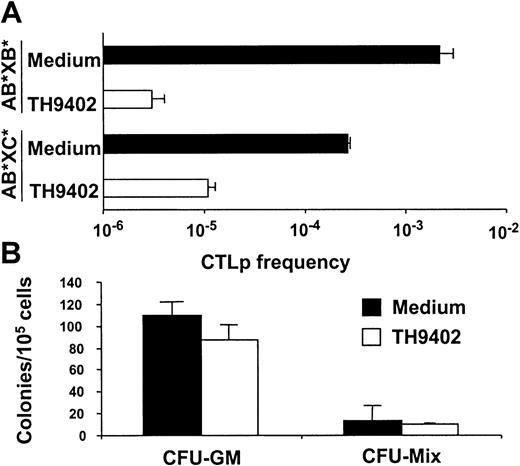
To confirm the specificity of PDCT for antihost T cells, CTLp cells were enumerated following treatment with TH9402 or medium using LDA. The number of CTLp cells active against B and C cells was determined after TH9402 PDCT of A cells MLR-primed against B cells (AB*) (Figure 4A). In untreated samples, more anti-B than anti-C CTLp cells were detected, a finding probably reflecting the primary nature of the immune response against C cells versus the secondary immune reaction against B cells. TH9402 PDCT eliminated anti-B cell CTLp cells by 1000-fold, but anti-C cell CTLp frequency was decreased by only 30-fold, confirming the preferential targeting of previously activated CTLp cells.
PDCT eliminates antistimulator and preserves anti–third-party CTLp and normal hematopoietic progenitor cells.
(A) Effect of TH9402 and medium treatment of MLR-activated cells (AB*) on the frequency of CTLp cells directed against stimulator B* and third-party C* cells. Results are expressed as mean ± SEM of 3 experiments. (B) Mobilized PB progenitors from 5 healthy donors were treated with TH9402 or medium. Results are expressed as mean ± SEM of CFU-GM and CFU-mix colonies.
PDCT eliminates antistimulator and preserves anti–third-party CTLp and normal hematopoietic progenitor cells.
(A) Effect of TH9402 and medium treatment of MLR-activated cells (AB*) on the frequency of CTLp cells directed against stimulator B* and third-party C* cells. Results are expressed as mean ± SEM of 3 experiments. (B) Mobilized PB progenitors from 5 healthy donors were treated with TH9402 or medium. Results are expressed as mean ± SEM of CFU-GM and CFU-mix colonies.
Progenitor recovery after PDCT
The effect of PDCT on mobilized PB cells from healthy individuals (n = 4) was used to assess the toxicity of the procedure for other spontaneously proliferating cells. These cells were exposed to the same PDCT conditions as for T-cell depletion. Survival of hematopoietic progenitor cells was evaluated using a semisolid culture assay (Figure 4B). These conditions, which induced 100- to 1000-fold decreases in activated T cells, did not cause a significant decrease in the growth of CFU-GM (P = .09) nor CFU-mix colonies (P = 0.1).
Kinetics of dye retention
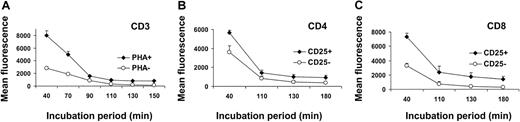
The specificity of TH9402-mediated killing for activated T cells could be due to a differential accumulation or retention of the dye in resting versus activated T cells. To test this hypothesis, TH9402 influx/efflux kinetics were evaluated in PHA-stimulated and resting lymphocytes. At the end of the incubation period, retention of the dye was higher in PHA-stimulated CD3+ cells than in resting lymphocytes (Figure 5A). In addition, activated lymphocytes continued to sequester more TH9402 over time than resting lymphocytes, even after reaching the plateau phase (P < .05). Moreover, after an MLR, CD25-expressing T lymphocytes, whether CD4+ or CD8+, retained more TH9402 compared to CD25− T cells (P < .05; Figure 5B-C). These data indicate that both TH9402 accumulation and TH9402 retention are increased in the proliferating and activated T cells.
Kinetics of incorporation of TH9402 in resting and activated lymphocytes.
(A) TH9402 dye retention was analyzed in the CD3+ cells from samples incubated (diamonds) or not (circles) with PHA. (B) Dye retention was also measured in activated (CD25+) and nonactivated (CD25−) CD4+ and (C) CD8+ cells within the same MLR-activated sample. MFI ± SEM of 3 to 6 experiments and P < .05 for all evaluations.
Kinetics of incorporation of TH9402 in resting and activated lymphocytes.
(A) TH9402 dye retention was analyzed in the CD3+ cells from samples incubated (diamonds) or not (circles) with PHA. (B) Dye retention was also measured in activated (CD25+) and nonactivated (CD25−) CD4+ and (C) CD8+ cells within the same MLR-activated sample. MFI ± SEM of 3 to 6 experiments and P < .05 for all evaluations.
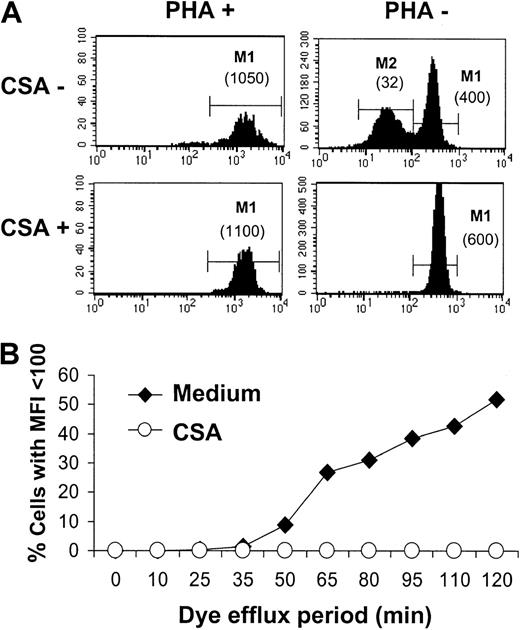
Interestingly, resting T cells not only incorporated lower levels of TH9402 than PHA-activated cells, but also a large proportion of the former cells demonstrated a second peak of lower fluorescence intensity (Figure 6A). This bimodal distribution indicates the existence among resting cells of 2 populations with a different propensity to eliminate the dye. Similar results obtained using MLR-activated T cells (data not shown) indicate that both TH9402 accumulation and TH9402 retention are increased in the proliferating and activated T cells.
Impact of PgP inhibition on TH9402 content in resting and activated lymphocytes.
(A) PHA-stimulated and resting lymphocytes were stained with TH9402 for 40 minutes and resuspended in medium alone or with CSA. Flow cytometric assessment of TH9402 content in CD3+ cells was performed 90 minutes after the end of the incubation period. Numbers in parentheses indicate the MFI of corresponding cell populations. (B) The impact of CSA exposure on the proportion of PHA-stimulated lymphocytes capable of eliminating TH9402 (MFI < 100 U) was measured over time, starting after completion of the 40-minute incubation period. The results are representative of 3 experiments.
Impact of PgP inhibition on TH9402 content in resting and activated lymphocytes.
(A) PHA-stimulated and resting lymphocytes were stained with TH9402 for 40 minutes and resuspended in medium alone or with CSA. Flow cytometric assessment of TH9402 content in CD3+ cells was performed 90 minutes after the end of the incubation period. Numbers in parentheses indicate the MFI of corresponding cell populations. (B) The impact of CSA exposure on the proportion of PHA-stimulated lymphocytes capable of eliminating TH9402 (MFI < 100 U) was measured over time, starting after completion of the 40-minute incubation period. The results are representative of 3 experiments.
Pgp involvement in TH9402 efflux
To study mechanisms of retention of the TH9402 rhodamine derivative, we focused on Pgp, which has been previously described as the main channel involved in rhodamine efflux.43 44 In resting T lymphocytes, inactivation of Pgp by CSA led to a disappearance of the peak of lower fluorescence intensity (M2: mean fluorescence intensity (MFI) = 32) and gave rise to a single peak of TH9402 fluorescence (M1) demonstrating slightly higher retention of the dye (MFI = 600) than the M1 peak of the CSA-unexposed sample (MFI = 400; Figure 6A). In contrast, inactivation of theMDR1 channel had no major impact on retention of the dye in PHA-stimulated lymphocytes. Moreover, the effect of CSA on TH9402 efflux was durable and prevented the appearance of CD3+ T cells with low concentrations of dye for more than 2 hours (Figure6B).
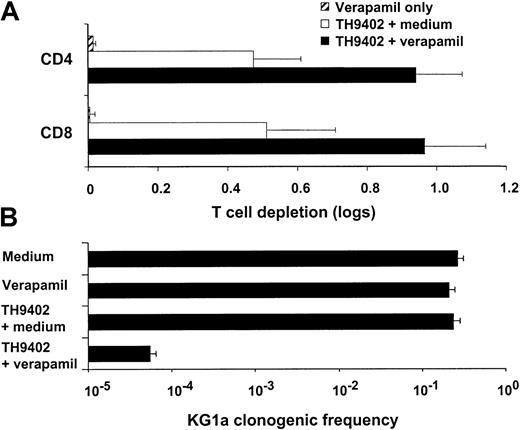
To determine if the higher retention of the dye caused by Pgp inhibition translated into higher cytotoxicity, we incubated resting T cells with verapamil and exposed them to PDCT. This MDR1inhibitor significantly enhanced the PDCT elimination of resting T cells (P < .01; Figure 7A). To investigate the extent of MDR1 involvement in TH9402-mediated effects, we repeated the same experiment using the KG1a cell line, which demonstrates high levels of constitutive expression of Pgp.45 Phototherapy with a lower dose (5 μM) of TH9402 was not cytotoxic to KG1a cells, but the addition of verapamil led not only to increased retention of the dye (data not shown), but also to depletion of 99.99% of clonogenic cells (Figure 7B). When used without PDCT, verapamil did not deplete T or KG1a cells. These findings clearly identify Pgp as the principal modulator of TH9402 cellular concentration and photodynamic cytotoxicity.
Down-modulation of MDR1 function enhances TH9402-mediated cytotoxicity.
(A) Resting PBMCs were exposed to 10 μM TH9402 in medium supplemented or not with verapamil. Elimination of CD4+ and CD8+ cell populations was measured 3 days after PDCT using flow cytometry and compared with untreated controls. Inhibition ofMDR1 function increased the photodynamic elimination of T cells. (B) Cytotoxicity of PDCT on KG1a cells, anMDR1-expressing cell line, was measured using an LDA. Verapamil alone or PDCT with 5 μM TH9402 had no effect on KG1a cells but combining the inhibition of MDR1 with verapamil to TH9402 PDCT resulted in the elimination of more than 3 logs of clonogenic cells. Results are expressed as mean ± SEM of at least 2 experiments.
Down-modulation of MDR1 function enhances TH9402-mediated cytotoxicity.
(A) Resting PBMCs were exposed to 10 μM TH9402 in medium supplemented or not with verapamil. Elimination of CD4+ and CD8+ cell populations was measured 3 days after PDCT using flow cytometry and compared with untreated controls. Inhibition ofMDR1 function increased the photodynamic elimination of T cells. (B) Cytotoxicity of PDCT on KG1a cells, anMDR1-expressing cell line, was measured using an LDA. Verapamil alone or PDCT with 5 μM TH9402 had no effect on KG1a cells but combining the inhibition of MDR1 with verapamil to TH9402 PDCT resulted in the elimination of more than 3 logs of clonogenic cells. Results are expressed as mean ± SEM of at least 2 experiments.
Discussion
Selective elimination of donor T-cell subsets recognizing host histocompatibility antigens represents an appealing strategy to eradicate GVHD. However, to limit complications with viral and fungal infections, graft rejection, and relapse, which occur after physical or functional T-cell depletion, T cells capable of generating an immune response toward foreign antigens must be preserved.5,11,46Nevertheless, the specific elimination of such host-reactive T cells represents a difficult task. In our present study, we uncovered a unique cytotoxic pathway that takes advantage of the intrinsic modulation of the Pgp channel transporter to eradicate immunoreactive T cells. Activated T lymphocytes demonstrated preferential accumulation and retention of the TH9402 rhodamine derivative over resting T cells. Indeed, we found that resting T lymphocytes, which expressMDR1,29 extruded TH9402 through this channel transporter, whereas the cellular activation process led to an impairment in MDR1-mediated TH9402 efflux. These kinetics of accumulation of TH9402 have resulted in the photodynamic eradication of responder cells immunized ex vivo against stimulator cells, in conditions simulating MHC-mismatched transplantation. In addition, this PDCT achieved drastic elimination of IL-2R–expressing CD4+ and CD8+ cell populations. Notably, these findings translated into highly efficient depletion of host-reactive CTLp cells. Moreover, the efficiency of Pgp spared resting T cells and preserved their ability to generate proliferative and cytotoxic responses against antigens other than host MHC.
Immunologic tolerance may result from a variety of mechanisms, including deletion, anergy, ignorance, and suppression.47-51 PDCT using the TH9402 photosensitizer abrogated antihost reactivity of T lymphocytes activated either with mitogens or with an allogeneic MLR. Flow cytometric evaluations demonstrated that most of the effect of TH9402 treatment was attributable to depletion of alloreactive T cells expressing CD25, the inducible high-affinity IL-2R. Both activated CD4+ and CD8+ cells were sensitive to the PDCT shown by the detection of less than 1% of the total pool of lymphocytes expressing CD25 after treatment. Although the scant number of CD25+ cells detected could represent activated T cells escaping photodynamic eradication, it is also possible that they correspond to cells bound to die from lethal damage of PDCT-mediated oxidative damage. Alternatively, these lymphocytes could represent nonactivated T lymphocytes, such as regulatory T cells, which have been found to constitutively express CD25.52-54 The preservation of a regulatory T-cell population would be particularly useful because it has been shown to play an important role in induction of tolerance to alloantigen via costimulatory blockade.55The latter scenarios would explain why the evaluation of the impact of PDCT on CTLp cells demonstrated greater elimination of antihost clonogenic cytotoxic precursors (Figure 4A) than of CD25+T-cell populations (Figure 3). Moreover, the 3 logs of depletion of CTLs observed with the LDA is of the same order as the threshold of 2 to 3 logs of T-cell depletion thought to be required for the prevention of GVHD.56
Because T-cell–receptor diversity after transplantation is decreased according to the number of T cells present in the graft, it is crucial that as many T cells as possible be spared.57Our results demonstrate that although phototherapy using TH9402 is highly toxic for activated T cells, it remains selective and preserves a large proportion of the CD4+ and CD8+ cells that do not express the IL-2 high-affinity receptor and other cell lineages such as myeloid and erythroid progenitors. Interestingly, the administration of such T cells has the potential to restore T-cell–receptor diversity through expansion in response to homeostatic signals of the host and to reconstitute the peripheral T-cell pool.58,59 Indeed, nonactivated T cells remain immunocompetent and able to proliferate in response to new antigenic stimuli, whether previously cultured in IL-2 only or mitogens, or stimulated with allogeneic cells. This is corroborated by the ability of TH9402-exposed cells to generate CTLp cells against third-party antigens, a finding that also confirms the selectivity of PDCT. Future studies will challenge us to determine if the small decrease in reactivity toward third-party cells observed after PDCT could reflect the elimination of T-cell clones with dual specificity, which demonstrate the capacity to react toward both host and third-party cells.60 61
Because only T cells recognizing an antigen expressed by stimulator cells will be eliminated, PDCT should spare T cells recognizing tumor antigens (developmentally regulated antigens or leukemia-specific antigens) provided care is taken to exclude neoplastic cells from the stimulator cell population.62,63 Natural killer (NK) cells also express high levels of Pgp and should be protected from PDCT toxic effects.28,64 Although we demonstrate here that PDCT is effective at eliminating T cells reactive to stimulator cell MHC antigens, the effect of PDCT on minor histocompatibility antigen (MiHA)–stimulated T cells has yet to be addressed.36,65In addition, the observation that TH9402-treated T cells respond to third-party MHC antigens indicates preservation of T-cell signaling and effector pathways that should translate into elimination of viral and fungal invaders.22 Moreover, the addition of donor T cells, although nonreactive toward host MHC antigens, could help lower the incidence of graft rejection associated with T-cell depletion.3,5,8,20 Indeed, T cells present after PDCT have the potential to act as veto cells to block antidonor reactivity of host T cells without requiring recognition of host alloantigens.66 67 In future studies, it will be important to delineate the nature of the various T and NK cell populations that escape elimination by TH9402 PDCT and their contribution to the prevention of immunologic and infectious complications after transplantation.
Our findings indicate that TH9402 PDCT does not exhibit a broad antiproliferative effect, but rather acts specifically against activated T cells according to intrinsic physiologic properties of target cells. Modulation of Pgp activity, which results in differential retention and cytotoxicity from TH9402, could reflect biomechanical modifications of such channel transporters with the activation process.68,69 Interestingly, Pgp could also be inactivated by PDCT itself,70 an inhibitory mechanism that would augment retention of the photosensitizer in those activated T cells with partial inhibition of MDR1 function, without affecting resting T cells that have already extruded most of the dye at the time of light application. The increased retention of TH9402, and potentially of its photoproducts, in activated cells could enhance the efficacy and specificity of the treatment. Although we cannot exclude a contribution of metabolic changes induced by T-cell activation to altered mitochondrial targeting by this rhodamine derivative,71 our findings clearly indicate that Pgp plays an important role in the intracellular handling of TH9402 and identify a novel approach that takes advantage of the functional inhibition of this pathway of resistance to selectively eradicate activated T cells.
The current photodynamic approach could be applied directly for the ex vivo treatment of stem cell grafts or donor lymphocyte infusions to prevent GVHD in the context of MHC-mismatched allogeneic transplantation.72,73 Moreover, recent identification and sequencing of several MiHAs and improvements in immunization strategies using dendritic cells should facilitate the activation of effector cells directed at host MiHAs.74 This may broaden the applicability of this PDCT to allogeneic HLA-matched transplant strategies. Finally, the selectivity of TH9402 PDCT for activated T lymphocytes could be exploited for the targeted elimination of both alloreactive T-cell clones that develop after solid organ transplants and autoreactive clones responsible for diseases such as lupus erythematosus, rheumatoid arthritis, and systemic sclerosis.75-78
The authors thank Drs B. Leonard, N. Beauger, and G. Krosl for insightful scientific advice; Dr M. A. Caligiuri for critical review of the manuscript; and C. LeHouillier and the members of the Cell Therapy Laboratory and Apheresis Unit for their excellent technical assistance. We thank all scientists at Theratechnologies Inc. for their close collaboration and technical support.
Prepublished online as Blood First Edition Paper, April 17, 2002; DOI 10.1182/blood-2001- 12-0353.
Supported by a grant from Theratechnologies-FRSQ and the Cancer Research Society of Canada. D.C.R. is the recipient of a clinician-scientist award of the FRSQ.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Denis Claude Roy, Department of Hematology-Immunology, Maisonneuve-Rosemont Hospital Research Center, 5415 L'Assomption Blvd, Montreal, QC, H1T 2M4, Canada; e-mail:denis-claude.roy@umontreal.ca.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal