Abstract
Extensive phenotype analysis of cutaneous T-cell lymphoma (CTCL) malignant cell lines revealed surface expression of receptors usually not detected on normal circulating CD4+CD45RO+lymphocytes. We previously found that CTCL malignant cells express the killer cell immunoglobulinlike receptor (KIR) KIR3DL2/CD158k, whereas they fail to express the other KIRs. In the present study, we report for the first time that the CD85j/immunoglobulin (Ig)–like transcript 2 (ILT2) receptor is found on Sézary cell lines and on circulating Sézary malignant CD4+ cells, while it is hardly detectable on circulating CD4+ lymphocytes from healthy individuals. We demonstrate that ILT2 is functional on CTCL cells, as its triggering leads to the recruitment of Src homology 2 domain-containing tyrosine phosphatase (SHP-1) and to the specific inhibition of CTCL malignant cell proliferation induced by CD3/T-cell receptor (TCR) stimulation. Interestingly, we found that separated CD4+ILT2+ circulating malignant Sézary cells are less susceptible to anti-CD3 monoclonal antibody (mAb)–induced cell death than autologous CD4+ILT2− lymphocytes. Therefore, the resistance to apoptosis of Sézary cells may result from distinct mechanisms including cytokine-induced high levels of bcl-2 and specific expression of inhibitory receptors involved in lymphocyte survival.
Introduction
Cutaneous T-cell lymphomas (CTCLs) are a heterogeneous group of lymphoproliferative disorders of the skin.1 The most frequent CTCLs, mycosis fungoides (MF) and its leukemic counterpart Sézary syndrome (SS), are both characterized by a clonal T-lymphocyte proliferation with a CD4+CD45RO+ phenotype. While MF presents with a skin-restricted infiltration of the clonal T-lymphocyte population and an indolent course, SS is an aggressive form of CTCL.2,3The factors that drive a T-cell clone to proliferate and to escape from the immune control are not clearly understood. The pathogenic role of viruses4-6; other epidermal cells, such as keratinocytes and dendritic cells7,8; or aberrant secretion or response to certain cytokines, such as transforming growth factor β (TGFβ), interferon γ (IFNγ), and interleukin 10 (IL-10),9-11have been evoked. CTCL may also be considered a result of defective negative regulation of cell growth, survival, or both. In agreement to this view, the lack of phosphotyrosine phosphatase SHP-1 expression in malignant T-cell lymphomas has been reported.12
In an attempt to define specific cell surface receptors restricted to CTCL that may elucidate the pathophysiology of the disease, we have recently identified the expression of the unique inhibitory killer cell Ig-like receptor (KIR) CD158k/KIR3DL2 on SS tumor cells.13In normal peripheral blood lymphocytes (PBLs), only natural killer (NK) cells and a subset of CD8+ memory cytolytic T lymphocyte (CTLs) express KIR in a clonotypic manner. The interaction of KIR with specific major histocompatibility complex (MHC)–I alleles determines the NK cell tolerance to self and maintains CD8+T lymphocyte survival.14-16
In the present study we further extended the phenotypic characterization of Sézary cells. We report for the first time that Sézary malignant cells express at their cell membrane an increased level of the CD85j/Ig-like transcript 2 (ILT2) molecule, which belongs to a family of receptors homologous to KIR and encoded by genes located in the leukocyte receptor complex.17-19 The ILT molecules have both inhibitory forms recruiting SHP-1 and short-tailed activating forms.20 They differ from KIRs in that they are mainly expressed on phagocytic and antigen-presenting cells such as monocytes, macrophages, dendritic cells, and B lymphocytes. Some inhibitory receptors, however, are detected on peripheral NK and T lymphocytes.21Interestingly, although it is detectable on the surface of a small proportion of CD8+ PBLs, ILT2 is present in the cytoplasm of all circulating T cells.22 We found that CTCL malignant cells express a significant amount of SHP-1 and that the latter is recruited by ILT2 on cell stimulation. Further on, we demonstrate that engagement of ILT2 surface molecule inhibits the anti-CD3 mAb-induced proliferation of CTCL tumor cells, whereas it fails to inhibit the proliferation induced by IL-7. These findings suggest that CTCL malignant cells express on their surface functional inhibitory receptors that interfere with signaling through CD3/TCRs. In addition, we showed that malignant CD4+ILT2+ cells separated from the blood of patients with SS are more resistant to CD3/TCR-dependent activation-induced cell death than the normal autologous CD4+ lymphocytes, which fail to express ILT2. Thus, the surface expression of KIR3DL2 and ILT2 on CTCL constitutes a powerful tool for the identification of malignant lymphocytes and for the study of the molecular basis of the disease.
Patients, materials, and methods
Patients
After informed consent and approval by an ethics committee (CCPPRB, Hôpital Henri Mondor, Créteil), we obtained blood samples from 12 SS patients with 20% to 90% circulating CD3+, CD4+, and CD8− malignant cells, and biopsies from involved skin of 2 patients with pagetoid reticulosis and 2 patients with MF. All patients had not previously been treated with chemotherapy. Diagnosis of SS was based on clinical criteria (erythroderma, pruritus, palmoplantar keratoderma, diffuse adenopathies), biological criteria including typical circulating Sézary cells (≥ 1000/μL), histological data (cutaneous epidermotropic T-cell lymphoma), and detection of an identical T-cell clone in the blood and in the skin by qualitative polymerase chain reaction (PCR) denaturing gradient gel electrophoresis (DGGE) γ.23 Skin biopsies were mechanically dispersed into single-cell suspensions, as described elswhere.24 The mononuclear cells were then washed and frozen in human serum plus 10% dimethyl sulfoxide for later use.
Cells and cell lines
Peripheral blood mononuclear cells (PBMCs) were isolated by the technique of Ficoll-Isopaque (Pharmacia Fine Chemicals, Piscataway, NJ) density gradient centrifugation, washed, and frozen in human serum plus 10% dimethyl sulfoxide for later use. Human CTCL malignant cell lines, Cou-L and Pno, were established from peripheral blood of CTCL patients and maintained in culture as previously described.24 25Cou-L is an IL-2–dependent cell line that has a CD3+CD4+Vβ13+ phenotype, whereas the Pno cell line has a CD3+Vβ22+CD4+CD8αα+phenotype and proliferates in response to IL-7. Both CTCL malignant cell lines have been maintained continuously in culture with stable clonotype for more than 36 months.
Monoclonal antibodies and flow cytometry studies
Indirect immunofluorescent staining was performed with hybridoma supernatants or ascites fluid; fluorescein isothiocyanate (FITC)–conjugated goat anti-mouse Ig (GAM) from Caltag laboratories (San Francisco, CA) was used. For 2-color analysis the immunofluorescent staining was performed by incubating 3 × 105 cells with anti-ILT2 mAb for 30 minutes at 4°C. Cells were then washed and further incubated with FITC-conjugated goat anti-mouse IgM + IgG (Caltag laboratories). After double washing, the free GAM binding sites were blocked with an excess of normal mouse Ig for 5 minutes at room temperature. Without further washing, the cells were incubated with a second phycoerythrin (PE)- or TriColor (TRI)-conjugated specific mAb. Stained cells were analyzed by means of a single argon flow cytometer analyzer (EPICS XL, Beckman-Coulter, Miami, FL), as described elsewhere.26 PE- or TRI-conjugated anti-CD3, anti-CD4, anti-T cell receptor variable (TCRV)β22, and anti-TCRVβ8 mAbs were purchased from Immunotech (Marseilles, France); PerCP-Cy5.5-conjugated anti-CD4 mAb was obtained from BD Biosciences (San Jose, CA); and the anti-TCRVβ13 mAb was obtained from BIOadvance (Emerainville, France). The mAb ICO-86 used as isotype control for SHP-1 coimmunoprecipitation studies was kindly provided by Anatoli Baryshikov (N. N. Blokhin Cancer Research Center, Moscow, Russia). Anti-ILT2 mAbs GHI/75 and VMP55 were obtained through the exchanges of the Fifth and Seventh International Workshops on Leukocyte Antigens. For intracellular labeling, cells were fixed in phosphate-buffered saline 4% p-formaldehyde for 20 minutes at 4°C, washed, and then permeabilized with staining buffer containing 0.1% saponin. The sorting experiments were done with PBMCs from 2 SS patients with a significant number of circulating Sézary cells (≥ 20% of PBLs). Briefly, cells were labeled with anti-CD4 and anti-ILT2 GHI/75 mAb and the CD4+ gated lymphocytes were sorted into ILT2+ and ILT2− fractions with an EPICS Elite cell-sorter (Beckman-Coulter). Separated cells were incubated for 9 hours with immobilized anti-CD3 mAbs or control mAb (10 μg/mL) and labeled with FITC-conjugated annexin V (Pharmingen, San Diego, CA) for 10 minutes at room temperature. After washing, propidium iodide (PI) (20 μg/mL) was added and the percentage of apoptotic cells was determined on the basis of annexin V binding and PI exclusion by means of a single argon flow cytometer analyzer (EPICS XL, Beckman-Coulter).
Biochemical studies
For the biochemical studies, 2 × 107 cells per sample for immunoprecipitation and 5 × 105 cells per sample for whole cell lysates were stimulated or not with 1 mM sodium vanadate and 0.03% H2O2 for 2 minutes at room temperature. Cells were washed in cold Tris-buffered saline and resuspended in lysis buffer (20 mM Tris-HCl pH 7.5, 150 mM NaCl, 1% NP40, 1 mM Na pervanadate, 10 mM NaF, 1 mM phenylmethanesulfonyl fluoride (PMSF), 1 μg/mL aprotinin, and 1 μg/mL leupeptin) for 1 hour at 4°C. Postnuclear supernatant was then precleared for 1 hour at 4°C with 25 μL protein A-Sepharose beads per sample, and whole cell lysates were resolved by discontinuous sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE). Alternatively, cell lysates were subjected to immunoprecipitation with 5 μL of GHI/75 ascites or isotype-matched anti-CD4 ascite (ICO-86) for 1 hour followed by protein G-Sepharose beads for 2 hours at 4°C. Immunoprecipitates were washed 4 times with washing buffer (20 mM Tris-HCl pH 7.5, 150 mM NaCl, 0.1% Triton X-100, 1 mM Na pervanadate, 10 mM NaF, 1 mM PMSF), and precipitated proteins were subjected to SDS-PAGE and transferred to Hybond hydrophobic polyvinylidene difluoride (PVDF) membranes (Amersham Pharmacia, Orsay, France). Immunoblotting was performed with anti–SHP-1 polyclonal rabbit antibody (Santa Cruz Biotechnology, Santa Cruz, CA) followed by horseradish peroxidase–conjugated goat anti-rabbit IgG and the enhanced chemiluminescence (ECL) detection system according to manufacturers' recommendations (Amersham Pharmacia).
Messenger RNA (mRNA) analysis by reverse transcriptase–PCR
Total RNA was extracted by means of the phenol-chloroform technique, and 3 μg of total RNA was reverse-transcribed according to Clontech (Palo Alto, CA) recommendations. Complementary DNA was amplified with ILT2- and G3PDH-specific primers with PCR (30 and 35 cycles), according to the described conditions.22 Samples were analyzed in a 1.5% agarose gel stained with ethidium bromide. The intensity of the bands was analyzed with Biorad Gel Doc 2000 (Biorad, Milan, Italy). Semiquantitative analysis was performed by comparing the intensity of ILT2-specific fluorescence in healthy and Sézary patients. For immunoscope analysis, the technique has been described in detail elswhere.27 28
Proliferation assays
For proliferation assays, 5 × 104 cells were cultured in triplicate in 96-well round-bottomed plates (Greiner, Nürtingen, Germany) in a final volume of 0.2 mL of culture medium. For stimulation of the Pno cell line, plates were precoated with anti-CD3 mAb (1 μg/well). Cells were cultured for 4 days and were pulsed with 1 μCi (0.037 MBq) of 3H[TdR] during the last 8 to16 hours of culture. Incorporation of3H[TdR] was measured in a liquid scintillation counter (Topcount, Packard Instrument, Meriden, CT).
Results
CTCL cell lines are stained by anti-ILT2 mAb
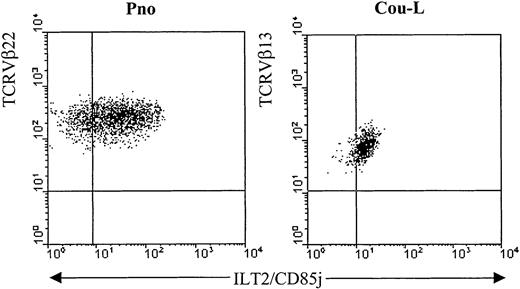
We have previously described 2 cytokine-dependent long-term CD4+ CTCL tumor cell lines, Cou-L (TCRVβ13+) and Pno (TCRVβ22+), that were derived from circulating tumor T lymphocytes.24,25 We have demonstrated by phenotypic and genetic analysis that both cell lines were identical to the malignant cells found in the patients' blood and skin. While not expressing most of the established Ig-like and lectinlike killer cell inhibitory receptors, both cell lines were reactive, with monoclonal antibodies recognizing p140/KIR3DL2.13 We extended the phenotypic analysis of these CTCL cell lines with the anti-ILT2 mAbs GHI/75 and VMP55 and established that both cell lines expressed ILT2 molecules on their surfaces (Figure1). Interestingly, as reported with normal T-cell clones,22 the levels of ILT2 surface expression on both malignant cell lines varied considerably in the course of a standard in vitro culture (data not shown).
Detection of ILT2 on CTCL cell lines.
The long-term CTCL cell lines Pno and Cou-L were analyzed by 2-color immunofluorescence and flow cytometry with an ILT2/CD85j-specific mAb (GHI/75), followed by FITC-conjugated anti-IgG + IgM GAM. After extensive washing to remove the excess of GAM and blocking with normal mouse Ig, the PE-conjugated anti-TCRVβ22 or anti-TCRVβ13 was added.
Detection of ILT2 on CTCL cell lines.
The long-term CTCL cell lines Pno and Cou-L were analyzed by 2-color immunofluorescence and flow cytometry with an ILT2/CD85j-specific mAb (GHI/75), followed by FITC-conjugated anti-IgG + IgM GAM. After extensive washing to remove the excess of GAM and blocking with normal mouse Ig, the PE-conjugated anti-TCRVβ22 or anti-TCRVβ13 was added.
Circulating Sézary malignant cells express ILT2
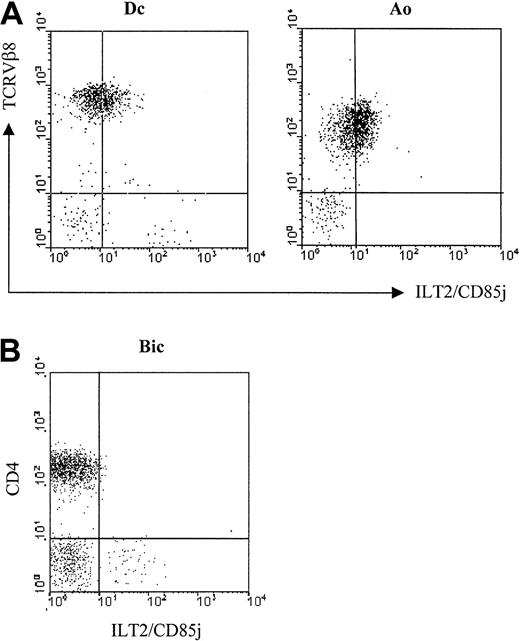
Previous results indicated that the ILT2 molecule was practically not detectable on CD4+ circulating lymphocytes22 (see control in Figure 3), whereas it was found on the cell surfaces of lymphocytes after in vitro stimulation and on IL-2–dependent CD4+ T cell clones. Therefore, it was important to determine whether the expression of ILT2 on the CTCL malignant cell lines was not attributable to their in vitro expansion with cytokines. We tested the surface expression of ILT2 with the GHI/75 mAb on freshly isolated PBLs from 4 untreated SS patients previously studied by immunoscope analysis for the TCRVβ usage of the expanded malignant clone (Ingen-Housz-Oro et al, manuscript in preparation). We found ILT2 on the surface of tumor cells in 4 of 4 patients studied. Figure 2A shows results obtained with 2 of these patients in which the malignant cells exhibited a significant level of TCR molecules detectable by flow cytometry. In both patients the profile of anti-ILT2 mAb binding to TCRVβ8+ cells corresponded to a weak homogeneous expression by the whole malignant clonal cell population. It should be noted that ILT2 cell membrane expression appeared to be restricted to circulating CD4+ SS malignant lymphocytes, as CD4+ lymphocytes isolated from involved skin of patients with pagetoid reticulosis or MF failed to exhibit at their membranes detectable levels of ILT2 molecules (Figure 2B).
Expression of ILT2/CD85j on fresh circulating SS cells.
(A) Freshly isolated PBMC from 2 representative SS patients, Dc and Ao—both with a TCRVβ8+ circulating malignant clone—were analyzed by 2-color immunofluorescence and flow cytometry with an ILT2/CD85j-specific mAb (GHI/75), followed by FITC-conjugated anti-IgG + IgM GAM. After extensive washing to remove the excess of GAM and blocking with normal mouse Ig, the PE-conjugated anti-TCRVβ8 mAb was added. (B) Lymphocytes isolated from involved skin of a MF patient, Bic, were analyzed by 2-color immunofluorescence and flow cytometry, as described above, with the ILT2/CD85j-specific mAb and a TRI-conjugated anti-CD4 mAb. No anti-ILT2 mAb reactivity with the CD4+ cell subset was detected with lymphocytes isolated from the skin biopsies of another MF patient and from 2 patients with pagetoid reticulosis.
Expression of ILT2/CD85j on fresh circulating SS cells.
(A) Freshly isolated PBMC from 2 representative SS patients, Dc and Ao—both with a TCRVβ8+ circulating malignant clone—were analyzed by 2-color immunofluorescence and flow cytometry with an ILT2/CD85j-specific mAb (GHI/75), followed by FITC-conjugated anti-IgG + IgM GAM. After extensive washing to remove the excess of GAM and blocking with normal mouse Ig, the PE-conjugated anti-TCRVβ8 mAb was added. (B) Lymphocytes isolated from involved skin of a MF patient, Bic, were analyzed by 2-color immunofluorescence and flow cytometry, as described above, with the ILT2/CD85j-specific mAb and a TRI-conjugated anti-CD4 mAb. No anti-ILT2 mAb reactivity with the CD4+ cell subset was detected with lymphocytes isolated from the skin biopsies of another MF patient and from 2 patients with pagetoid reticulosis.
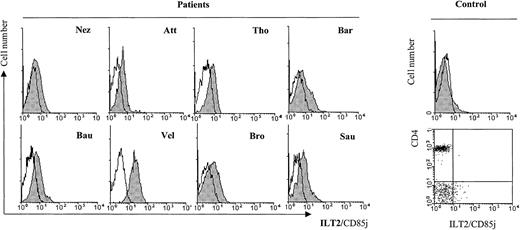
We further tested freshly isolated PBLs from 8 SS patients with cytomorphological data for blood involvement and established an increased ILT2 expression on the CD4+ subset (Figure3) as compared with healthy control individuals (control, Figure 3). It should be noted, however, that individual variations in the intensity of ILT2 surface labeling existed between the patients, independent of the percentage of morphologically detected malignant cells.
Profiles of ILT2/CD85j expression on CD4+ PBL from SS patients in comparison with a representative healthy control patient.
Freshly isolated PBMC from SS patients with cytomorphological data for blood involvement were analyzed by 2-color immunofluorescence and flow cytometry with the ILT2/CD85j-specific mAb GHI/75 (shaded histograms) or an isotype-matched control mAb (unfilled histograms), followed by FITC-conjugated anti-IgG + IgM GAM. After extensive washing to remove the excess of GAM and blocking with normal mouse Ig, a TRI-conjugated anti-CD4 mAb was added. CD4+ gated lymphocytes are represented on the histograms. The same staining performed with PBMC from a representative healthy control patient is shown in the histogram and the dot plot to the right.
Profiles of ILT2/CD85j expression on CD4+ PBL from SS patients in comparison with a representative healthy control patient.
Freshly isolated PBMC from SS patients with cytomorphological data for blood involvement were analyzed by 2-color immunofluorescence and flow cytometry with the ILT2/CD85j-specific mAb GHI/75 (shaded histograms) or an isotype-matched control mAb (unfilled histograms), followed by FITC-conjugated anti-IgG + IgM GAM. After extensive washing to remove the excess of GAM and blocking with normal mouse Ig, a TRI-conjugated anti-CD4 mAb was added. CD4+ gated lymphocytes are represented on the histograms. The same staining performed with PBMC from a representative healthy control patient is shown in the histogram and the dot plot to the right.
Expression of ILT2-specific mRNA in PBLs from SS patients is not increased
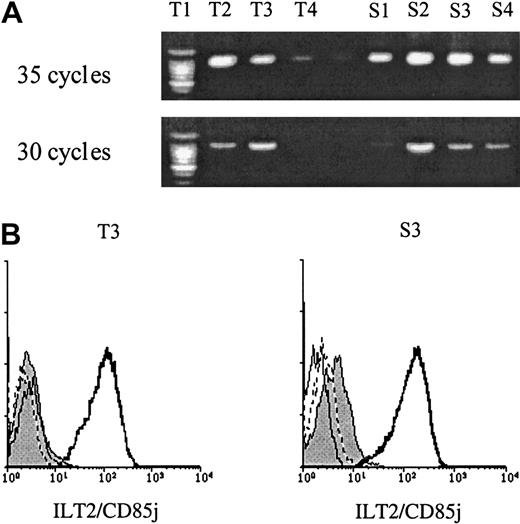
As normal T lymphocytes were reported to express significant amounts of ILT2 in their cytoplasm, it was important to determine whether the surface expression of ILT2 on CD4+ CTCL cells reflected an increased traffic from the intracellular compartments rather than an enhancement of the transcriptional activity.22 To clarify this issue, we compared, as described elsewhere,22 the levels of specific-ILT2 mRNA in the PBLs of 4 patients with a dominant CD4+ Sézary cell clone (> 50% of PBLs) with levels in 4 healthy controls who had only a small subset of CD8+ lymphocytes bearing a surface expression of ILT2. The results (Figure4A) revealed no significant differences in the level of specific ILT2 mRNA between the 2 groups. Interestingly, this lack of difference in the expression of ILT2 mRNA between healthy individuals and SS patients was correlated with the lack of difference in the intracellular anti-ILT2 mAb staining obtained in the 2 groups (Figure 4B).
ILT2 expression in PBL from healthy control patients and SS patients.
(A) PCR analysis of ILT2-specific mRNA expression in patient and control PBLs. Complementary DNA was amplified with ILT2- and G3DH-specific primers with 30 and 35 cycles of PCR. Samples were analyzed in a 1.5% agarose gel stained with ethidium bromide. No significant differences were detected in levels of ILT2-specific mRNA between PBLs from control patients (T1 to T4) and SS patients (S1 to S4). (B) Intracellular expression of ILT2 in CD4+ PBLs from a SS patient (S3) and a healthy control donor (T3). Freshly isolated PBMCs were permeabilized or not and stained with ILT2/CD85j or an isotype-matched control mAb, followed by FITC-conjugated GAM Ig. After extensive washing and blocking with normal mouse Ig, PerCP-Cy5.5-conjugated anti-CD4 mAb was added. Only CD4+gated cells were analyzed. The histograms presented correspond to permeabilized anti-ILT2–stained CD4+ cells (thick line), permeabilized isotype control–stained CD4+ cells (dotted line), nonpermeabilized anti-ILT2–stained cells (thin line, shaded), and nonpermeabilized isotype control–stained cells (thin line, unshaded).
ILT2 expression in PBL from healthy control patients and SS patients.
(A) PCR analysis of ILT2-specific mRNA expression in patient and control PBLs. Complementary DNA was amplified with ILT2- and G3DH-specific primers with 30 and 35 cycles of PCR. Samples were analyzed in a 1.5% agarose gel stained with ethidium bromide. No significant differences were detected in levels of ILT2-specific mRNA between PBLs from control patients (T1 to T4) and SS patients (S1 to S4). (B) Intracellular expression of ILT2 in CD4+ PBLs from a SS patient (S3) and a healthy control donor (T3). Freshly isolated PBMCs were permeabilized or not and stained with ILT2/CD85j or an isotype-matched control mAb, followed by FITC-conjugated GAM Ig. After extensive washing and blocking with normal mouse Ig, PerCP-Cy5.5-conjugated anti-CD4 mAb was added. Only CD4+gated cells were analyzed. The histograms presented correspond to permeabilized anti-ILT2–stained CD4+ cells (thick line), permeabilized isotype control–stained CD4+ cells (dotted line), nonpermeabilized anti-ILT2–stained cells (thin line, shaded), and nonpermeabilized isotype control–stained cells (thin line, unshaded).
CTCL cell lines express SHP-1 that is recruited by ILT2 on cell activation
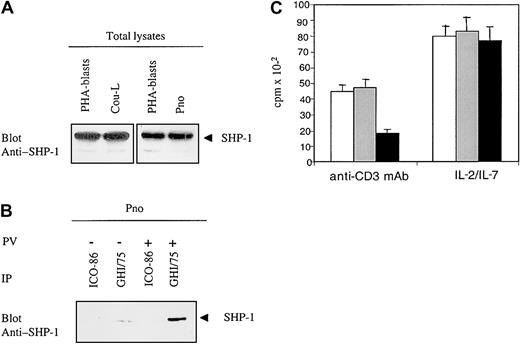
Engagement of ILT2 induces tyrosine-phosphorylation of the immunoreceptor tyrosine-based inhibitory motifs (ITIMs), which subsequently recruit SHP-1 phosphatases that dephosphorylate adaptors involved in TCR signaling.29 However, lack or dysfunction of SHP-1 was reported to be frequent in T-cell lymphoma cell lines.12 To determine whether the ILT2 receptors that we detected in CTCL malignant cell lines could exhibit inhibitory function, we studied the expression of SHP-1 protein in Pno and Cou-L cells. SHP-1 was detected in both cell lines, and its expression was equivalent to that in normal phytohemagglutinin (PHA)-activated PBLs (Figure 5A). Next, to determine whether ILT2 molecules recruited SHP-1 phosphatase on phosphorylation, we immunoprecipitated ILT2 from Pno cells, either unstimulated or stimulated with sodium pervanadate (the latter inducing substantial tyrosine phosphorylation of cellular substrates), and studied the precipitates for the presence of SHP-1. We found that ILT2 was constitutively associated with SHP-1 and that this association significantly increased after pervanadate treatment (Figure 5B). Finally, to demonstrate that ILT2 expressed by CTCL malignant cells can deliver a negative signal sufficient to inhibit cell proliferation, we studied the functional consequence of ILT2 molecule engagement on Pno CTCL cells triggered to proliferate through their CD3/TCR receptors. We have previously demonstrated that immobilized anti-CD3 mAb induced a weak but significant proliferation of Pno cells. Figure 5C shows that the cross-linking of ILT2 receptors (with their specific mAb followed by GAM Ig) resulted in a significant decrease in the proliferation rate of CTCL cells triggered by immobilized anti-CD3 mAb. Interestingly, the cross-linking of ILT2 receptors alone did not influence the IL-7–dependent proliferation of Pno malignant cells (Figure5C).
CTCL cells express a functional ILT2/CD85j receptor.
(A) Probing of the CTCL-derived cell lines Pno and Cou-L for presence of SHP-1: Western blot of whole cell lysates with an anti–SHP-1 polyclonal antibody. T-cell-rich PHA–stimulated PBLs served as positive controls. (B) ILT2/CD85j recruits SHP-1 in Pno cells: ILT2 immunoprecipitates from pervanadate-untreated and pervanadate-treated Pno cells were blotted with an anti–SHP-1 antibody. (C) Cross-linking of surface ILT2 inhibits the anti-CD3–induced proliferation while not affecting the cytokine-driven proliferation of Pno cells. Pno cells (3 × 104) were activated with immobilized anti-CD3 mAb or IL-7 as described in “Patients, materials, and methods,” in the presence of medium alone (white histograms), in the presence of isotype control mAb (gray histograms), or in the presence of anti-ILT2 mAb (black histograms), cross-linked with GAM Ig.
CTCL cells express a functional ILT2/CD85j receptor.
(A) Probing of the CTCL-derived cell lines Pno and Cou-L for presence of SHP-1: Western blot of whole cell lysates with an anti–SHP-1 polyclonal antibody. T-cell-rich PHA–stimulated PBLs served as positive controls. (B) ILT2/CD85j recruits SHP-1 in Pno cells: ILT2 immunoprecipitates from pervanadate-untreated and pervanadate-treated Pno cells were blotted with an anti–SHP-1 antibody. (C) Cross-linking of surface ILT2 inhibits the anti-CD3–induced proliferation while not affecting the cytokine-driven proliferation of Pno cells. Pno cells (3 × 104) were activated with immobilized anti-CD3 mAb or IL-7 as described in “Patients, materials, and methods,” in the presence of medium alone (white histograms), in the presence of isotype control mAb (gray histograms), or in the presence of anti-ILT2 mAb (black histograms), cross-linked with GAM Ig.
ILT2+ circulating Sézary cells are resistant to CD3/TCR-dependent activation-induced cell death
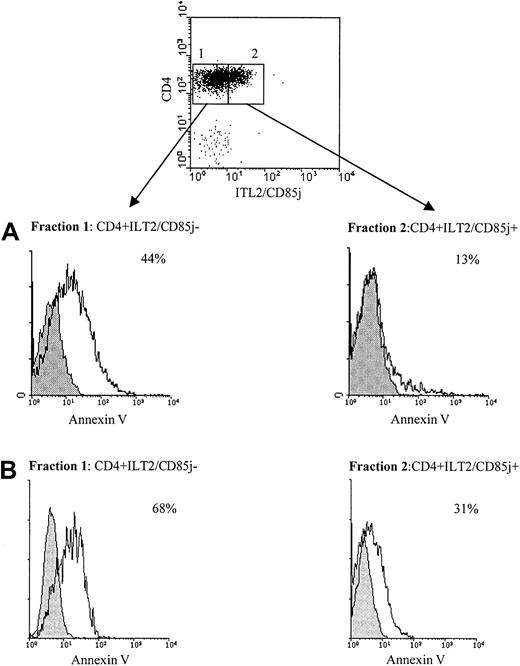
To study the difference in survival ability following CD3/TCR engagement between freshly isolated CD4+ Sézary cells and normal autologous CD4+ lymphocytes, we used cell sorting to separate CD4+ILT2+ and CD4+ILT2− subpopulations from the blood of patients with significant numbers of circulating Sézary cells. Figure 6 shows representative results obtained with 2 of 4 patients studied. The experiment presented in Figure 6A was performed with PBMCs from the previously described patient Cou, expressing 30% of CD4+TCRVβ13+circulating malignant cells.24 We verified with the anti-TCRVβ13 mAb that the CD4+ILT2+ isolated lymphocytes corresponded to the malignant Sézary cells (data not shown). The sorted fractions were then stimulated for 9 hours with an immobilized anti-CD3 mAb or an isotype-matched control mAb, followed by labeling with annexin V and PI. We found that the malignant CD4+ILT2+ population was less sensitive to anti-CD3 mAb–induced cell death than the CD4+ normal population (Figure 6A, lower panel). Similar results were obtained with a different patient, expressing 20% of CD4+ILT2+ malignant cells (Figure 6B). It must be noted that almost no annexin V–positive cells were obtained when both sorted populations were incubated with the isotype control mAb (Figure 6, shaded histograms).
Differential resistance to CD3/TCR-dependent activation-induced cell death of ILT2+CD4+CTCL cells and autologous ILT2− CD4+ T cells.
(A) Upper panel: PBMCs from a patient with 30% circulating Sézary cells were stained with anti-ILT2 GHI/75 mAb followed by FITC-conjugated GAM Ig. After extensive washing to remove the excess of GAM Ig and blocking with normal mouse Ig, TRI-conjugated anti-CD4 mAb was added. The CD4+ILT2+ and CD4+ILT2− subpopulations were sorted. Lower panel: The ILT2+CD4+ and ILT2−CD4+ cell fractions were incubated for 9 hours with 10 μg/mL immobilized anti-CD3 (unshaded histogram) or isotype control mAb (shaded histogram) and analyzed for binding of annexin V and PI exclusion by flow cytometry. Percentages indicated for each fraction correspond to anti-CD3–stimulated apoptotic cells and were calculated after exclusion of PI-stained cells. (B) PBMCs from a different SS patient with 20% circulating Sézary cells were separated into CD4+ILT2+ and CD4+ILT2− subpopulations and tested for their resistance to cell death induced through CD3/TCR engagement, as described above.
Differential resistance to CD3/TCR-dependent activation-induced cell death of ILT2+CD4+CTCL cells and autologous ILT2− CD4+ T cells.
(A) Upper panel: PBMCs from a patient with 30% circulating Sézary cells were stained with anti-ILT2 GHI/75 mAb followed by FITC-conjugated GAM Ig. After extensive washing to remove the excess of GAM Ig and blocking with normal mouse Ig, TRI-conjugated anti-CD4 mAb was added. The CD4+ILT2+ and CD4+ILT2− subpopulations were sorted. Lower panel: The ILT2+CD4+ and ILT2−CD4+ cell fractions were incubated for 9 hours with 10 μg/mL immobilized anti-CD3 (unshaded histogram) or isotype control mAb (shaded histogram) and analyzed for binding of annexin V and PI exclusion by flow cytometry. Percentages indicated for each fraction correspond to anti-CD3–stimulated apoptotic cells and were calculated after exclusion of PI-stained cells. (B) PBMCs from a different SS patient with 20% circulating Sézary cells were separated into CD4+ILT2+ and CD4+ILT2− subpopulations and tested for their resistance to cell death induced through CD3/TCR engagement, as described above.
Discussion
ILT2 is an inhibitory receptor specific for a broad range of MHC class Ia and Ib molecules and the viral class I–like protein UL18.30,31 All myeloid and B lymphocytes and a subset of NK lymphocytes express ILT2. In addition, this molecule along with KIR is expressed by peripheral CD8+ T cells with memory/effector phenotype.32 According to previous studies with the anti-ILT2 mAb GHI/75, most circulating CD4+ lymphocytes fail to express ILT2 on their cell surfaces, whereas the molecule is present in the cytoplasm of all T cells, including the CD4+ subpopulation.22This broad expression reflects a key role in the negative regulation of important immune functions, including antigen presentation, antigen- and superantigen-driven activation, and generation of effector and memory T cells.20,33 We previously demonstrated that CD4+ CTCL malignant cells express KIR3DL2/CD158k, while lacking expression of the other killer cell inhibitory receptors, including the lectinlike molecules.13 In addition, we recently reported an apparently novel molecule expressed at the cell surface of circulating Sézary cells, which provides inhibitory signals to CD3/TCR engagement of CTCL malignant cells.34
The present study addresses for the first time the expression of ILT2 in patients with CD4+ SS malignant cells. Initially, we established the surface expression of ILT2 in cytokine-dependent CD4+ malignant T-cell lines. Because ILT2 surface expression increases during lymphocyte activation and has been reported on all CD4+ T-cell clones studied,22 it was important to confirm our observation with peripheral circulating Sézary cells. Indeed, ILT2 was detected on the cell membrane of peripheral blood Sézary cells that were distinguished from normal reactive CD4+ lymphocytes with the appropriate anti-TCRVβ mAb. The TCRVβ use of the clonal Sézary cells was determined by immunoscope analysis of the PBLs of each patient. It should be noted that the profile of ILT2 mAb binding to the TCRVβ8+population corresponded to a weak homogeneous expression by the whole malignant clonal cell population. Interestingly, we found that CD4+ lymphocytes isolated from involved skin of patients with MF and pagetoid reticulosis fail to express detectable ILT2 molecules at their cell membranes. Thus, it appears that ILT2 membrane expression in CD4+ lymphocytes may be specific to SS malignant cells. Further, in 8 SS patients with various percentages of circulating malignant cells we found reactivity of the anti-ILT2 GHI/75 mAb with the CD4+ lymphocytes. Likewise, a malignant cell-restricted expression of ILT2 was recently reported for clonal expansions of CD8+ T large granular lymphocytes in asymptomatic patients.35 However, the level of cell membrane ILT2 detected within the CD4+ subset varied among the patients and did not necessarily correlate with the percentage of circulating malignant cells. Such heterogeneity in the level of ILT2 expression was observed by Saverino et al with normal T-cell clones.22 CTCLs do not correspond phenotypically to recently activated cells, since they do not systematically express the standard early activation markers,36,37 but rather correspond to memory cells that have undergone antigen-driven activation. Further, only a short-term increase of ILT2/CD85 expression is observed after in vitro activation.38 In this context, the increased surface expression of ILT2 on CTCL cells was unexpected and may reflect either aberrant regulation of the molecular traffic between the cytoplasm and the cell surface or a repeated stimulation of the tumor cells by a continuously present antigen. It should be noted that we failed to detect an increase of both ILT2-specific mRNA (PBLs) and the intracellular specific protein in the CD4+ lymphocytes from patients with SS. Thus, most probably the increased membrane expression of ILT2 reflects enhanced traffic from intracellular compartments.
Previous studies established a constitutive activation of the IL-2R Janus kinase-signal-transducing activator of transcription (Jak-STAT) signaling pathway in a number of malignant T-cell lines as a possible explanation for their uncontrolled growth.39,40Further, a lack of expression of the SHP-1 phosphatase due to extensive methylation of its gene promoter was recently reported in T-cell lymphomas as a possible explanation for the persistent activation of IL-2R/Jak and other signaling pathways.12 Considering that SHP-1 is essential for the generation of negative signals through ILT2, it might be speculated that the overexpression of ILT2 in Sézary cells simply reflects deficient ILT2/SHP-1 signaling. Unexpectedly, however, we detected the SHP-1 protein in both CTCL cell lines studied, and it was quantitatively identical to the amounts of SHP-1 found in normal PBLs (Figure 5A).
A persistence of IL-2R/Jak3 phosphorylation in the presence of SHP-1 had already been observed in few malignant T-cell lines and it was supposed that the SHP-1 protein expressed in CTCL cells was not functional.40 In the present study we demonstrated that in vitro phosphorylation of ILT2 molecules on tumor cells effectively results in the recruitment of SHP-1. Moreover, the ILT2/SHP-1 pair was functional, since the engagement of ILT2 by its specific mAb inhibited the anti-CD3–induced tumor cell line proliferation. The results we obtained with CTCL are similar to those reported for normal T cells in which cross-linking of ILT2 inhibits CD3-induced intracellular Ca++ mobilization and the proliferation of CD4+T cells.22
The existence of a functional ILT2 inhibitory pathway in CTCL does not preclude the constitutive IL-2R/Jak phosphorylation, because, according to our results, ILT2 does not interfere with cytokine receptor signaling. SHP-1 may not be available to IL-2R because it has already been recruited by inhibitory receptors. Indeed, we observed not only recruitment but also a basal level of SHP-1/ILT2 association (Figure 5B), which has not been reported for normal ILT2+cells.20,29 It has already been noted that TCR signaling in CTCL tumor cells is reduced, along with decreased activity of Zap70, Syk, and membrane Csk.41 Our results suggest that this deficiency may result at least in part from the overexpression of functional ILT2 inhibitory receptors.
Finally, we found that circulating malignant Sézary cells may be separated from the nonmalignant reactive CD4+ autologous T lymphocytes by means of anti-ILT2 mAb GHI/75. We found that these malignant cells, as compared with the autologous reactive CD4+ lymphocytes, are resistant to anti-CD3 mAb–induced cell death. These results suggest that CD4+ CTCL resistance to activation-induced cell death is associated with the expression of inhibitory receptors, as has been already reported for normal circulating effector or memory CD8+ T cells expressing ILT2 and KIR.32
In conclusion, the expression of functional inhibitory signaling molecules may perpetuate survival of CTCL malignant cells by protecting them from activation-induced apoptosis following CD3/TCR engagement. Furthermore, cytokines such as IL-7 were found to maintain the survival of CTCL by increased expression ofbcl-2.25,42 Thus, malignant Sézary cells exhibit receptors capable of inhibiting signaling through TCR, whereas these receptors do not inhibit proliferation induced by cytokines. These results are in agreement with the finding that IL-7 overexpression in transgenic mice causes cutaneous lymphomas.43 44 Finally, the identification of functional receptors expressed on CTCL malignant cells constitutes an important step toward an understanding of the molecular basis of the disease and the elaboration of novel therapeutic approaches.
Prepublished online as Blood First Edition Paper, April 30, 2002; DOI 10.1182/blood-2001-12-0303.
Supported by grants from INSERM, Paris, France, Association de la Recherche contre le Cancer, Villejuif, France, and GEFLUC, Ivry sur Seine, France.
M.N. and P.M. contributed equally to this work.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Armand Bensussan, INSERM 448, Faculté de Médecine de Créteil, 8 rue du Général Sarrail, 94010 Créteil, France; e-mail:armand.bensussan@im3.inserm.fr.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal