Abstract
Platelets, although not phagocytotic, have been suggested to release O. Since O-producing reduced nicotinamide adenine dinucleotide (phosphate) (NAD(P)H) oxidases can be specifically activated by certain agonists and are found in several nonphagocytotic tissues, we investigated whether such an enzyme is the source of platelet-derived O. We further studied which agonists cause platelet Orelease and whether platelet-derived O influences thrombus formation in vitro. Collagen, but not adenosine 5′-diphosphate (ADP) or thrombin, increased O formation in washed human platelets. This was a reduced nicotinamide adenine dinucleotide (NADH)–dependent process, as shown in platelet lysates. Consistent with a role of a platelet, NAD(P)H oxidase expression of its subunits p47phox and p67phoxand inhibition of platelet O formation by diphenylene-iodoniumchloride (DPI) and by the specific peptide-antagonist gp91ds-tat were observed. Whereas platelet-derived O did not influence initial aggregation, platelet recruitment to a preformed thrombus following collagen stimulation was significantly attenuated by superoxide dismutase (SOD) or DPI. It was also inhibited when ADP released during aggregation was cleaved by the ectonucleotidase apyrase. ADP in supernatants of collagen-activated platelets was decreased in the presence of SOD, resulting in lower ADP concentrations available for recruitment of further platelets. Exogenous Oincreased ADP- concentrations in supernatants of collagen-stimulated platelets and induced irreversible aggregation when platelets were stimulated with otherwise subthreshold concentrations of ADP. These results strongly suggest that collagen activation induces NAD(P)H oxidase–dependent O release in platelets, which in turn enhances availability of released ADP, resulting in increased platelet recruitment.
Introduction
Platelet activation is thought to be a key event in acute vascular thrombosis. Therefore, prevention of enhanced platelet activation is a major target of therapeutic strategies fighting cardiovascular and cerebrovascular diseases.1-3 An important stimulus for physiologic platelet activation and thrombus formation is the contact of platelets with components of the subendothelial matrix, like collagen.4 Although Marcus et al have shown as early as 1977 that platelets have the ability to release superoxide anions (O),5 it was only recently proposed that platelets stimulated by collagen produce reactive oxygen species (ROS) such as hydrogen peroxide,6hydroxyl radicals,7 or O.7,8 While O, a highly reactive radical, damages cells in high concentrations by reacting with proteins, lipids, and DNA, in low concentrations its continuous production, with similarity to second messengers, has been suggested to indirectly affect signal transduction processes.9,10 Platelet agonists other than collagen, such as thrombin or ADP, do not seem to induce ROS formation during aggregation.8 This difference raises the question whether O formation could serve a modulating function when thrombus formation is induced by collagen.
The cellular source of platelet O is unclear. Growing evidence supports the assumption that platelet activation by collagen is specifically due to binding to the glycoprotein VI (GPVI)–receptor,11,12 resulting in a cascade of tyrosine phosphorylation events ultimately leading to activation of phospholipase Cγ (PLCγ),13 which is known to strongly activate protein kinase C (PKC) through production of diacylglycerol.4 Recently, evidence for the existence of a neutrophil-type reduced nicotinamide adenine dinucleotide (phosphate) (NAD(P)H) oxidase in platelets that can be activated by PKC and is involved in O formation has been presented,14-16 similar as in other O-generating systems, like the vascular endothelium. In endothelial cells, an NAD(P)H oxidase is the main source of O.17 As O readily reacts with NO, this has been suggested to result in attenuated NO-dependent vessel relaxation,18,19 and the role of O in the regulation of vascular tone has become a major focus of interest.17 Moreover, antioxidants likeN-acetylcysteine (NAC) have been shown to exert direct antiaggregatory effects.20 Although these findings raise the possibility that platelet-derived O is involved in regulating platelet activation, evidence for a role of platelet-derived O in platelet function is rare. In a canine model of coronary arterial thrombosis, thrombus formation was regulated by intraplatelet redox state.21 Leo and colleagues have shown that platelets subjected to anoxia/reoxygenation are more reactive, due to an enhanced Ogeneration.14 However, so far it remains unclear whether an enhanced O production occurs also during direct platelet activation, such as with collagen, and how this could affect thrombus growth. Whereas conventional methods of measuring platelet aggregation in vitro are limited by the amount of cells used,22 the in vivo situation provides the thrombus with a continuous further supply of circulating platelets. These additional platelets, however, are activated not only by the stimulus that originally initiated thrombus formation, but also by substances like ADP, thromboxane A2, Ca++, and others that are released from platelet granules after activation.4 Therefore, the extent of thrombus formation in vivo is determined not only by the initial exposure to an agonist, but also by the release of further stimuli and the recruitment of further platelets caused by these substances.
In this study we sought to determine which agonists cause platelet O production, whether a neutrophil-type NAD(P)H oxidase is the source of this phenomenon, and how platelet function is affected by platelet release of O. We sought to discriminate between the aggregation induced by the initial agonist and the effect of released substances on unstimulated platelets, as the resulting recruitment represents a crucial element of thrombus growth. As ADP is one important mediator of recruitment, we investigated whether O increases ADP-dependent platelet reactions.23 Availability of this nucleotide is determined not only by the amount released, but also by ectonucleotidases metabolizing it,24 which, again, are known to be influenced by ROS in other systems.25 Therefore, we also investigated whether platelet-derived O can indirectly influence ADP metabolism, possibly by modulating the activity of ectonucleotidases.
Materials and methods
Platelet and neutrophil preparation
Venous blood was drawn from healthy volunteers who had not taken any medication for at least 10 days. Informed consent was obtained from all subjects. Blood that had been anticoagulated by 3.13% sodium citrate was centrifuged at 150g for 15 minutes. For experiments investigating platelet function, only the upper third of the supernatant was used to prevent leukocyte contamination. Washed platelets (WP) were prepared by another centrifugation step at 600g for 10 minutes and resuspended in calcium-free modified Tyrode buffer containing 138 mM sodium chloride, 2.7 mM potassium chloride, 12 mM sodium hydrogen carbonate, 400 μM disodium phosphate, 1 mM magnesium chloride, 5 mM D-glucose, and 5 mM HEPES (buffer A). WP were used for experiments within 2 hours. Polymorphonuclear neutrophils (PMNs) for control experiments were isolated from the buffy coat of blood that had been centrifuged at 150g for 15 minutes according to a magnetic antibody separation technique as previously described.26 Platelet and leukocyte counts were obtained using a resistance particle counter (Coulter Z2, Beckman Coulter, Krefeld, Germany).
Measurement of superoxide formation by chemiluminescence assay
O production was measured by a chemiluminescence assay using the dye L-012. We have previously described the high specificity and sensitivity of this assay for O.27 The reaction volume of 300 μL contained 150 000-300 000/μL WP, calcium chloride (1 mM), and L-012 (100 μM). After measuring the background signal, stimulating substances such as phorbol-12-myristate 13-acetate (PMA, 1 μM), collagen (6 μg/mL), adenosine diphosphate (ADP, 1-50 μM), or thrombin (0.1-1 U/mL) were added. Photon emission was expressed as percent increase in relative light units versus control conditions. Maximum leukocyte contamination in the reaction buffers was 9/μL.
Measurement of superoxide formation by cytochromec assay
In an alternative method to assess platelet Oproduction, the cytochrome c method was used with minor modifications as described before.28 WP (10 × 108) were incubated with cytochrome c(40 μM), which was dissolved in phosphate-buffered saline with or without superoxide dismutase (SOD, 250 U/mL) and the respective stimuli. All stimulations were performed in the presence of 1 mM calcium chloride. The reduction of cytochrome c was measured in a spectrophotometer (Ultrospec 2000, Tecan, Grödig, Austria) at 550 nm over a 30-minute period. The superoxide-dependent part of cytochrome c reduction was calculated from the difference between samples incubated with or without SOD (ε550 nM = 21.1 mM−1cm−1).
Measurement of NADH/NAD(P)H oxidase activity
WP were stimulated as indicated. After the reaction was stopped by adding 2 mM ethyleneglycoltetraacetic acid (EGTA), platelets were pelleted by centrifugation at 600g for 10 minutes at 4°C and resuspended in lysis buffer containing 1 mM sodium fluoride, 150 μM tetra-sodium-diphosphate-10-hydrate, 1 mM ethylene-diaminetetraacetic acid (EDTA), 400 μM sodiumorthovanadate, 4 mM disodiumphosphate, 10 μM phenylmethylsulfonyl fluoride, and 10 μg/mL each of leupeptin, pepstatin, and aprotinin. The platelets were then disrupted by passing them 5 times through a 29-gauge needle, followed by storage on ice for 24 hours. Disruption was confirmed microscopically. For detection of O formed by the lysates, the cytochrome creduction method was used as described above using 10 μg protein per experiment. Various concentrations of NAD(P)H or reduced nicotinamide adenine dinucleotide (NADH) as indicated were used to start the reaction, and reduction of cytochrome cwas measured for 30 minutes. Protein content was determined by the method of Bradford.29
Immunoblotting studies
Platelets were washed in the presence of prostacyclin analog (iloprost, 0.01 μg/mL) and resuspended in buffer A. After being adjusted to a final volume of 500 000/μL in the presence of iloprost (0.1 μg/mL) and 1 mM EGTA and subsequent stimulation, lysis was performed by adding an equal volume of ice-cold lysis buffer containing Tris, 50 mM; EDTA, 1 mM; sodium orthovanadate, 1 mM; sodium fluoride, 10 mM; tetra-sodium-diphosphate 10-hydrate, 1.5 mM; disodium hydrogen phosphate, 4 mM; leupeptin, aprotinin, pepstatin, 1 μM each; phenylmethylsulfonyl fluoride, 1 mM; Triton-X 100, 1%; sodium dodecyl sulfate (SDS), 0.1%; and desoxycolic acid, 0.5% (buffer B) to the platelet suspension. Lysates were centrifuged at 10 000g for 10 minutes to remove debris, and the supernatants were used for experiments. Proteins were separated by SDS–polyacrylamide gel electrophoresis following standard procedures and transferred to a nitrocellulose membrane. After blocking for 30 minutes in blocking buffer (sodium chloride, 200 mM; Tris pH 7.5, 50 mM; bovine serum albumin (BSA), 3%; Tween 20, 0.05%; and horse serum, 10%; membranes were incubated with the respective primary antibody in blocking buffer for 1 hour at room temperature. They were then washed 4 times using TBS-T (Tris base, 50 mM; sodium chloride, 150 mM; Tween 20, 0.1%) and incubated with horseradish peroxidase–conjugated secondary antibody for 1 hour at room temperature. The signal intensities were measured using an enhanced chemiluminescence (ECL) detection system following the instructions of the kit used (ECL Western blotting detection reagents, Amersham, Freiburg, Germany). Bands were recorded with a videodensitometric system from Bio-Rad (Gel Doc 1000), Munich, Germany.
Aggregation studies
Platelet aggregation was measured using the turbidimetric method described by Born.22 WP were adjusted to a concentration between 150 000 and 300 000/μL. Calcium chloride (1 mM) was added 2 minutes before starting the reaction, and aggregation was measured photometrically using a 2-chamber aggregometer (elvi 840, Logos, Milan, Italy) under continuous stirring at 1000 rpm at 37°C.
Recruitment studies
To determine platelet recruitment, 2 different approaches were used. One was performed according to a method described by Freedman and colleagues.30 Aggregation was measured for 7 minutes. Then an equal portion of untreated WP was added to the tube, which increased the density of the solution and hence led to a reduction of light transmission. Aggregation of the newly added platelet-portion in the presence of an existing thrombus was then measured for 5 minutes and expressed as percentage of the aggregation that had been reached initially. In the second assay, recruitment was assessed according to a method described by Valles et al.31 In this assay, platelets were stimulated under various conditions as indicated at 37°C without stirring. After stimulation, cells and collagen were pelleted at 10 000g for 50 seconds, and the cell- and stimulus-free supernatant was immediately transferred as a stimulus to another platelet suspension placed in an aggregometer. Concentration in the final suspensions was adjusted to 200 000/μL. Then aggregation was measured as described above.
Platelet ADP release
WP were counted and adjusted to a final concentration of 500 000/μl in buffer A. To prevent aggregation and activation due to exogenously added ADP, iloprost (0.05 μg/mL) was added. Platelets were then stimulated with collagen, and samples of the supernatant were taken at 7 minutes after stimulation. Supernatant samples were obtained by centrifugation at 1500g to pellet the platelets and remove the supernatant, which was immediately mixed with perchloric acid (400 mM) to stop potential enzymatic activities of nucleotidases. After addition of perchloric acid, samples were centrifuged at 10 000g for 5 minutes at 4°C to remove precipitates and the amount of ADP in the supernatant quantified by high-performance liquid chromatography (HPLC). As previously described,32the acidified samples were applied to an EC 250/4 nucleosil carbohydrate column and eluted with 10 mM NH4H2PO4 (A; at pH 3.5) or 0.5 mM NH4H2PO4 (B; at pH 3.0) using a gradient of 100% of A for 15 minutes, then 30% of B for 1 minute, 40% of B for 4 minutes, and 100% of A at minute 20. Retention time for ADP was 10.5 minutes.
Materials
Collagen was purchased as fibrils from Nycomed Pharma, Munich, Germany; 8-amino-5-chloro-7-phenylpyridol(3,4-d)pyridazine-1,4(2H,3H)dione (L-012) was obtained from Aventis, Frankfurt, Germany; and superoxide dismutase from Roche Molecular Biochemicals, Penzberg, Germany. Anti-p47phox, anti-p67phox, and anti-gp91phox were obtained from Santa Cruz Biotechnology (Heidelberg, Germany). Prostacyclin analog (iloprost) was purchased from Schering (Berlin, Germany). Erbstatine analog was from Bachem (Heidelberg, Germany); the peptides gp91ds-tat and scrambled gp91ds-tat (scrmb-tat) were kind gifts from Dr Patrick Pagano (Detroit, MI). EC 250/4 nucleosil carbohydrate columns were from Macherey-Nagel (Düren, Germany). All other substances were obtained from Sigma Chemicals, Darmstadt, Germany.
Statistical analysis
For descriptive purposes, all data are expressed as means ± SEM. Data were analyzed using one-way ANOVA or Student t test. For paired recruitment experiments, paired t tests were performed. Differences were considered significant when the error probability level wasP < .05.
Results
Superoxide formation in platelets is stimulus specific
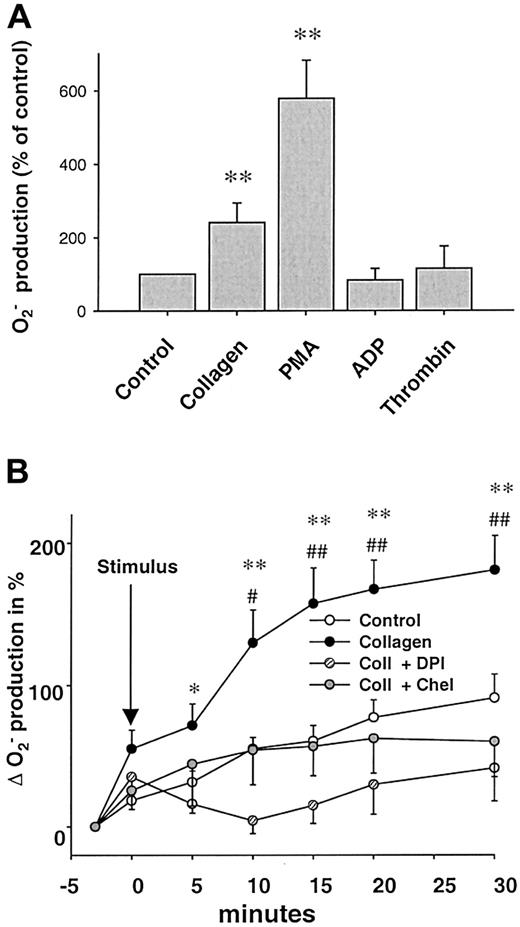
Stimulation with collagen (6 μg/mL) or phorbol ester (PMA, 1 μM) led to a significant, long-lasting 2.4-fold and 5.8-fold increase in O production compared to control platelets (n = 24, P < .05, n = 21, andP < .01, respectively, 10 minutes after stimulation). In contrast, ADP in a concentration range from 1 to 50 μM as well as thrombin (0.1-1 U/mL) did not enhance platelet Oformation significantly (Figure 1A). Both SOD and NAC abolished the collagen- or PMA-induced Osignal (Figure 2A), whereas boiled SOD was without effect. Collagen-induced O production was furthermore completely prevented by the specific PKC-inhibitor chelerythrine (50 μM, n = 10, P < .01, Figure 1B) or the tyrosine kinase inhibitor erbstatine analog (1 μM, n = 4,P < .05, not shown). To control our findings, platelet O production was also assessed using the cytochromec reduction method (Figure 2C). In these experiments, collagen also induced a 2.1-fold increase in Oproduction over control conditions (0.0323 ± 0.008 vs 0.0155 ± 0.004 nmol/min/105 cells, n = 6,P < .05). To exclude that neutrophils contaminating the solution accounted for the observed O release, control experiments with increasing concentrations of neutrophils (10-100/μL) in buffer alone or in platelet suspensions were performed. Neutrophils alone or stimulated with collagen did not show an increase of L-012 chemiluminescence (L-012-CL). These neutrophils, however, showed a substantial O production when stimulated with the calcium ionophore A23187 (1 μM, Table 1).
Collagen induces platelet O production.
(A) Effects of various stimuli on platelet superoxide (O) production. Collagen (6 μg/mL, n = 24) and phorbol-12-myristate 13-acetate (PMA, 1 μM, n = 21), but not ADP (5-50 μM, n = 9) or thrombin (0.1-1 U/mL, n = 12), increased O formation by platelets (10 minutes after stimulation). O was measured by L-012 chemiluminescence. (B) Influence on NAD(P)H oxidase and PKC inhibitors on collagen-induced (6 μg/mL) O formation. Kinetics of collagen-induced O production and its inhibition by DPI (100 μM, n = 6) and chelerythrine (50 μM, n = 10). Data are shown as mean ± SEM; * (#), ** (##) significantly different at P < .05, .01 vs stimulus and DPI (*) or chelerythrine (#).
Collagen induces platelet O production.
(A) Effects of various stimuli on platelet superoxide (O) production. Collagen (6 μg/mL, n = 24) and phorbol-12-myristate 13-acetate (PMA, 1 μM, n = 21), but not ADP (5-50 μM, n = 9) or thrombin (0.1-1 U/mL, n = 12), increased O formation by platelets (10 minutes after stimulation). O was measured by L-012 chemiluminescence. (B) Influence on NAD(P)H oxidase and PKC inhibitors on collagen-induced (6 μg/mL) O formation. Kinetics of collagen-induced O production and its inhibition by DPI (100 μM, n = 6) and chelerythrine (50 μM, n = 10). Data are shown as mean ± SEM; * (#), ** (##) significantly different at P < .05, .01 vs stimulus and DPI (*) or chelerythrine (#).
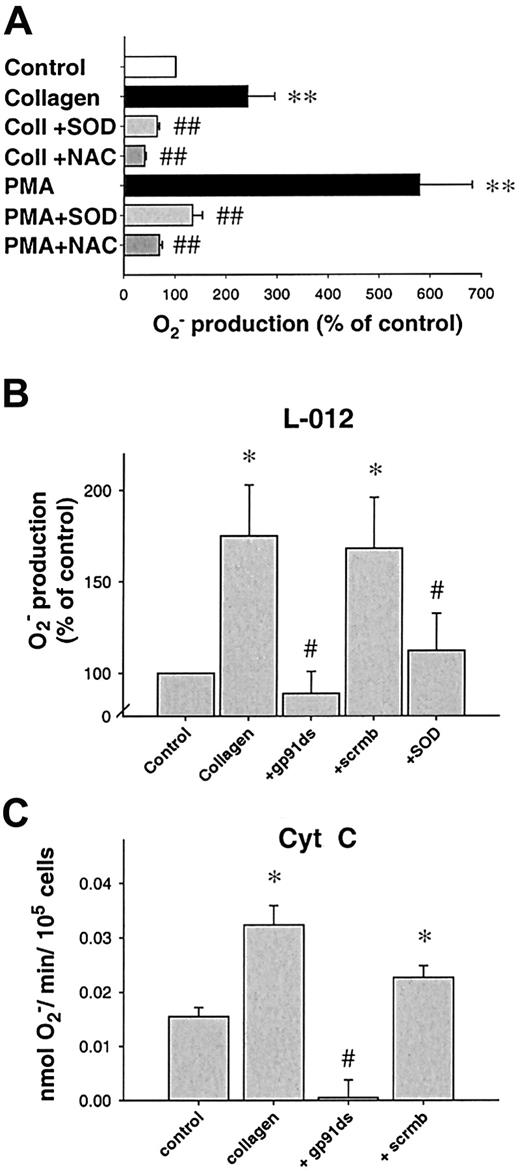
Inhibition of platelet Orelease.
(A) Effect of O scavengers on platelet release of O. N-acetylcysteine (NAC, 1 mM, n = 9) or superoxide dismutase (SOD, 250 U/mL, n = 9) significantly decreased O release after PKC stimulation using PMA, whereas boiled SOD was without effect (not shown). When collagen was used as a stimulus, similar results were obtained (6 μg/μL, n = 7 each, 10 minutes after stimulation). (B) Specific NAD(P)H oxidase inhibitors prevent platelet O production. A peptide specifically inhibiting the interaction between gp91phox(or its analogs) and p47phox (gp91ds-tat, 100 μM) but not its scrambled analog (scrmbl-tat, 100 μM) inhibits platelet O production as measured by L-012 chemiluminescence (n = 9, at 10 minutes). (C) Amount of O production as measured by cytochrome creduction. Platelet suspensions were stimulated in cytochromec dissolved in PBS, after preincubation with the respective inhibitors when indicated (n = 6, at 10 minutes). Data are shown as mean ± SEM; *, ** significantly different versus control atP < .05 and .01, respectively; #, ##P < .05 and .01 versus stimulus.
Inhibition of platelet Orelease.
(A) Effect of O scavengers on platelet release of O. N-acetylcysteine (NAC, 1 mM, n = 9) or superoxide dismutase (SOD, 250 U/mL, n = 9) significantly decreased O release after PKC stimulation using PMA, whereas boiled SOD was without effect (not shown). When collagen was used as a stimulus, similar results were obtained (6 μg/μL, n = 7 each, 10 minutes after stimulation). (B) Specific NAD(P)H oxidase inhibitors prevent platelet O production. A peptide specifically inhibiting the interaction between gp91phox(or its analogs) and p47phox (gp91ds-tat, 100 μM) but not its scrambled analog (scrmbl-tat, 100 μM) inhibits platelet O production as measured by L-012 chemiluminescence (n = 9, at 10 minutes). (C) Amount of O production as measured by cytochrome creduction. Platelet suspensions were stimulated in cytochromec dissolved in PBS, after preincubation with the respective inhibitors when indicated (n = 6, at 10 minutes). Data are shown as mean ± SEM; *, ** significantly different versus control atP < .05 and .01, respectively; #, ##P < .05 and .01 versus stimulus.
Neutrophils do not contribute to superoxide (O) production of platelet suspensions stimulated with collagen
| . | O production % of control . | P (vs control) . |
|---|---|---|
| Platelets + collagen, n = 4 | 207.47 ± 22.86 | < .05 |
| 10 PMN/μL + collagen, n = 4 | 87.49 ± 4.42 | NS |
| 50 PMN/μL + collagen, n = 4 | 101.61 ± 29.32 | NS |
| 100 PMN/μL + collagen, n = 4 | 112.84 ± 8.54 | NS |
| 100 PMN/μL, n = 4 | 115.52 ± 10.75 | NS |
| Platelets + 100 PMN/μL + collagen, n = 4 | 220.61 ± 28.06 | < .05 |
| 100 PMN/μL + A23187, n = 4 | 985.26 ± 30.46 | < .01 |
| . | O production % of control . | P (vs control) . |
|---|---|---|
| Platelets + collagen, n = 4 | 207.47 ± 22.86 | < .05 |
| 10 PMN/μL + collagen, n = 4 | 87.49 ± 4.42 | NS |
| 50 PMN/μL + collagen, n = 4 | 101.61 ± 29.32 | NS |
| 100 PMN/μL + collagen, n = 4 | 112.84 ± 8.54 | NS |
| 100 PMN/μL, n = 4 | 115.52 ± 10.75 | NS |
| Platelets + 100 PMN/μL + collagen, n = 4 | 220.61 ± 28.06 | < .05 |
| 100 PMN/μL + A23187, n = 4 | 985.26 ± 30.46 | < .01 |
NS indicates not significant.
NAD(P)H oxidase–dependent superoxide formation in platelets
Next, we sought to investigate the enzymatic source of collagen-induced O production in platelets. We used various inhibitors of possible enzymatic sources. Rotenone (10-50 μM), indomethacin (1-10 μM), or oxypurinol (100-300 μM) were without effect on collagen-induced O production (not shown), making mitochondrial electron transport, cyclooxygenase, or xanthine oxidase improbable sources. In contrast, DPI (100 μM), an unspecific inhibitor of NAD(P)H oxidase, completely abolished O formation caused by collagen (n = 6,P < .01) and PMA (n = 7, P < .0001, Figure 1B), as did the specific inhibitor of NAD(P)H oxidase, the chimeric peptide gp91ds-tat. When preincubated for 30 minutes, gp91ds-tat (100 μM) completely abolished collagen-induced O production as measured by L-012-CL (n = 6, P < .05, Figure 2B) or cytochromec reduction (Figure 2C). In both cases, the same concentration of the scrambled peptide (scrmb) was without effect (n = 6).
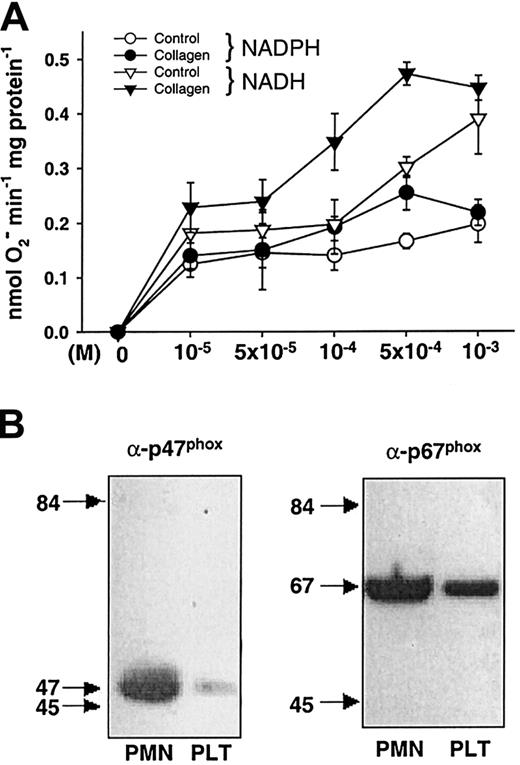
To further determine whether O production in platelets was NAD(P)H oxidase dependent, we measured O formation in lysates of untreated or collagen-stimulated platelets in the presence or absence of various concentrations of NADH or NAD(P)H. Platelet superoxide production in the presence of NADH had a dose-dependent effect, with highest levels observed at 500 μM (Figure 3A). NAD(P)H also increased the signal, although to a lesser extent than NADH. Treatment with collagen before lysis increased O production in the presence of both substrates (n = 3, Figure 3A). PMA also increased it to 0.64 ± 0.12 nmol/mg protein/min (P < .01, n = 13, not shown), whereas pretreatment with ADP (50 μM) as a control did not significantly enhance it (not shown).
NADH/NAD(P)H oxidase activity and expression.
(A) NADH/NAD(P)H oxidase activity in stimulated platelets. Addition of NADH led to a dose-dependent increase in O production in lysates of platelets (n = 3). When platelets had been stimulated with collagen, it was further increased compared to lysates of control platelets. NAD(P)H as a cosubstrate caused less O production (n = 3). (B) NAD(P)H oxidase subunit expression in platelets. Immunoblots of platelet protein expression (PLT) of the NAD(P)H oxidase subunits p47phox and p67phox compared to neutrophil protein (PMN) as a positive control. As described in “Materials and methods,” 40 μg of protein lysed was subjected to SDS–polyacrylamide-gel-electrophoresis, blotted on nitrocellulose membranes, and visualized using polyclonal antibodies against the indicated proteins.
NADH/NAD(P)H oxidase activity and expression.
(A) NADH/NAD(P)H oxidase activity in stimulated platelets. Addition of NADH led to a dose-dependent increase in O production in lysates of platelets (n = 3). When platelets had been stimulated with collagen, it was further increased compared to lysates of control platelets. NAD(P)H as a cosubstrate caused less O production (n = 3). (B) NAD(P)H oxidase subunit expression in platelets. Immunoblots of platelet protein expression (PLT) of the NAD(P)H oxidase subunits p47phox and p67phox compared to neutrophil protein (PMN) as a positive control. As described in “Materials and methods,” 40 μg of protein lysed was subjected to SDS–polyacrylamide-gel-electrophoresis, blotted on nitrocellulose membranes, and visualized using polyclonal antibodies against the indicated proteins.
As NAD(P)H oxidases are composed of at least 4 subunits in neutrophils and other vascular cells,17 of which the small g-protein rac, p22phox, and p67phox have previously been shown to be expressed in platelets,16 33 we performed immunoblotting studies investigating the presence of the remaining unidentified subunits p47phox, gp91phox, and p67phox. Although we could demonstrate protein expression of p47phox and of p67phox in all of our platelet preparations (n = 5), we could not detect gp91phox using commercially available antibodies (Figure3B).
Aggregation of platelets is differentially affected by superoxide
Aggregation induced by various doses of collagen was not influenced by endogenous O, since SOD (500 U/mL) did not significantly affect the extent of aggregation (Table2). Likewise, treatment with the inhibitor of NAD(P)H oxidase, DPI (30 μM), had no significant effect on aggregation. SOD or DPI also had no effect when aggregation was induced by various doses of ADP. In contrast, the platelets were reactive to NO-synthase inhibition. When collagen-stimulated platelets were incubated with the NO-synthase inhibitorN-nitro-L-arginine (L-NA, 30 μM), aggregation was significantly enhanced (Table 2).
No influence of platelet-derived O on aggregation
| . | Collagen control n = 10 . | Collagen + SOD n = 10 . | Collagen + DPI n = 9 . | Collagen + L-NA n = 9 . | ADP control n = 10 . | ADP + SOD n = 10 . |
|---|---|---|---|---|---|---|
| Aggregation at 3 minutes (% of maximal aggregation) | 67.2 ± 3.87 | 74.5 ± 4.68 | 70.5 ± 6.50 | 90.5 ± 3.56 | 83.3 ± 1.01 | 80.7 ± 2.85 |
| P | NS | NS | NS | < .01 | NS | NS |
| . | Collagen control n = 10 . | Collagen + SOD n = 10 . | Collagen + DPI n = 9 . | Collagen + L-NA n = 9 . | ADP control n = 10 . | ADP + SOD n = 10 . |
|---|---|---|---|---|---|---|
| Aggregation at 3 minutes (% of maximal aggregation) | 67.2 ± 3.87 | 74.5 ± 4.68 | 70.5 ± 6.50 | 90.5 ± 3.56 | 83.3 ± 1.01 | 80.7 ± 2.85 |
| P | NS | NS | NS | < .01 | NS | NS |
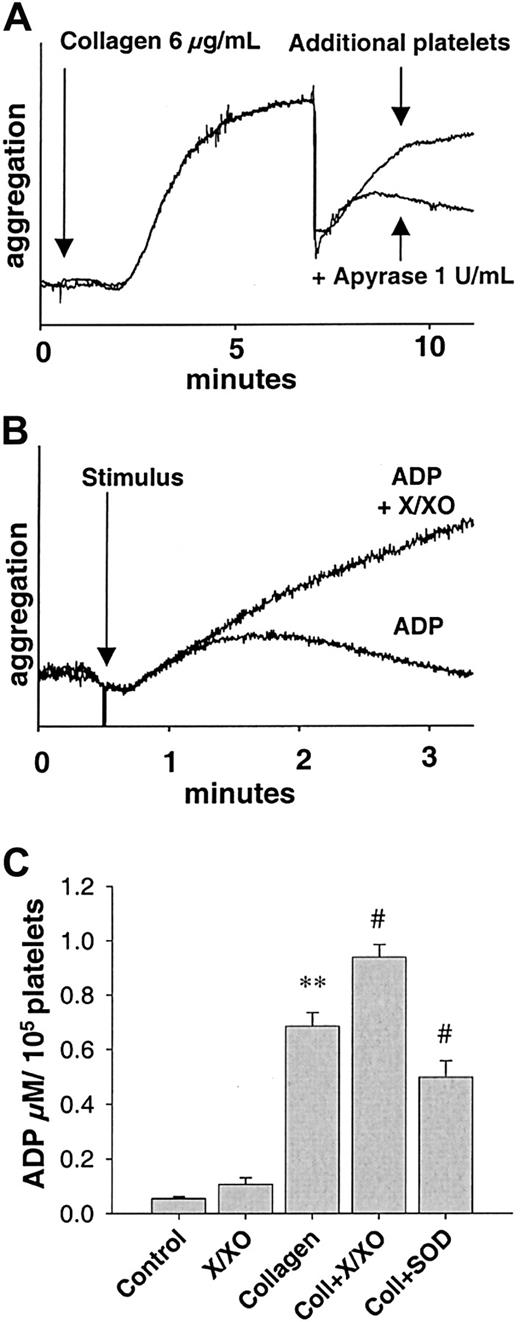
Preincubation of platelets with exogenous Ousing the O-generating system xanthine (300 μM)/xanthine oxidase (2 mU/mL, 30 seconds) showed different results depending on the stimulus. While aggregation in response to collagen was not altered, O markedly enhanced the sensitivity for ADP. At a concentration of 1 μM, ADP induced only a reversible aggregation under control conditions, while it induced irreversible aggregation in the presence of O(n = 9, Figure 4B). Xanthine/xanthine oxidase (X/XO) alone did not induce aggregation during the period observed in these experiments (not shown).
ADP-dependent recruitment following collagen stimulation.
(A) Tracing of parallel recruitment experiments showing dependence of recruitment on ADP release following collagen stimulation. Aggregation was induced by 6 μg/mL collagen fibrils. At 7 minutes following stimulation, additional platelets were added simultaneously with 1 U/mL apyrase or the same volume of vehicle. Apyrase, which cleaves ADP, inhibited recruitment of further platelets (graph representative of 9 similar experiments). (B) Enhancement of ADP-dependent platelet aggregation by reactive oxygen species. Tracing of a parallel experiment showing enhanced aggregation of a subthreshold concentration of ADP (1 μM) in the presence of exogenously created O using xanthine (300 μM, X) and xanthine oxidase (2 mU/mL, XO, graph representative of 9 similar experiments). (C) ADP in platelet supernatants. ADP measured in the supernatants of collagen-stimulated platelets is decreased in the presence of SOD (500 U/mL) and increased in the presence of X/XO (concentrations as above). In supernatants of unstimulated platelets, there was little release of ADP, which was not significantly increased by treatment with X/XO alone (n = 11). **Significantly different versus control atP < .01; #, P < .05 versus collagen.
ADP-dependent recruitment following collagen stimulation.
(A) Tracing of parallel recruitment experiments showing dependence of recruitment on ADP release following collagen stimulation. Aggregation was induced by 6 μg/mL collagen fibrils. At 7 minutes following stimulation, additional platelets were added simultaneously with 1 U/mL apyrase or the same volume of vehicle. Apyrase, which cleaves ADP, inhibited recruitment of further platelets (graph representative of 9 similar experiments). (B) Enhancement of ADP-dependent platelet aggregation by reactive oxygen species. Tracing of a parallel experiment showing enhanced aggregation of a subthreshold concentration of ADP (1 μM) in the presence of exogenously created O using xanthine (300 μM, X) and xanthine oxidase (2 mU/mL, XO, graph representative of 9 similar experiments). (C) ADP in platelet supernatants. ADP measured in the supernatants of collagen-stimulated platelets is decreased in the presence of SOD (500 U/mL) and increased in the presence of X/XO (concentrations as above). In supernatants of unstimulated platelets, there was little release of ADP, which was not significantly increased by treatment with X/XO alone (n = 11). **Significantly different versus control atP < .01; #, P < .05 versus collagen.
Platelet recruitment is ADP dependent and enhanced by superoxide
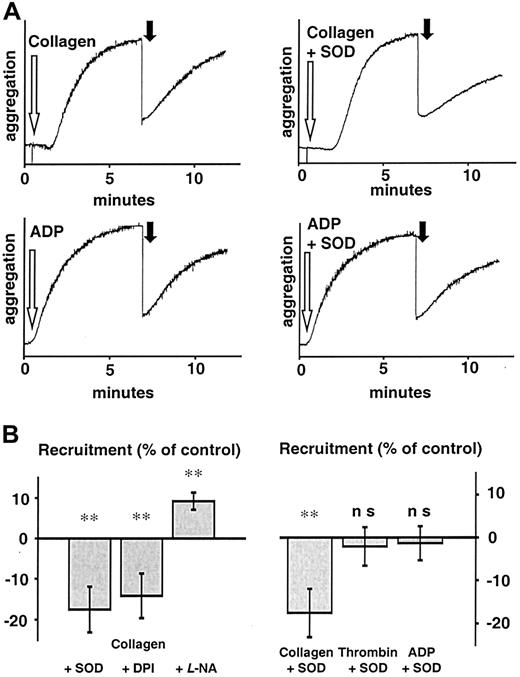
As O production by platelets was observed to be sustained for up to 30 minutes (Figure 1B), we further studied the role of its release on the recruitment of platelets that were brought in contact with the thrombus 7 minutes after initial stimulation of aggregation. At this time, significant amounts of Ohad been observed to be released by the collagen-stimulated thrombus (Figure 1A-B). To investigate the effect of Ogenerated by collagen-stimulated platelets, we performed recruitment experiments using either the O scavenger SOD (500 U/mL) or DPI (100 μM; representative traces are shown in Figure5A). Only following collagen stimulation (6 μg/mL) did SOD and DPI decrease platelet recruitment by 19% and 16%, respectively, whereas recruitment following thrombin- or ADP-induced aggregation was not affected by SOD or DPI (n = 10 each,P < .01 for collagen vs collagen + SOD or collagen vs collagen + DPI, Figure 5A-B). We could also confirm the observation that platelet recruitment is dependent on platelet NO production by preincubating platelets with L-NA (30 μM) for 15 minutes. These platelets showed not only an increased velocity of aggregation, but also a 13% increase in recruitment (P < .001, Figure 5B), as measured by the method of Freedman et al.30 Furthermore, the extent of recruitment following ADP-induced aggregation was significantly less than that following collagen stimulation (6 μg/mL, n = 10,P < .05), in spite of the fact that high doses of ADP (50 μM) were used.
Influence of antioxidants on recruitment following collagen stimulation.
(A) Representative tracings of platelet recruitment experiments. Recruitment induced by various agonists showed different reactions to scavenging of O and inhibition of NAD(P)H oxidase. Seven minutes after platelets had been stimulated (white arrow) in the presence of either vehicle or SOD (500 U/mL), an equal amount of untreated platelets was added (black arrow) and recruitment of additional platelets to the preformed thrombus measured for another 5 minutes. Aggregation of recruited platelets resembled that of ADP-induced aggregation, indicating that ADP release from platelets during aggregation is the main stimulus acting on recruitable platelets. (B) Left: Aggregation of recruited platelets at 5 minutes after addition to a preformed thrombus as percent of control (recruitment following collagen stimulation). SOD (500 U/mL) and DPI (100 μM) significantly decreased recruitment, whereas L-NA (30 μM) enhanced it (n = 10 each). Right: SOD only decreased recruitment when stimulation was due to an agonist that induces platelet O production like collagen, but not when it was due to thrombin or ADP (n = 10 each). Boiled SOD was without effect (not shown). **Significantly different versus control atP < .01.
Influence of antioxidants on recruitment following collagen stimulation.
(A) Representative tracings of platelet recruitment experiments. Recruitment induced by various agonists showed different reactions to scavenging of O and inhibition of NAD(P)H oxidase. Seven minutes after platelets had been stimulated (white arrow) in the presence of either vehicle or SOD (500 U/mL), an equal amount of untreated platelets was added (black arrow) and recruitment of additional platelets to the preformed thrombus measured for another 5 minutes. Aggregation of recruited platelets resembled that of ADP-induced aggregation, indicating that ADP release from platelets during aggregation is the main stimulus acting on recruitable platelets. (B) Left: Aggregation of recruited platelets at 5 minutes after addition to a preformed thrombus as percent of control (recruitment following collagen stimulation). SOD (500 U/mL) and DPI (100 μM) significantly decreased recruitment, whereas L-NA (30 μM) enhanced it (n = 10 each). Right: SOD only decreased recruitment when stimulation was due to an agonist that induces platelet O production like collagen, but not when it was due to thrombin or ADP (n = 10 each). Boiled SOD was without effect (not shown). **Significantly different versus control atP < .01.
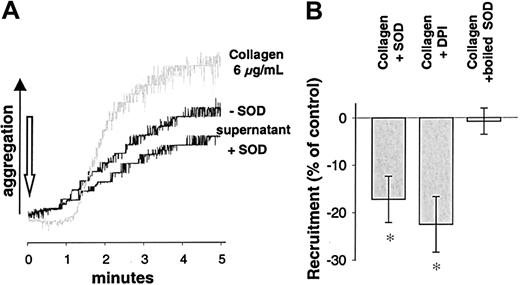
To control these findings, an alternative model of measuring platelet recruitment was performed as described by Valles et al.31Supernatants of platelet suspensions stimulated by collagen in the presence of SOD induced a decreased aggregation as compared to supernatants of platelets exposed to collagen alone (−17.2% ± 4.9% for SOD, n = 7, P < .05; and 22.5% ± 5.9% for DPI, n = 7, P < .05, Figure6B). Again, this was not the case when ADP or ADP in the presence of SOD was used as the initial stimulus (n = 7). Aggregation induced by the supernatants of collagen-stimulated platelets showed different kinetics and a faster onset than that directly induced by collagen (Figure 6A, gray tracing). These tracings rather resembled tracings of aggregation induced by ADP. Therefore, we tested whether the recruitment observed after stimulation with collagen was due to ADP release. Apyrase, an ectonucleotidase that hydrolyzes ADP to adenosine monophosphate (AMP) and free phosphate, was used to address this issue. Apyrase (1 U/mL) completely abolished platelet recruitment (n = 9, Figure 4A) in both models of platelet recruitment, indicating that release of ADP was indispensable for recruitment following collagen stimulation.
Aggregation induced by supernatants of collagen-stimulated platelets.
(A) Recruitment measured as aggregation induced by supernatants of activated platelets. Aggregation tracings of WP stimulated with supernatants of differentially activated platelets. WP were stimulated with collagen (6 μg/mL) in the presence or absence of SOD (500 U/mL) and pelleted by centrifugation. The supernatants were collected and immediately transferred to an aggregation cuvette with unstimulated platelets to measure aggregation of these untreated WP. As a background tracing collagen-induced aggregation (6 μg/mL) is shown (gray). (B) Recruitment is inhibited by SOD or DPI. Recruitment measured as aggregation induced by supernatants obtained as described above was significantly inhibited by SOD (500 U/mL) when collagen was used as a stimulus. Boiled SOD was without effect. Similar results were obtained when inhibiting NAD(P)H oxidase using DPI (100 μM, n = 7). *Significantly different versus control (stimulus without SOD or DPI) at P < .05.
Aggregation induced by supernatants of collagen-stimulated platelets.
(A) Recruitment measured as aggregation induced by supernatants of activated platelets. Aggregation tracings of WP stimulated with supernatants of differentially activated platelets. WP were stimulated with collagen (6 μg/mL) in the presence or absence of SOD (500 U/mL) and pelleted by centrifugation. The supernatants were collected and immediately transferred to an aggregation cuvette with unstimulated platelets to measure aggregation of these untreated WP. As a background tracing collagen-induced aggregation (6 μg/mL) is shown (gray). (B) Recruitment is inhibited by SOD or DPI. Recruitment measured as aggregation induced by supernatants obtained as described above was significantly inhibited by SOD (500 U/mL) when collagen was used as a stimulus. Boiled SOD was without effect. Similar results were obtained when inhibiting NAD(P)H oxidase using DPI (100 μM, n = 7). *Significantly different versus control (stimulus without SOD or DPI) at P < .05.
Oxidative influence on ADP released after collagen stimulation
We had observed that collagen stimulation led to a long-lasting production of O and that ADP release was a crucial element of platelet recruitment following collagen stimulation. Furthermore, platelets that had been pretreated with X/XO had shown an increased aggregation response to ADP. We speculated that an altered activity of a platelet ectonucleotidase could have been involved. Inactivation of ectonucleotidases resulting in increased concentrations of ADP has been described before.25 Therefore, we investigated whether there is evidence for altered amounts of ADP available to recruit further platelets under different circumstances, by measuring amounts of ADP released from platelets. Supernatants of unstimulated platelets contained little ADP (0.06 ± 0.01 μmol/min/100 000 cells, n = 11), which was not significantly altered when experiments were performed in the presence of exogenous ROS using the X/XO system (0.11 ± 0.02 μmol/min/100 000 cells, n = 5). In supernatants of collagen-stimulated platelets, however, there were high amounts of ADP (0.69 ± 0.05 μmol/min/100 000 cells, n = 11, n < .01). They were even increased (to 0.94 ± 0.05 μmol/min/100 000 cells, n = 5,P < .05), when collagen stimulation was performed in the presence of exogenous ROS produced through the X/XO system (X: 300μM, XO: 2mU). Scavenging O derived from collagen-stimulated platelets by SOD (500 U/mL) reduced amounts of ADP in the supernatants to 0.49 ± 0.06 μM/min/100 000 cells (n = 11, P < .05; Figure 4C).
Discussion
This study demonstrates that collagen induces an enhanced release of O from platelets through activation of an NAD(P)H oxidase. The released O modulates platelet function in an autocrine manner by increasing ADP-dependent recruitment of further platelets to a forming thrombus, whereas the initial aggregation is not affected by O.
While in accordance with other observations, all untreated platelets showed a basal O release5; only collagen stimulated the platelets to increase Orelease substantially. This response was specific for collagen,8,34 since neither ADP nor thrombin increased platelet O release although they also induced a strong aggregation. Collagen-induced platelet signaling is thought to be mainly mediated by binding to the surface receptor glycoprotein VI (GPVI),12 whereas ADP acts via several purinergic receptors35 and thrombin via protease-activated receptors36 and, to a lesser degree, via GPIb.37 GPVI binding induces activation and phosphorylation of PLCγ through a pathway involving numerous tyrosine phosphorylation steps.13 As collagen-dependent platelet O production was prevented by a blocker of tyrosine kinases and also by inhibition of PKC, collagen-specific tyrosine phosphorylation events eventually leading to PKC activation might explain that superoxide production is specific for collagen. However, collagen activation of PKC alone cannot fully explain this, as thrombin is also known to induce PKC activation.36 Recent findings showing that LPS-stimulated platelet O release is abolished by an inhibitor of PKC,38 however, support involvement of PKC. The collagen-induced Oproduction was furthermore inhibited by the flavoenzyme inhibitor DPI, consistent with an NAD(P)H oxidase being the source of O. Such O-generating NAD(P)H oxidases have been identified in a variety of tissues, including leukocytes and endothelial cells.17,39-41 NAD(P)H oxidase is known to be a multicomponent protein complex assembled by the cellular subunits p47phox, p67phox, and the membrane-bound proteins p22phox and gp91phox, which together with the small GTPDase rac1/2 associate to form the active enzyme complex.17,41 In fact, rac is known to be activated in platelets upon collagen stimulation.33 The presence of the subunits p22phox and p67phox as parts of a putative platelet NAD(P)H oxidase has also recently been demonstrated.16 Indeed, we could confirm existence of platelet p67phox of a neutrophil-type NAD(P)H oxidase at the protein level. In addition, we were also able to show the presence of p47phox in platelets for the first time. However, we did not succeed in detecting the gp91phox protein. It could be that platelets express a different isoform of this important subunit, which could not be detected by the antibody used. Recently a peptide (gp91ds-tat) has been designed to specifically inhibit the interaction of the NAD(P)H oxidase subunit p47phox with the large membrane spanning subunit gp91phox. This peptide inhibited the interaction of p47phox with gp91phox by binding to the docking sequence (ds) of p47phox at gp91phox.42 In our experiments, it completely abolished platelet Oproduction in response to collagen. Its effect on platelet O production is not in contradiction to the missing proof of gp91phox as the docking sequence for p47phox at gp91phox shows high homology between gp91phox and its so-far characterized isoforms nox1 and nox4 (renox).39,43 Existence of a platelet NAD(P)H oxidase is further supported by the finding that addition of NADH or NAD(P)H to platelet lysates increased O production significantly and particularly so when the platelets had been pretreated with collagen. Preference of NADH as a substrate can also be seen in endothelial NAD(P)H oxidases,40 giving additional evidence for an isoform of gp91phox being present in platelets.
When characterizing the role of a NAD(P)H oxidase in forming O, it is important to rule out a contribution of leukocytes to the O production observed. In our experiments, the highest leukocyte contamination amounted to 9 cells/μL. Control experiments using 10 to 100 leukocytes/μL in either platelet buffer or in the presence of platelets revealed that in this concentration the leukocytes, in contrast to platelets, did not induce O production in response to collagen. They were, however, fully functioning, since their response to A23187 showed the expected effects.
SOD in amounts sufficient to scavenge all Oreleased by platelets did not affect aggregation of platelets in response to collagen stimulation. This is in accordance with previous studies.44-46 There are, nevertheless, several controversial reports on effects of exogenous ROS on platelet function. Although there is one report of exogenous hydrogen peroxide (H2O2) impairing aggregation in response to ADP,47 the high amounts of H2O2needed for this observation are unlikely to exist in vivo. On the other hand, several reports support an enhancing role of ROS on platelet activity. Handin et al found that X/XO aggregated washed platelets and caused [14C]-serotonin release.45O derived from pyrogallol increased aggregation and adhesion,48 whereas inhibition of the O-producing enzyme NAD(P)H oxidase by DPI inhibited aggregation.49 It has also been reported that platelets exposed to anoxia/reoxygenation generate O and hydroxyl radicals, being the cause of enhanced spontaneous aggregation.14 However, all these studies failed to show an effect of platelet-derived O on thrombus formation directly. Our studies show a more differential role of O in the course of aggregation and thrombus growth. While the initial formation of a thrombus caused by collagen stimulation was not sensitive to O, the recruitment of further platelets for thrombus growth clearly was. Moreover, platelet-derived O formation was involved in the recruitment of platelets to a preformed thrombus only when a stimulus that increased platelet O release was the initial stimulus for thrombus formation. When thrombus formation was induced by ADP or thrombin, which we show not to increase Oformation in platelets, recruitment was not affected by the O scavenger SOD. The finding that recruitment following collagen stimulation could be abolished by apyrase suggests that recruitment is independent of a direct effect of collagen. Furthermore, kinetics of collagen-induced aggregation and of aggregation induced by supernatants of collagen-stimulated platelets showed different behavior, giving additional evidence for a released substrate rather than the original stimulus being responsible for recruitment of further platelets. It favors the concept of an effect of platelet-derived O on platelet function, which is independent of O merely scavenging platelet-derived NO. However, a contribution of O scavenging NO and thereby influencing platelet recruitment cannot be ruled out.
It is well known that release of ADP from platelet-dense granules is a major factor for platelet recruitment.50 When washed platelets pretreated with exogenously generated Owere stimulated with ADP, otherwise subthreshold concentrations of ADP were sufficient to induce irreversible aggregation, suggesting ADP-dependent effects could be modulated by O. Direct comparison of the quantities of O produced by platelets, neutrophils, and the O-generating system X/XO revealed that the amount of O in supernatants of platelets was less than that of the other systems when appropriate stimuli were used (data not shown). Thus, the importance of this “priming” of ADP-dependent aggregation might be even more pronounced in the presence of adequately activated neutrophils.
By directly measuring the amount of ADP released from resting or collagen-activated platelets, we provide a possible explanation for the effects of collagen-induced O production on platelet recruitment. Under basal conditions, there was no substantial release of ADP. Collagen induced a strong release of ADP, but the amounts of ADP in supernatants of collagen-stimulated platelets were decreased when O was scavenged by SOD. Moreover, amounts of ADP were even increased when stimulation was performed in the presence of exogenous radicals produced by X/XO. As X/XO added to platelets that were not stimulated with collagen did not induce significant ADP increase, the increase in collagen-stimulated platelets could not have been due to an increased release induced by X/XO. While these data cannot exclude that O alters affinity of ADP to one of the purinergic ADP receptors identified on platelets,35a more likely mechanism is that O increases the availability of ADP by inactivating a platelet ectonucleotidase (Ecto-ATPDase). ATPDases hydrolyze ATP to ADP and ADP to AMP, which no longer induces aggregation. When located on the outer membrane surface, they can be called ecto-ATPDases. Indeed, in endothelial cells, an ecto-ATPDase that has been shown to be identical to CD39,51 is inhibited in an autocrine manner by ROS released during activation with TNFα.25 Platelets have been shown to also express CD39, which also contains ecto-ATPDase activity,52 but further characterization of this nucleotidase is so far lacking. Hence, oxidative inactivation of platelet CD39 or another platelet ATPDase would fully explain our findings, although we cannot give direct evidence for this. We therefore suggest that the influence of platelet-derived O on platelet recruitment might be due to oxidative modification of a platelet ATPDase during thrombus formation.
The release of O from platelets upon exposure to collagen might be an efficient, physiological method of amplification of thrombus formation in situations where prolonged thrombus formation takes place. Enhanced platelet activation, as it may, for example, occur in acute coronary syndromes,1,53 might be influenced by platelet O production, which in turn may critically depend on the initial mode of platelet activation. Also in other pathophysiologic situations, like hyperglycemia, enhanced collagen-induced platelet activation seemed to be due to platelet overproduction of O.54 Furthermore, platelets from diabetic patients have been shown to release increased amounts of O, which in turn increased ADP-dependent Ca++-signaling.55 Therefore, enhanced platelet O formation might be one mechanism explaining increased susceptibility of certain risk groups for arterial thrombosis. Further studies are needed to identify patient populations with increased platelet O formation.
We propose that platelet recruitment due to alterations in oxidative state might contribute to collagen-dependent platelet activation. It can be speculated that increased platelet recruitment due to platelet O release could shift the balance between thrombotic and antithrombotic influences, resulting in enhanced susceptibility to acute thrombotic events. Then, assessment of the activity of a platelet NAD(P)H oxidase and of collagen-specific signaling pathways might help to identify patients at risk and to develop new therapeutic strategies.
We thank Dr Patrick Pagano for providing gp91ds-tat and its scrambled analog. We also thank Professor B. Engelmann, S. Zieseniss, and I. Mueller for support and expert discussion; and D. Kiesl for technical assistance.
Supported by a grant from the Friedrich-Baur-Stiftung of the Ludwigs- Maximilians-University.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Florian Krötz, Institut für Physiologie, Schillerstr 44, 80336 Munich, Germany; e-mail:fkroetz@lmu.de.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal