Abstract
Numerous studies have implicated bacteria in cardiovascular disease, but there is a paucity of information on the mechanism involved. In this study we show how the common oral bacteriumStreptococcus sanguis can directly interact with platelets, resulting in activation and aggregate formation. Platelet aggregation was dependent on glycoprotein IIb/IIIa (GPIIb/IIIa) and thromboxane. Platelets could also directly bind to S sanguis, but this interaction was not inhibited by GPIIb/IIIa antagonists. Antibodies to GPIb could inhibit both platelet aggregation and platelet adhesion to bacteria. This suggested a direct interaction between GPIb and S sanguis; however, this interaction did not require von Willebrand factor, the normal ligand for GPIb. By use of a range of monoclonal antibodies to GPIb and the enzyme mocharagin, which cleaves GPIb at amino acid 282, the interaction was localized to a region within the N-terminal 1-225 portion of GPIbα. Furthermore S sanguisfailed to induce aggregation of platelets from a patient with Bernard-Soulier disease, the organism bound to Chinese hamster ovary cells transfected with the GPIbα gene but did not bind to mock-transfected cells and biotin-labeled S sanguis cells bound to purified GPIb in ligand blots. It is suggested that the interaction between S sanguis and GPIb is important in the pathogenesis of infective endocarditis and may also play a contributory role in some cases of myocardial infarction.
Introduction
Recent reports suggest a role for infectious agents in cardiovascular disease. Much of this work is in the form of clinical evidence of infection1-6 or the effect of antibiotics on the incidence of cardiovascular disease.7,8Although studies have found evidence of bacteria in atherosclerotic plaques,1,2,4,5 9-13 their role in the etiology of cardiovascular disease is uncertain. In contrast, the role of bacteria in infective endocarditis is well established and the molecular mechanisms involved may also occur in other forms of cardiovascular disease.
Infective endocarditis involves inflammation of the heart valves due to infection and if untreated can lead to valve failure and death. In most cases there is one or more predisposing factors, which results in damage to the endothelium on or adjacent to the valves. This area of damage becomes covered with a platelet-fibrin vegetation and these can become colonized by bacteria that gain access to the blood. The 2 species most commonly involved are Streptococcus sanguis14 and Staphylococcus aureus.15 Historically oral streptococci have been referred to as Streptococcus viridans, but this name was never accepted as a recognized taxon because of the biochemical and serologic heterogeneity among isolates. Subsequent detailed biochemical and genetic studies allowed the definition of at least 17 taxa within what was originally called S viridans. However, the term viridans has survived but is now used as viridans group of streptococci to recognize the existence of various taxa and S sanguis is one taxon within this group of organisms. Studies using animal models of endocarditis caused by S sanguis have shown that the severity of the disease is associated with an ability of the infecting organism to adhere to and cause aggregation of platelets.16 17
Platelets are anucleated cellular fragments of megakaryocytes, which, when activated, aggregate to form a thrombus. Activation can be mediated by many different agonists that lead to fibrinogen binding to its receptor, glycoprotein (GP) IIb/IIIa, and cross-linking of platelets into aggregates.18,19 When the endothelium is damaged, platelet GPIb can bind to von Willebrand factor (VWF) in the subendothelial matrix resulting in platelet activation20and deposition of platelets on the area of damage. Thrombus formation on ruptured atherosclerotic plaques is also important in myocardial infarction21 and has led to the use of antiplatelet agents for treatment and prevention.22
Streptococcus sanguis has been shown to induce platelet aggregation in a thromboxane-dependent manner requiring adenosine diphosphate (ADP) secretion.23 It has also been shown to be complement and Fc-receptor (FcγRIIA)–dependent24,25 and inhibited by an antibody to GPIb.26 In addition, Gong and colleagues have reported an interaction between a S sanguis protein and 2 platelet proteins (175 and 230 kd),27 one or both of which may be platelet collagenlike receptors.28 29 These various studies used different strains of S sanguis making comparison of the data difficult but suggesting that either strains differ in their mechanism of interaction with platelets or that there is more than one interaction operating simultaneously.
In this study, we have further investigated the roles of GPIb and FcγRIIA in the interaction between S sanguis and platelets and show that the organism interacts directly with platelet GPIb.
Materials and methods
Materials
The ADP, arachidonic acid, and collagen were from Bio-Data (Horsham, PA). Thrombin receptor-activating peptide SFLLRN (TRAP) was synthesized by Dr Patrick Harriot (Queens University, Belfast, Northern Ireland). Monoclonal antibody AN51 was purchased from Dako (Glostrup, Denmark), VM16-d from Sanbio (Uden, The Netherlands), SZ-2 from Calbiochem (Nottingham, United Kingdom), P6/40 from Serotec (Oxford, United Kingdom), and the anti-FcγRIIA antibody (CD32), IV-3, from Medarex (Annandale, NJ). Botrocetin was purchased from Pentapharm (Basle, Switzerland) and ristocetin from Biopoole (Ventura, CA). Abciximab was obtained from Lilly (Leiden, The Netherlands), orbofiban was a gift from Dr Robert Anders (GD Searle, Skokie, IL), and eptifibatide was a gift from Dr David Philips (COR Therapeutics, San Francisco, CA). The 546-labeled goat antimouse antibody and fluorescein isothiocyanate (FITC)–antimouse antibody were from Molecular Probes (Leiden, The Netherlands). All tissue culture reagents were purchased from Gibco (Paisley, United Kingdom). Bovine serum albumin (BSA) was purchased from Calbiochem-Novabiochem (La Jolla, CA). The Chinese hamster ovary (CHO) GPIbβ/IX cells were a gift from Dr José Lopez (Department of Molecular and Human Genetics, Baylor College of Medicine, Houston, TX). Platelet glycoprotein kits were obtained from BioCytex (Marseille, France). Mocarhagin was a gift from Dr Michael Berndt (Baker Research Medical Institute, Praham, Victoria, Australia). Thromboxane B2(TXB2) was measured by enzyme immunoassay (EIA) kits purchased from R & D Systems (Minneapolis, MN). Brain-heart infusion (BHI) media was from Oxoid (Basingstoke, United Kingdom). Other laboratory reagents were from Sigma (Poole, United Kingdom).
Bacterial strains and growth
Streptococcus sanguis strain 133-79 was originally isolated from a blood culture of a confirmed case of bacterial endocarditis and was a kind gift from Dr Mark Herzberg (Department of Preventive Science, University of Minnesota, Minneapolis). Other clinical strains were supplied by hospital laboratories in the United Kingdom as isolates from infective endocarditis cases. The laboratory strains of S sanguis that were used were strain NCTC 7863, the type strain obtained from the National Collection of Type Cultures, Colindale, London and SK96, which was a kind gift from Dr R.A. Whiley (St Bartholomew's and the London Hospital Medical and Dental College, United Kingdom). Strains were maintained on blood agar and subsequently grown in BHI broth (Oxoid, Poole, United Kingdom) overnight at 37°C for use in experiments. Bacteria were harvested and washed by centrifugation at 15 000g for 5 minutes and finally suspended in phosphate-buffered saline (PBS), pH 7.5. For platelet aggregation studies, bacterial suspensions were adjusted to 7 × 109 cells/mL and for adhesion studies adjusted to 3 × 109 cells/mL. For experiments in which bacteria were used to probe Western blots of glycocalicin, 133-79 and NCTC 7863 were labeled with biotin-NHS N-hydroxysuccinimide ester (Sigma) in PBS, pH 7.7 for 4 hours at room temperature, followed by extensive washing in PBS. For experiments in which bacteria were assessed for the ability to adhere to glycocalicin in enzyme-linked immunosorbent assay (ELISA) wells, 133-79 and NCTC 7863 were labeled with FITC (10 μg/mg wet weight of bacteria in 50 mM carbonate-bicarbonate buffer, pH 8.2 overnight at 4°C with mixing) and unconjugated FITC was removed by extensive washing with centrifugation. Suspensions were adjusted to an absorbance of 1.0 at 600 nm.
Platelet preparation
For routine assays, blood was drawn (using a 19-gauge butterfly needle) from the antecubital vein of healthy human volunteers who had not taken any nonsteroidal anti-inflammatory drugs during the previous 10 days. Nine volumes of blood were added to 1 volume of 3.8% sodium citrate or acid-citrate-dextrose (ACD). Platelet-rich plasma (PRP) was prepared by centrifugation of anticoagulated whole blood at room temperature at 150g for 10 minutes. The blood remaining after removing the PRP was centrifuged at 630g for 10 minutes at room temperature to yield platelet-poor plasma (PPP).
In some experiments platelets were collected from patients with specific bleeding disorders. Whole blood collected from a well-characterized patient with Bernard-Soulier syndrome30was allowed sediment at room temperature for 3 hours to obtain PRP. Platelets were tested for normal responses to arachidonic acid (0.5 mg/mL) or ADP (2 mM). Changes in light transmission were recorded against autologous PPP (100% light transmission). Blood was also collected from 2 patients with Glanzmann thrombasthenia, who lack normal levels of GPIIb/IIIa on their platelets. PRP was prepared by the normal procedure described above and gel-filtered platelets were prepared as described below.
Glanzmann thrombasthenia was diagnosed by immunofluorescence using kits from BioCytex. Whole blood was diluted 1:4 with kit buffer and 20 μL of this added to 20 μL antibody. Three antibodies were used: anti-GPIb, anti-GPIIIa, and an isotype control. After 20 minutes' incubation, 20 μL FITC-labeled secondary antibody was added. At the same time 20 μL of the secondary was also added to 40 μL of calibration beads (4 populations of 2-μm beads containing known quantities of antibody). Samples were then analyzed by flow cytometry on a FACSCalibur (Becton Dickinson, Oxford, United Kingdom). The relationship between fluorescence and antibody molecules bound was determined allowing the number of platelet glycoprotein molecules to be determined.
Gel-filtered platelets
Platelets were separated from plasma proteins by gel filtration. PRP (collected in ACD) was adjusted to pH 6.5 with ACD and apyrase (1 U/mL) and prostaglandin-E1 (2 μM) were added prior to centrifugation at room temperature at 630g for 10 minutes. The PPP was removed and the platelet pellet was resuspended in 2 mL modified Hepes-Tyrode buffer (JNL; 6 mM dextrose, 130 mM NaCl, 9 mM NaCl2, 10 mM Na citrate, 10 mM Tris base, 3 mM KCl, 0.8 mM KH2PO4, and 0.9 mM MgCl2). The platelet suspension was then applied to a chromatography column containing 5 mL packed Sepharose 2B-300, which was previously equilibrated with JNL buffer. The resultant platelet fractions were pooled. The platelet concentration was determined using a Sysmex-100 particle counter (Sysmex, Kobe, Japan).
Albumin-gradient platelets
For preparation of highly purified platelets free from platelet-associated proteins an albumin gradient technique was used. A 2-mL aliquot of PRP was layered on a gradient of high-grade BSA (50%, 25%, 17%, 12%, and 10%) in a 15-mL tube. The tubes were then centrifuged at 1200g for 15 minutes. The resultant platelet layer (lying on top of the 50% solution) was removed using a Pasteur pipette.
Platelet aggregation
Platelet aggregation was assayed by light transmission at 37°C using a PAP-4 aggregometer (Bio-Data). The gel-filtered and albumin-gradient platelets were adjusted to a final concentration of 2 × 108 platelets/mL. Physiologic concentrations of CaCl2 (1.8 mM) and fibrinogen (1 mg/mL) were added to the platelets prior to aggregation studies.
Platelet adhesion assay
The 96-well microtiter plates were coated with 100 μL bacteria (OD 1.0), fibrinogen (20 μg/mL), or BSA (20 μg/mL). The plate was incubated at 37°C for 2 hours. Following this, the plate was washed and blocked with 1% BSA for a further 1 hour at 37°C. The plate was washed 3 times in JNL buffer to remove any unbound protein. Then 50 μL gel-filtered platelets (2 × 108 platelets/mL) was added to each well and allowed to adhere for 30 minutes at 37°C. Each well was gently washed 3 times with 100 μL JNL to remove any nonadhered platelets. Adherent platelets were then lysed with 100 μL lysis buffer containing a substrate for acid phosphatase (0.1 M Na acetate pH 5.5, 0.1% Triton X-100, 10 mM p-nitrophenol phosphate) and incubated for 2 hours at 37°C. The reaction was stopped by the addition of 1 M NaOH and the resultant color was read at 410 nm in a microtiter plate reader (Wallac Victor2, Perkin Elmer, Cambridge, United Kingdom). Adhesion data were expressed as the color produced by platelets adherent to bacteria as a percentage of that produced by platelets adherent to fibrinogen.
Transient transfection
The CHOβ/IX cells were grown to 30% confluence in a tissue-culture Petri dish and transfected as previously described.30 pcI-neoGPIbα or unligated pcDNA for the control (9 mg) was mixed with 60 μL lipofectamine and 1.6 mL optiMEM for 15 minutes in the dark. This transfection mixture was added to the CHO cells and incubated at 37°C with 5% CO2 for 5 hours. Fresh media was added and the CHO cells incubated for 60 hours at 37°C with 5% CO2. After 60 hours, the cells were harvested by scraping from the tissue culture Petri dish, washed twice in α-minimal essential medium (MEM) and finally resuspended in Hanks buffered saline solution. To examine for transfection efficiency, the transfected cells were incubated with 10 μg/mL primary antibody (mouse-anti-GPIbα antibody, clone SZ-2) or isotype control antibody for 20 minutes at room temperature. Antibody binding was determined using 10 μg/mL specific goat antimouse FITC-labeled secondary antibody for 20 minutes at room temperature. The samples were then diluted in 2 mL PBS and analyzed on a FACSCalibur flow cytometer (Becton Dickinson).
Immunofluorescent microscopy
For binding studies, stably transfected CHOβ/IX cells transiently transfected with GPIbα were mixed with 100 μL S sanguis (OD 1.0) and gently agitated for 10 minutes at room temperature. The cells were then applied to the slides. CHO cells were incubated with a “primary” antibody raised against GPIbα (5 μg/mL) for 45 minutes at room temperature. Unbound antibody was removed by gentle washing in Tris (hydroxymethyl)aminomethane-buffered saline (TBS). The CHO cells were finally incubated with fluorescent 546-labeled goat antimouse antibody (4 μg/mL) or no antibody for 10 minutes in the dark. Slides were mounted for fluorescent antibody visualization using a confocal microscope (LSM 510; Zeiss, Herts, United Kingdom). Images of stained cells were acquired using an argon laser at 488 nm, whereas nonstained cells images were acquired using the differential interference contrast mode.
Mocarhagin-treated platelets
Platelet-rich plasma was pretreated with a proteinase-disintegrin, mocharagin (5 μg/mL)31 for 45 minutes at 37°C. Treated platelets were tested for their ability to aggregate in response to ADP (20 μM), ristocetin (1.5 mg/mL), andS sanguis (OD 1.6).
Thromboxane ELISA
Production of TXB2 following platelet stimulation by the agonists arachidonic acid (1.5 mM), S sanguis (OD 1.6), and ristocetin (1.5 mg/mL) was measured using a commercial ELISA kit according to the manufacturer's instructions. Aliquots (50 μL) of each reaction were snap frozen in liquid nitrogen for analysis of TXB2 levels.
Preparation of glycocalicin
Glycocalicin, the soluble cleavage fragment of GPIb, was prepared according to the method of Loscalzo and Handin,32which involved incubation of platelets in 3 M KCl at 37°C to allow calpain cleavage of GPIb, wheat germ agglutinin affinity chromatography followed by column chromatography of the eluted material on Sepharose CL-6B. Positive column fractions were identified by reaction with an anti-GPIb antibody (PM6/40) and stored at −20°C until required.
Ligand blotting
Purified glycocalicin was run on 7.5% sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) gels, blotted onto nitrocellulose and after blocking in 5% (wt/vol) hemoglobin, incubated with biotin-labeled S sanguis cells overnight at room temperature. Blots were then washed in PBS, incubated in avidin-conjugated horseradish peroxidase (Sigma), washed, and developed in 4-chloro-1-napthol (Sigma; 0.6 mg/mL in 100 mM Tris-HCl buffer, pH 7.5) and H2O2 (5 μL/mL).
Adhesion to glycocalicin
Aliquots of glycocalicin were diluted to 10 μg/mL in 50 mM carbonate-bicarbonate buffer, pH 9.6, and coated onto ELISA wells overnight at 4°C. After washing and blocking wells with 2% (wt/vol) BSA, FITC-labeled S sanguis cells were added to coated wells and incubated at 37°C for 90 minutes. Wells were then washed with PBS several times and adherent bacteria detected using a fluorescence plate reader (POLARStar Galaxy; BMG Lab Technologies, Aylesbury, United Kingdom) at 490exc nm and 540emmnm.
Results
S sanguis–induced platelet aggregation
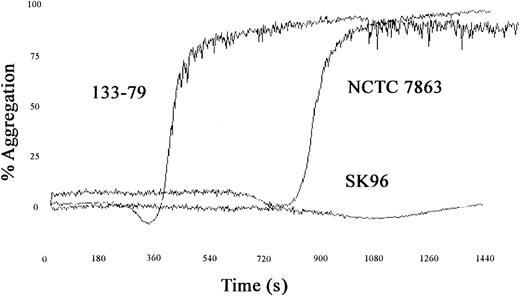
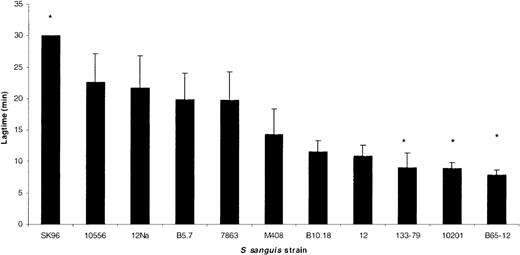
A number of strains of S sanguis were tested for their ability to induce platelet aggregation and typical examples of the traces obtained are shown in Figure1. Aggregation occurred in an all-or-nothing manner, and altering the concentration of bacteria affected the lag time but not the maximum aggregation. Lag time is defined as the time taken from addition of bacteria to PRP until the first recognizable signs of aggregation. A range of lag times was observed with the strains used, but the lag time was consistent for each strain. Comparison of the lag times of strains with that of NCTC 7863, the type strain of the species and which has previously been shown to induce aggregation,24 3 significantly different groups could be recognized (P < .001, analysis of variance [ANOVA]). One group had relatively short lag times (around 5 minutes) and included strain 133-79. A second group had longer lag times (around 16 minutes), similar to NCTC 7863, and finally one strain (SK96) failed to induce aggregation within 30 minutes (Figure 2).
S sanguis–induced aggregation.
Traces showing the effects of different strains of S sanguison platelet aggregation with a short and long lag time and one strain that fails to induce aggregation.
S sanguis–induced aggregation.
Traces showing the effects of different strains of S sanguison platelet aggregation with a short and long lag time and one strain that fails to induce aggregation.
S sanguis strain-dependent aggregation.
The lag time in platelet aggregation for a number of different strains of S sanguis. All strains are clinical isolates from patients with infective endocarditis, except for the laboratory strains NCTC 7863, which is the type strain, 12, 12Na, 10556, and SK96. The asterisk indicates a lag time significantly different from NCTC 7863 (P < .05). SK96 failed to aggregate within 30 minutes.
S sanguis strain-dependent aggregation.
The lag time in platelet aggregation for a number of different strains of S sanguis. All strains are clinical isolates from patients with infective endocarditis, except for the laboratory strains NCTC 7863, which is the type strain, 12, 12Na, 10556, and SK96. The asterisk indicates a lag time significantly different from NCTC 7863 (P < .05). SK96 failed to aggregate within 30 minutes.
S sanguis–platelet adhesion
The adhesion assay used relied on platelets binding to bacteria that had been immobilized on polystyrene wells and made use of the intracellular platelet enzyme, acid phosphatase, for quantification. The method proved to be sensitive and reproducible and the level of acid phosphatase in platelets was much higher than the levels in the streptococci (data not shown). The assay had the advantage that the adhesion event could be studied in the absence of any surface activation of the platelets, which would have occurred if the platelets had been bound to the polystyrene wells. In early experiments we used an assay that measured adhesion of radiolabeled bacteria to immobilized platelets. This yielded similar data to that reported here but had the disadvantage of requiring the use of radioisotopes to enumerate the adherent bacteria (data not shown).
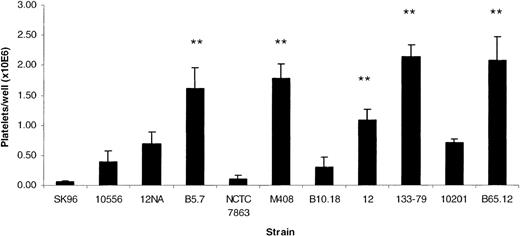
Strains differed in their ability to bind platelets using the above assay (P < .0001, ANOVA). Certain strains supported high levels of platelet adhesion, whereas others were less adhesive or did not support adhesion at all (Figure 3). The extent of platelet attachment to the adhesive strains was usually similar to the level of adhesion seen to a fibrinogen-coated surface. For example, the adhesion of strain 133-79 was 139% ± 28% (n = 5) of the adhesion of platelets to fibrinogen-coated wells. In addition, adhesion to strain 133-79 did not require activation of platelets, as it was not inhibited by pretreating platelets with aspirin or PGE1 (7.5% ± 6% inhibition, n = 4). Strain 133-79 was selected as a representative of the adherent, fast-aggregating strains and used for further studies.
S sanguis strain-dependent platelet adhesion.
Platelet adhesion to a number of different strains of S sanguis. All strains are clinical isolates from patients with infective endocarditis, except for the laboratory strains NCTC 7863, which is the type strain, 12, 12Na, 10556, and SK96. The asterisk indicates a lag time significantly different from NCTC 7863 (P < .05).
S sanguis strain-dependent platelet adhesion.
Platelet adhesion to a number of different strains of S sanguis. All strains are clinical isolates from patients with infective endocarditis, except for the laboratory strains NCTC 7863, which is the type strain, 12, 12Na, 10556, and SK96. The asterisk indicates a lag time significantly different from NCTC 7863 (P < .05).
Platelet thromboxane production
Platelet aggregation by strain 133-79 was inhibited by aspirin (100% inhibition, n = 3), a cyclooxygenase inhibitor, suggesting a role for thromboxane-A2 in the aggregation response. Platelets exposed to S sanguis (133-79) produced thromboxane (248 ± 11 ng/mL; n = 3) at levels that were intermediate between that produced with arachidonic acid (441 ± 21 ng/mL) and ristocetin (48 ± 24 ng/mL). However, aggregation was not dependent on the release reaction because apyrase (10 U/mL) failed to inhibit 133-79–induced aggregation (51% ± 2% versus 48% ± 4% with apyrase, n = 3, P = NS), whereas it completely inhibited ADP-induced aggregation (61% ± 3% versus 4% ± 1% with apyrase, n = 3, P = .003).
Platelet GPIIb/IIIa
Platelet aggregation induced by S sanguis was inhibited by PGE1 (100% inhibition, n = 3), which inhibits platelet activation. The GPIIb/IIIa receptor antagonists eptifibatide and abciximab also inhibited (98% ± 1% inhibition, n = 3 and 98% ± 0.5% inhibition, n = 4, respectively). However, the specific GPIIb/IIIa antagonists, eptifibatide and abciximab, did not inhibit adhesion of platelets to S sanguis (13% ± 13% inhibition n = 3; 12% ± 4% inhibition n = 7, respectively). Platelets from 2 patients with Glanzmann thrombasthenia (absence of GPIIb/IIIa on the platelet surface) also adhered to bacteria as effectively as control platelets (82% of control adhesion, n = 2). These patients had less than 2000 GPIIb/IIIa receptors/platelet (as determined by flow cytometry), whereas normal platelets have about 50 000 receptors/platelet.
Platelet GPIb
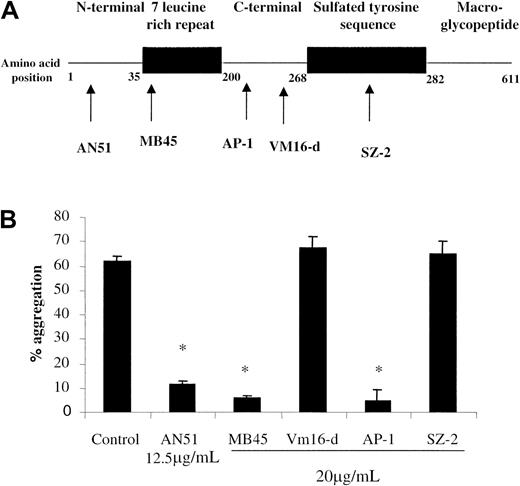
The anti-GPIb antibody AN51 inhibited platelet aggregation induced by all strains of S sanguis (88% ± 2% inhibition, n = 6) suggesting a role for GPIb. To further confirm this we tested a panel of GPIb antibodies directed against different regions of the GPIb molecule (Figure 4). Antibodies that recognize the extreme N-terminal portion of GPIbα between amino acids 1 and 225 inhibited aggregation. Furthermore, cleavage of GPIbα from platelets with mocarhagin, an enzyme specific for GPIbα at amino acid 282/28331 resulted in 97.5% ± 2.5% inhibition of aggregation (n = 2), confirming the involvement of the N-terminal region. This was further confirmed by the failure of S sanguis to induce aggregation (2% ± 1% aggregation, n = 2) of platelets from a patient with Bernard-Soulier syndrome (with no detectable GPIb30) even though they responded to ADP (34% ± 2% aggregation, n = 2).
Role of GPIb in S sanguis–induced aggregation.
The effect of anti-GPIb antibodies on S sanguis–induced platelet aggregation. The proposed binding sites for the antibodies are shown.36 37
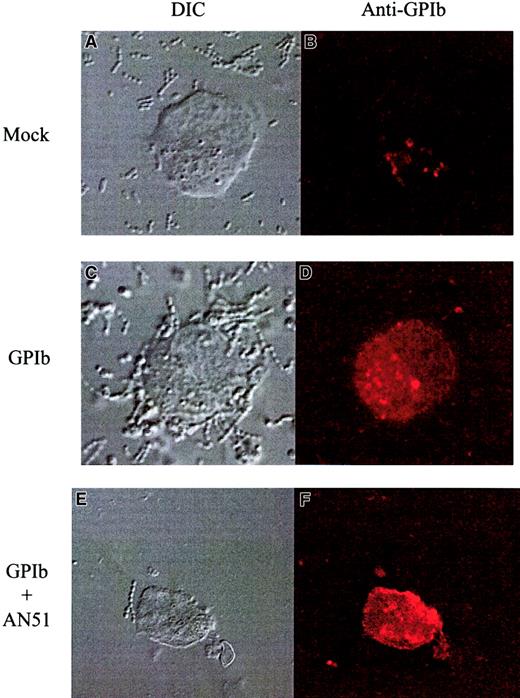
The S sanguis strain 133-79 was able to interact directly with GPIb in a number of experiments. First, platelet adhesion to strain 133-79 was inhibited by the anti-GPIb antibody AN51 (46% ± 9% inhibition) at concentrations that completely inhibited aggregation, whereas antibodies to GPIIb/IIIa were without effect. Second, S sanguis 133-79 was able to bind to CHOβ/IX cells transfected with GPIbα (transfection efficiency of 26% by flow cytometry) using confocal microscopy, whereas they did not bind to mock-transfected CHOβ/IX cells (Figure5). This adhesion was also inhibited by the anti-GPIb antibody, AN51. Third, biotin-labeled 133-79 cells bound to a single band in ligand blots of SDS-PAGE gels of purified GPIb. In contrast, strain NCTC 7863 failed to bind to either (Figure6B). Finally, fluorescent-labeled 133-79 adhered to immobilized glycocalicin, whereas neither NCTC 7863 nor SK96 bound (Figure 6A).
S sanguis interaction with cells expressing GPIb.
Binding of S sanguis to GPIb-transfected CHO cells. The panels on the right show binding of 546-labeled anti-GPIb antibody (SZ2, noninhibitory). The panels on the left show the cells by differential contrast microscopy. The top row shows mock-transfected cells, the middle row, transfected cells, and the bottom row transfected cells with an anti-GPIb antibody (AN51). These show representative images from at least 3 separate experiments. Original magnification × 1000-1300.
S sanguis interaction with cells expressing GPIb.
Binding of S sanguis to GPIb-transfected CHO cells. The panels on the right show binding of 546-labeled anti-GPIb antibody (SZ2, noninhibitory). The panels on the left show the cells by differential contrast microscopy. The top row shows mock-transfected cells, the middle row, transfected cells, and the bottom row transfected cells with an anti-GPIb antibody (AN51). These show representative images from at least 3 separate experiments. Original magnification × 1000-1300.
S sanguis binding to glycocalicin.
(A) Binding of fluorescently labeled strains of S sanguis (SK96 and NCTC 7863 do not adhere to platelets) to glycocalicin. The P value is for the comparison of 133-79 with SK96 and NCTC 7863. (B) Binding of biotin-S sanguis to glycocalicin (apparent molecular weight, 118 kd) Western blotted from 7.5% SDS-PAGE gel. The blot was probed with lane 1, anti-GPIb (P6/40); lane 2, biotinylated S sanguis 133-79 cells; lane 3, biotinylated S sanguis NCTC 7863 cells, and lane 4, PBS. After washing, lane 1 was probed with horseradish peroxidase–conjugated goat antimouse IgG and lanes 2 to 4 were probed with horseradish peroxidase–conjugated avidin. All lanes were then developed in chlor-1-naphthol and H2O2.
S sanguis binding to glycocalicin.
(A) Binding of fluorescently labeled strains of S sanguis (SK96 and NCTC 7863 do not adhere to platelets) to glycocalicin. The P value is for the comparison of 133-79 with SK96 and NCTC 7863. (B) Binding of biotin-S sanguis to glycocalicin (apparent molecular weight, 118 kd) Western blotted from 7.5% SDS-PAGE gel. The blot was probed with lane 1, anti-GPIb (P6/40); lane 2, biotinylated S sanguis 133-79 cells; lane 3, biotinylated S sanguis NCTC 7863 cells, and lane 4, PBS. After washing, lane 1 was probed with horseradish peroxidase–conjugated goat antimouse IgG and lanes 2 to 4 were probed with horseradish peroxidase–conjugated avidin. All lanes were then developed in chlor-1-naphthol and H2O2.
The role of the Fc receptor
NCTC 7863–mediated aggregation was previously shown to involve binding of antibody and to be dependent on the platelet FcγRIIA receptor.24 The antibody IV3, which is directed to the FcγRIIA receptor inhibited aggregation induced by 133-79 (100% inhibition, n = 3). However, it had no effect on platelet adhesion to 133-79 (5% inhibition).
A role for plasma proteins
To determine whether any plasma components were required for aggregation by S sanguis, platelets purified on an albumin gradient were used because this method has been shown to be the most effective at separating platelets from plasma proteins.33Albumin gradient platelets did not aggregate with S sanguis133-79 unless fibrinogen was added (47% ± 7% aggregation, n = 4), suggesting that neither antibody nor other plasma factors were required. Also, gel-filtered platelets adhered to S sanguis 133-79 (139% ± 28%, n = 5) showing that plasma factors were not required for adhesion.
Role of VWF
VWF is one of the natural ligands for GPIb.34 Because it is possible that VWF may contaminate the purified platelet preparations, we investigated whether VWF played a role in platelet aggregation by S sanguis. An antibody to VWF that inhibited ristocetin-induced aggregation (AVW-3) had no effect on S sanguis–induced aggregation (60% ± 2% aggregation, n = 3).
Discussion
The strains of S sanguis tested here showed 3 phenotypes with regard to the nature of their interaction with platelets. Type I induced rapid aggregation and were highly adhesive to platelets (eg, strain 133-79), type II induced aggregation with longer lag times and were not adhesive to platelets (eg, NCTC 7863), and type III neither induced aggregation nor supported adhesion of platelets (eg, SK96). Similar phenotypes have been described by Herzberg et al.35
Platelet aggregation induced by types I and II S sanguis strains was dependent on activation and required fibrinogen binding to its receptor, GPIIb/IIIa. This was true aggregation and bacteria did not bind directly to GPIIb/IIIa because adhesion was not inhibited by GPIIb/IIIa antagonists. Rapidly aggregating strains (type I) did not, however, require ADP or VWF secretion. Because bacteria can cross-link platelets, it is surprising that there is no evidence of agglutination. This is likely due to the fact that platelet aggregometry only detects large aggregates and that the strength of the bacterial-platelet interaction may only support the formation of microaggregates. We have previously shown that platelet aggregation induced by NCTC 7863 (type II) depends on IgG binding to the FcγRIIA and complement assembly on the bacterial surface24 and so it seems likely that strains that induce relatively rapid platelet aggregation, such as 133-79, may use a different mechanism.
Because we had previously shown that the anti-GPIb antibody, AN51, inhibited NCTC-7863-induced platelet aggregation,24 we turned our attention to GPIb. After GPIIb/IIIa it is the most abundant glycoprotein on platelets and it plays an essential role in platelet adhesion in vivo.20 However, here we have extended that work to include a panel of monoclonal antibodies against GPIb and studied their effect on strain 133-79. S sanguis–induced platelet aggregation was inhibited by antibodies that inhibit ristocetin-induced aggregation (AN51, MB45, and AP-1) but not by antibodies that inhibited thrombin binding or botrocetin-induced aggregation (Vm16-d and SZ2).36,37 The latter result differs from that reported by Sullam and coworkers38 who found that SZ2 inhibited S sanguis–induced platelet aggregation, but this is likely to be due to the use of different strains.
Platelet aggregation by S sanguis (type I and type II) was also inhibited by antibody to the FcγRIIA receptor, which is a platelet IgG receptor. Whereas in the case of the type II strain NCTC 7863 this is due to an interaction with bacterial-bound IgG, in the case of 133-79 antibody is not required for aggregation and the anti-FcγRIIA antibody does not inhibit adhesion suggesting no direct role for a FcγRIIA-IgG interaction.
Although our data apparently provide evidence for a role of GPIb and FcγRIIA, the findings might possibly be explained on the basis that anti-GPIb antibodies sterically block the FcγRIIA interacting with bacterial-bound IgG and Sullam and coworkers38 have shown that FcγRIIA is physically associated with GPIb. However, we found that S sanguis (133-79) directly interacts with glycocalicin in an adhesion assay, a ligand-blotting assay, and to cells expressing GPIb. Equally platelets without GPIb (Bernard-Soulier disease or mocharagin-treated cells) fail to aggregate to S sanguis. These data together indicate the involvement of a direct interaction with GPIb. Equally, plasma proteins were not required for adhesion and only fibrinogen was required for aggregation, indicating the platelet-bacterial interaction was independent of IgG. The inhibition seen with the anti-FcγRIIA antibody may be due to an effect on GPIb-mediated signaling.39
Our data and those of others then suggest that strains of S sanguis vary in the mechanisms by which they interact with platelets but all may well involve GPIb, the FcγRIIA, and in some cases a complement receptor. Strain NCTC 7863 requires complement and IgG for aggregation,24 which mediate the interaction with platelets through an interaction with FcγRIIA and indirectly through GPIb. In contrast, 133-79 interacts directly with platelets through GPIb and indirectly with FcγRIIA. One model for the platelet interaction by S sanguis that could explain these data involves receptor clustering of GPIb with FcγRIIA, which are known to be closely associated on the platelet surface,38 into a signaling complex. S sanguis either interacts with the FcγRIIA through an antibody bridge or directly with GPIb. In either case blockade of either GPIb or FcγRIIA will inhibit aggregation. The importance of receptor clustering in platelets has been previously recognized, for example, cross-linking of GPIIb/IIIa,40CD9, or FcγRIIA41 with antibodies can lead to platelet activation.
Although nothing is currently known about the S sanguisGPIb-binding molecule, it is likely to be protein in nature because protease treatment of the bacteria reduced adhesion to platelets (data not shown). This suggests that there may be a prothrombotic virulence factor on some strains of S sanguis and it is tempting to speculate that it may be a VWF-like molecule on the surface of the bacteria. Previously, a collagenlike protein termed platelet aggregation-associated protein (PAAP) on 133-79 was implicated in aggregation.28,29 Whereas some collagens can bind to GPIb via VWF,42 our data would suggest that a protein other than PAAP is involved due to the absence of a role for VWF, although it has been reported that the snake venom lectin aggretin is able to bind to both the α2β1 collagen receptor and GPIb to induce aggregation.43 It is possible then that the PAAP is multifunctional and might bind to both α2β1 and GPIb or act in conjunction with a GPIb-binding protein. However, in our hands a peptide (PGEQGPL) reported to represent the interactive domain of PAAP and to inhibit platelet aggregation by 133-7944 failed to affect either aggregation or adhesion (data not shown).
Binding of GPIb to VWF only occurs under shear conditions20 or with chemical modification of VWF by ristocetin or botrocetin and is accompanied by a weak thromboxane signal. However, the interaction of S sanguis with GPIb is unusual in that it requires neither shear nor chemical modification to facilitate binding and appears to induce a thromboxane-dependent activation signal leading to GPIIb/IIIa-mediated aggregation. Previous work has shown that engagement of GPIb can lead to activation of GPIIb/IIIa,45-47 but this is the first direct evidence of activation of GPIIb/IIIa that is mediated by thromboxane production due to a ligand binding to GPIb.
Bacterial polymorphisms are known to be important factors in disease48 and presence of the GPIb-binding protein in some strains may explain the strong association between S sanguisand infective endocarditis. Despite this, S sanguis is not unique in its ability to interact with GPIb and may be a more general mechanism for bacterial-platelet interactions. Recently we have shown that Helicobacter pylori binds to GPIb and induces aggregation, although via a different mechanism.49 Also,Staphylococcus aureus protein A has been shown to interact with platelets via VWF-GPIb and IgG-FcγRIIA.50
Our data suggest that the use of antiplatelet agents may be useful in both the prevention and treatment of infective endocarditis. It also has implications for conditions other than infective endocarditis. Recent reports have suggested that periodontal disease is an independent risk factor for myocardial infarction51 andS sanguis has been identified in atherosclerotic plaque.9 Another oral bacterium Porphyromonas gingivalis has recently been shown to activate platelets via a secreted enzyme acting on the protease activated receptor 1 (thrombin receptor) on platelets52 and to increase atherosclerosis in an animal model.53 It is known that oral bacteria can gain access to the circulation as a result of periodontal disease. In patients with damaged heart valves this can lead to infective endocarditis. However, in patients with unstable atherosclerotic plaques this may aggravate existing unstable angina by increasing platelet activation. The interaction of bacteria with platelets may therefore play an important role in cardiovascular diseases.
We would like to thank Ms Teresa Keane for her assistance in the confocal microscopy experiments. We would also like to thank Dr José Lopez, Department of Molecular and Human Genetics, Baylor College of Medicine, Houston, TX; Dr Michael Berndt, Baker Research Medical Institute, Praham, Victoria, Australia; and Dr Dermot Kenny, Department of Clinical Pharmacology, Royal College of Surgeons in Ireland for gifts of reagents.
Supported by grants from the Royal College of Surgeons in Ireland, the University of Sheffield, and the British Heart Foundation.
This work was a poster presentation at the American Society of Hematology meeting in 1999.54
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Dermot Cox, Department of Clinical Pharmacology, Royal College of Surgeons, 123 St Stephens Green, Dublin 2, Ireland; e-mail: dcox@rcsi.ie.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal