Abstract
Extracorporeal photochemotherapy (ECP) has been shown to be an effective therapy for patients with acute and chronic graft-versus-host disease (GVHD) following allogeneic bone marrow transplantation, but its biologic mechanism is not understood. We reported that clinical response to ECP was associated not only with normalization of skewed CD4/CD8 ratios but also with an increase in CD3−/CD56+ natural killer cells and a decrease in the number of CD80+ and CD123+ circulating dendritic cells (DCs). To further elucidate the effects of ECP on activated lymphocyte subpopulations and the interaction between effector lymphocytes and antigen-presenting DCs, we isolated and characterized DC populations from patients with chronic GVHD undergoing ECP therapy. Antigen-presenting activity of DCs was measured as proliferation of antigen-stimulated autologous and allogeneic T cells by mixed-lymphocyte reaction (MLR). In MLR assays the proliferation of T cells was decreased in all 10 patients by a mean of 84% (range, 75%-95%; P ≤ .002) after a 2-day cycle of ECP and longitudinally over the 12-month course of therapy. Immunophenotypic analysis of DC populations revealed a preponderance of DC1 monocytic dendritic cells in all patients before the initiation of ECP. Nine of 10 patients demonstrated a shift from DC1 to DC2 and as a concordant shift from a predominantly Th1 (interleukin-2 [IL-2], interferon-γ) to Th2 (IL-4, IL-10) cytokine profile after ECP, and 8 of 10 had a clinical response to ECP. Our results suggest that ECP alters alloreactivity by affecting allo-targeted effector T cells and antigen-presenting DCs.
Introduction
Acute and chronic graft-versus-host disease (aGVHD and cGVHD) represent a major cause of morbidity and mortality following allogeneic bone marrow transplantation (BMT).1 The incidence of aGVHD ranges from 20% to 50% and is dependent on factors related to the graft and to the effects of conditioning regimens on host tissue. cGVHD affects 30% to 50% of successfully engrafted patients and is thought to be related to ongoing alloreactivity against minor HLA-antigen mismatch between donor and recipient.2
Extracorporeal photochemotherapy (photopheresis, ECP) involves ex vivo exposure of leukapheresed peripheral blood mononuclear cells to ultraviolet A (UVA) light in the presence of a DNA-intercalating agent, 8-methoxypsoralen (8-MOP), with subsequent reinfusion of the treated cells. The total number of lymphocytes treated ex vivo per cycle has been estimated to be between 5% and 15% of the total circulating lymphocytes, and the total energy delivered by UVA light is estimated to be 2 J/cm2 per lymphocyte.3 ECP has demonstrated efficacy in the treatment of cutaneous T-cell lymphoma4 (CTCL) and recently in the management of T-cell–mediated diseases such as scleroderma and alloreactivity in patients with solid organ and bone marrow allografts.5
Immunomodulatory effects of ECP have been explored most extensively in the context of cutaneous T-cell lymphoma, in which the induction of tumor-specific CD8+ effector cells has been demonstrated. Induction of apoptosis in circulating tumor cells exposed to UVA and 8-MOP is believed to prime antigen-presenting dendritic cells (DCs) with processed tumor antigens capable of inducing an antitumor immune response. In patients with autoimmune diseases and GVHD, ECP appears to induce tolerance to alloreactive or autoreactive antigen-generated T-cell responses, but the immunomodulatory effects of ECP in autoimmune disorders are unclear. In scleroderma, identification of autoreactive T-cell clones supports a mechanism similar to that in CTCL.
Clinical responses to ECP were demonstrated in patients with cGVHD initially by Rosetti et al,1 who demonstrated a selective effect of ECP on cytotoxic effector CD8+ T cells with no change in CD4+ T-helper populations in 5 children with cGVHD, suggesting a differential effect on T-cell subsets without a clear explanation of how this effect was manifest. Recently, we demonstrated not only an effect of ECP on T-cell subsets in cGVHD patients but also a direct effect on circulating antigen-presenting DCs and natural killer cell populations in patients with cGVHD who had clinical response to ECP, and we hypothesized that the mechanism of action of ECP in cGVHD, and perhaps in autoimmune diseases, may be related to the effects of the treatment on antigen presentation rather than on effector T-cell populations.6
Dendritic cells are highly potent antigen-presenting cells (APCs) of bone marrow origin that have been shown to stimulate primary and secondary T- and B-cell responses.7 DCs are strategically distributed in tissues, they are constitutively rich in major histocompatibility complex (MHC) class II molecules, and they can be readily induced to express the costimulatory molecules necessary for activation of naive or resting T cells. To elicit an immune response, DCs must undergo a maturation process initiated by inflammatory signals and completed after contact with T cells. Maturation enables DCs to migrate from peripheral tissues to lymphoid organs and to acquire a potent antigen-presenting capacity. In addition, mature DCs are the most relevant and initial source of cytokines that govern the development of Th1 response.8
Two distinct types of DC precursors have been identified: myeloid monocytes (pre-DC1) and plasmacytoid DC precursors (pre-DC2). In mouse spleen, CD8+ “lymphoid” and CD8−“myeloid” DC subsets have been identified. Whereas myeloid DC1 derived from monocytes produce a large amount of interleukin-12 (IL-12) and induce T-helper cells to differentiate into Th1 cells, lymphoid DC2 derived from plasmacytoid precursors produce lower amounts of IL-12 and induce a Th2 cytokine profile.9 Recent studies have shown that CD1d-expressing DCs stimulate murine and human natural killer T cells (NKT cells). Thus, the type of DC may also influence NKT cell differentiation into NKT1 or NKT2 cells.10
To further elucidate the functional effects of ECP on alloantigen presentation and cytokine production by effector T cells in cGVHD, we examined DC function and helper T cell (Th1/Th2) differentiation. We demonstrate that before ECP, DC from patients with cGVHD were capable of inducing brisk autologous and allogeneic lymphocyte proliferation in 7 of 10 patients, whereas after in vivo exposure to ECP, autologous and allogeneic T-cell proliferation was significantly attenuated. Further, there was a significant decrease in DC1 (CD80+, CD123+) compared with DC2 (CD83+, CD86+) dendritic cells, with a resultant shift from a predominantly Th1 (interferon-γ [IFN-γ]) to a Th2 (IL-4, IL-10) cytokine profile as early as 3 months after the initiation of ECP, which was maintained during the 12 months of ECP therapy. Our results suggest that ECP may induce tolerance to alloantigens in cGVHD by inducing a shift in the DC1/DC2 balance toward DC2, thus attenuating ongoing alloreactivity mediated, in part, by Th1 cytokine secretion.
Materials and methods
Reagents
Tetanus toxoid was provided by Massachusetts Public Health Biological Laboratories (Boston, MA). Staphylococcal enterotoxin B and phorbol-12-myristate 13-acetate (PMA) were purchased from Sigma Chemical (St Louis, MO); granulocyte macrophage–colony-stimulating factor (GM-CSF), tumor necrosis factor-α (TNF-α), and IL-4 were purchased from R&D Systems (Minneapolis, MN). AIM-V medium and human AB serum were obtained from Life Technologies (Rockville, MD).
Patients
Patients had a diagnosis of extensive cGVHD, refractory to standard therapy (at least 4 weeks of 1 mg/kg prednisone or equivalent), with therapeutic levels of cyclosporin A. All patients had progressive or symptomatic nonresponsive cGVHD. Five patients did not respond to mycophenolic acid therapy on an institutional phase 2 study, and 2 did not respond to tacrolimus therapy. All patients had extensive sclerodermatous skin changes. All patients had ECOG performance status of 3 or less. Patients with history of photosensitive disease, psoralen allergy, or active uncontrolled infection were ineligible. All patients signed informed consent conforming to an institutional review board–approved protocol. ECP was performed on 2 consecutive days every 2 weeks as previously described. Toxicity and response was assessed on day 0 and at the beginning of each 2-week treatment cycle using the National Cancer Institute common toxicity criteria. Patients continued taking their immunosuppressive agents, which were adjusted for cGVHD activity.
Generation of dendritic cells from monocytes
Fresh peripheral blood mononuclear cells (PBMCs) from patients were isolated by Ficoll-Hypaque (Amersham-Pharmacia Biotech, Uppsala, Sweden) gradient centrifugation. Nonadherent cells were removed by incubation of cells in culture flasks for 16 hours in RPMI 1640 complete medium (10% human AB serum, 50 μg/mL glutathione, 50 μg/mL streptomycin, and 50 μg/mL penicillin) at 5% CO2and 37°C. Nonadherent cells were frozen at −80°C.
To induce DC differentiation, monocytes were cultured in 24-well plates (Costar, Cambridge, MA) at 5 × 105 cells/well in AIM V supplemented with 3% heat-inactivated human AB serum, 1000 IU/mL IL-4, and 1000 IU/mL GM-CSF at 37°C in 5% CO2 for 4 days. DCs were pulsed once with tetanus toxoid concentration at 0.01 fL/mL on day 3. Antigen was removed by washing the cells and renewing the supplemented medium. The maturation-inducing agent TNF-α was added to the supplemented culture medium at day 4 (20 ng/mL) and at day 6 (10 ng/mL). DCs were stimulated with PMA (10 ng/mL) at day 6 for 2 days. Cells were collected at the end of the culture (day 8) by incubating with phosphate-buffered saline (PBS) containing 0.2 mM EDTA for 5 minutes.
Flow cytometry
Cell staining was performed on 1 × 105 cells/mL with the following phycoerythrin (PE) or fluorescein isothiocyanate (FITC)–conjugated monoclonal antibodies: CD3 FITC, CD4 FITC, CD8 PE, CD25 PE, CD28 PE, CD56 PE, CD69 FITC, CD80 PE (B7-1), CD83 PE, CD86 PE (B7-2), and CD123 PE (Becton Dickinson, San Jose, CA). Cells were stained for 30 minutes in PBS with 0.05% fetal bovine serum (FBS), 2 mM EDTA, and 0.01% sodium azide. Cells were washed twice for 5 minutes in PBS with 0.05% FBS, 2 mM EDTA, and 0.01% sodium azide and were fixed with 1% paraformaldehyde in PBS. Ten thousand cells were analyzed by means of a FACScan (Becton Dickinson) equipped with a 488-nm argon laser. All isotype controls were set to be less than 2% positive for statistical analysis.
Mixed-lymphocyte reaction
Autologous lymphocytes from GVHD patients treated with ECP or allogeneic lymphocytes from healthy donors were cultured at 103 cells/well in 96-well plates (Costar) in AIM V supplemented with 3% heat-inactivated human AB serum with increasing numbers of irradiated DCs (30 Gy from a cesium Cs 137 source). Cells were pulsed with tetanus toxoid. Thymidine incorporation was measured in triplicate on day 6 by an 18-hour pulse with [3H]-thymidine (1 μCi/well [0.037 MBq/well]) (NEN, Boston, MA). Cells were harvested, and [3H]-thymidine incorporation was measured by β-liquid scintillation counter.
Antigen presentation
Isolated pre- and post-ECP autologous lymphocytes were cultured at 103 cells/well in 96-well plates in AIM V and 3% heat-inactivated human AB serum. Autologous DCs were irradiated and added 1 × 104, 5 × 103, 2.5 × 103, and 1.25 × 103 cells per well to obtain T/DC ratios of 10:1, 20:1, 40:1, and 60:1, respectively. Proliferation of antigen-specific lymphocytes was evaluated in triplicate after 6-day exposure to tetanus toxoid by measuring [3H]-thymidine uptake during the last 18 hours.
Separation of Th-cell subpopulations
PBMCs were pulsed to staphylococcal enterotoxin-B protein (10 μg/mL) for 16 hours in complete medium. Cells (1 × 106) were washed with PBS supplemented with 0.5% bovine serum albumin (BSA) and 2 mM EDTA. Cells were incubated with cytokine-catch reagent (IFN-γ, IL-4, or IL-10) in complete medium for 45 minutes at 37°C. Cells were labeled with PE- or FITC-conjugated cytokine detection antibodies CD8 PE–IFN-γ FITC, CD4 FITC–IL-4 PE, and CD4 FITC–IL-10 PE (Miltenyi Biotech, Auburn, CA)11 for 10 minutes. Cells were washed twice with PBS supplemented with 0.5% BSA and 2 mM EDTA. Cytokine-secreted T-cell subsets were detected by flow cytometry using a 388-nm argon laser. Data were analyzed using the Student t test and were shown as the mean ± SE. P ≤ .05 was considered significant.
Results
Ten consecutive patients undergoing ECP for cGVHD after allogeneic bone marrow transplantation were studied. As we previously reported, 9 of 10 responded to treatment with improvement in sclerodermatous skin changes and in oral, musculoskeletal, hepatic, and lung involvement. Patients received ECP for 2 consecutive days every 2 weeks. Lymphocyte and dendritic cell subsets and functional analysis were performed before initiation and at the completion of day 2 (after photopheresis) of each photopheresis cycle. Patients were treated for a minimum of 6 months and until the response plateau. Peripheral blood DCs were differentiated from monocytes and stem cells in the presence of GM-CSF, IL-4, and TNF-α for 8 days. DCs were harvested and stained for the expression of CD80 (B7.1, preDC1), CD83 (interdigitating reticulum DC and circulating DC), CD86 (B7.2, preDC2), and CD123. Dendritic cell yields and DC1/DC2 distribution were similar among 10 patients and are shown in Table1. Before ECP therapy, the number of CD80+ and CD123+ DC1 cells in the cGVHD patients was increased compared with CD83+ and CD86+ DC2 cells. After one cycle of ECP, the number of DC1 cells decreased whereas the number of DC2 increased, as shown in Figure1. Similar results were obtained at 6 and 12 months of therapy, and the overall change in DC populations during treatment was consistent among patients, as shown in Table 1.
DC yield (baseline to last ECP cycle day 2)
| Patients . | CD80+ . | CD83+ . | CD86+ . | CD123+ . |
|---|---|---|---|---|
| 1 | − 3 | + 2 | + 2 | − 2 |
| 2 | − 6 | + 2 | + 2 | − 3 |
| 3 | − 2 | + 2 | + 3 | − 4 |
| 4 | − 3 | + 11 | + 9 | − 4 |
| 5 | − 5 | + 3 | + 2 | − 2 |
| 6 | − 3 | + 2 | + 2 | − 2 |
| 7 | − 4 | + 6 | + 5 | − 3 |
| 8 | − 3 | + 1.2 | + 3 | − 2 |
| 9 | − 3 | + 2 | + 6 | 0 |
| 10 | − 4 | − 3 | − 2 | − 5 |
| Patients . | CD80+ . | CD83+ . | CD86+ . | CD123+ . |
|---|---|---|---|---|
| 1 | − 3 | + 2 | + 2 | − 2 |
| 2 | − 6 | + 2 | + 2 | − 3 |
| 3 | − 2 | + 2 | + 3 | − 4 |
| 4 | − 3 | + 11 | + 9 | − 4 |
| 5 | − 5 | + 3 | + 2 | − 2 |
| 6 | − 3 | + 2 | + 2 | − 2 |
| 7 | − 4 | + 6 | + 5 | − 3 |
| 8 | − 3 | + 1.2 | + 3 | − 2 |
| 9 | − 3 | + 2 | + 6 | 0 |
| 10 | − 4 | − 3 | − 2 | − 5 |
Cultured monocyte- and lymphocyte-derived DC subpopulations were stained with CD80, CD123 (DC1) and CD83, CD86 (DC2) and were analyzed by flow cytometry at baseline, before ECP treatment, and at 12 months after initiation of ECP at the completion of day 2 of the treatment cycle. Results are shown as -fold difference, with each column demonstrating increases (+) or decreases (−). P< .005.
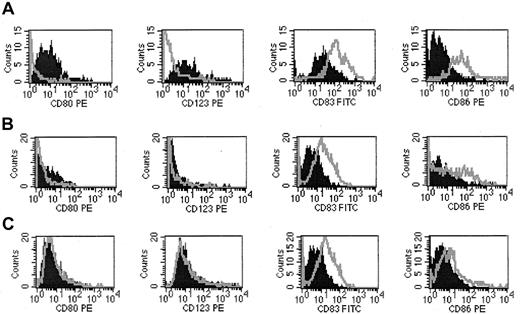
Modulation of DC subtypes before and after ECP treatment.
(A) At baseline, PBMCs were stained with CD80, CD123 (DC1) and CD83, CD86 (DC2). DC subpopulations were gated and analyzed by FACScan. Pre-ECP (dark-filled peaks); post-ECP (outlined peaks). (B) At 6 months of ECP treatment. (C) At last cycle of ECP treatment.
Modulation of DC subtypes before and after ECP treatment.
(A) At baseline, PBMCs were stained with CD80, CD123 (DC1) and CD83, CD86 (DC2). DC subpopulations were gated and analyzed by FACScan. Pre-ECP (dark-filled peaks); post-ECP (outlined peaks). (B) At 6 months of ECP treatment. (C) At last cycle of ECP treatment.
DC functional activity before and after ECP was measured by antigen presentation to autologous and allogeneic lymphocytes in MLR assays. DCs generated from PBMCs both before and after ECP at 3 different times over the course of 1 year of treatment were irradiated and incubated with autologous and allogeneic lymphocytes in variable T/DC numbers in the presence of tetanus toxoid for 6 days. Cell proliferation was measured by [3H]-thymidine uptake. Before ECP treatment, brisk proliferation of autologous and allogeneic T cells was noted. Interestingly, the proliferation of autologous T cells was greater than that of allogeneic T cells in 7 of 10 patients (Table2). Of the 3 patients in whom there was greater stimulation with allogeneic T cells, 2 responded to ECP and one had no response but had received a donor lymphocyte infusion (DLI) for recurrent lymphoma. At the completion of the first ECP treatment, allogeneic and autologous lymphocyte proliferation were significantly blunted in all 10 patients, as shown in Figure2, demonstrating an effect of ECP on antigen presentation by DCs.
ECP effects on antigen presentation function of DCs
| Patient . | Organs involved . | Drugs at start of ECP . | Drugs at end of trial . | Onset of ECP from transplantation, d . | Months of ECP . | Response . | MLR . |
|---|---|---|---|---|---|---|---|
| 1 | Skin, joints, mouth | Prednisone, 20 mg/d FK506, 2 mg/d Tacrolimus, 2 mg/d | Prednisone, 10 mg/d | 600 | 9 | PR | Autologous |
| 2 | Skin, joints, mouth | Prednisone, 6 mg/d Tacrolimus, 1 mg/d | Prednisone, 3.5 mg/d Cellcept, 1000 bid Tacrolimus, DIC | 2928 | 9 | PR | Autologous |
| 3 | Skin, mouth | Prednisone, 25 mg/d MMF, 2 g/d CSA, 200 mg/d | Prednisone, 12.5 mg/d CSA, 150 mg/d | 101 | 11 | PR | Autologous |
| 4 | Skin, joints, mouth, liver | Prednisone, 20 mg/d MMF, 2 g/d CSA, 200 mg/d | Prednisone, 10 mg/d CSA, 50 mg/d | 204 | 10 | PR | Allogeneic |
| 5 | Skin, lungs | Prednisone, 20 mg/d | Prednisone, 20 mg/d | 122 | 4 | NC* | Allogeneic |
| 6 | Skin, lungs | Prednisone, 22.5 mg/d Cellcept, 500 mg bid | Prednisone, 17.5 mg/d | 221 | 9 | PR skin improved, lungs unchanged | Allogeneic |
| 7 | Skin, eye, liver | Prednisone, 20 mg/d Plaquenil, 200 mg/d | Prednisone, 10 mg/d Plaquenil, DIC | 457 | 4 | PR | Autologous |
| 8 | Skin, joints, mouth | Prednisone, 10 mg/d MMF, 2 g/d CSA, 200 mg/d | Prednisone, 10 mg/d MMF, 1.5 g/d | 496 | 16 | PR | Autologous |
| 9 | Skin, mouth | Prednisone, 20 mg QOD Cellcept, 1000 mg/d | Cellcept, 500 bid | 1342 | 9 | PR skin improved | Autologous |
| 10 | Skin, joints, mouth | Prednisone, 10 mg/d MMF, 2 g/d CSA, 200 mg/d | Prednisone, 10 mg/d CSA, 50 mg/d | 189 | 12 | NC | Autologous |
| Patient . | Organs involved . | Drugs at start of ECP . | Drugs at end of trial . | Onset of ECP from transplantation, d . | Months of ECP . | Response . | MLR . |
|---|---|---|---|---|---|---|---|
| 1 | Skin, joints, mouth | Prednisone, 20 mg/d FK506, 2 mg/d Tacrolimus, 2 mg/d | Prednisone, 10 mg/d | 600 | 9 | PR | Autologous |
| 2 | Skin, joints, mouth | Prednisone, 6 mg/d Tacrolimus, 1 mg/d | Prednisone, 3.5 mg/d Cellcept, 1000 bid Tacrolimus, DIC | 2928 | 9 | PR | Autologous |
| 3 | Skin, mouth | Prednisone, 25 mg/d MMF, 2 g/d CSA, 200 mg/d | Prednisone, 12.5 mg/d CSA, 150 mg/d | 101 | 11 | PR | Autologous |
| 4 | Skin, joints, mouth, liver | Prednisone, 20 mg/d MMF, 2 g/d CSA, 200 mg/d | Prednisone, 10 mg/d CSA, 50 mg/d | 204 | 10 | PR | Allogeneic |
| 5 | Skin, lungs | Prednisone, 20 mg/d | Prednisone, 20 mg/d | 122 | 4 | NC* | Allogeneic |
| 6 | Skin, lungs | Prednisone, 22.5 mg/d Cellcept, 500 mg bid | Prednisone, 17.5 mg/d | 221 | 9 | PR skin improved, lungs unchanged | Allogeneic |
| 7 | Skin, eye, liver | Prednisone, 20 mg/d Plaquenil, 200 mg/d | Prednisone, 10 mg/d Plaquenil, DIC | 457 | 4 | PR | Autologous |
| 8 | Skin, joints, mouth | Prednisone, 10 mg/d MMF, 2 g/d CSA, 200 mg/d | Prednisone, 10 mg/d MMF, 1.5 g/d | 496 | 16 | PR | Autologous |
| 9 | Skin, mouth | Prednisone, 20 mg QOD Cellcept, 1000 mg/d | Cellcept, 500 bid | 1342 | 9 | PR skin improved | Autologous |
| 10 | Skin, joints, mouth | Prednisone, 10 mg/d MMF, 2 g/d CSA, 200 mg/d | Prednisone, 10 mg/d CSA, 50 mg/d | 189 | 12 | NC | Autologous |
MLR data refer to maximal proliferation (pre– vs post–8-MOP/UVA exposure in cycle 3 of therapy; autologous vs allogeneic lymphocytes).
CSA indicates cyclosporin A.
DLI for recurrent lymphoma after third cycle of ECP.
Effects of ECP on antigen presentation.
DC antigen presenting activity is represented in autologous and allogeneic MLR using different T/DC ratios. (A) Baseline. (B) After 6 months of ECP treatment. (C) After last cycle of ECP treatment.
Effects of ECP on antigen presentation.
DC antigen presenting activity is represented in autologous and allogeneic MLR using different T/DC ratios. (A) Baseline. (B) After 6 months of ECP treatment. (C) After last cycle of ECP treatment.
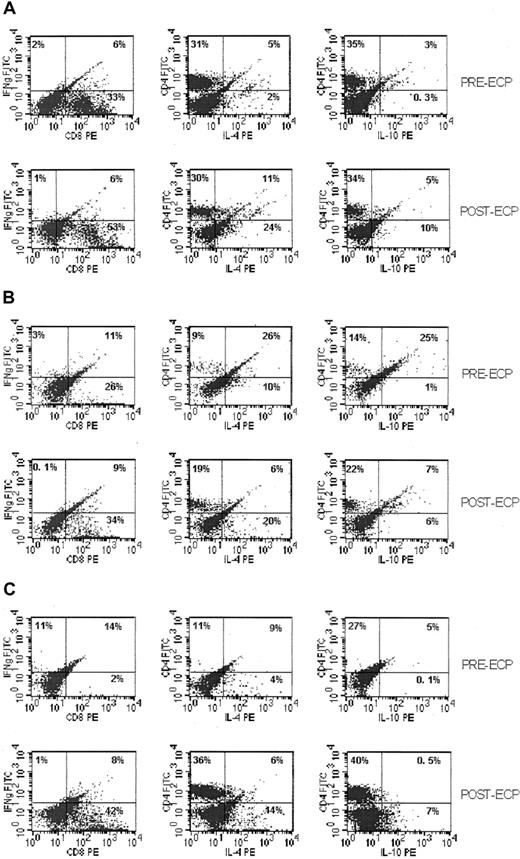
Because the observed effects of ECP on DC subpopulations would be anticipated to result in functional skewing of cytokine secretion from Th1 to Th2 cytokines, we evaluated Th1/Th2 ratios before and after ECP using cytokine capture assays. After antigen stimulation of peripheral blood lymphocytes with staphylococcal enterotoxin B, IFN-γ–secreting Th1 cells decreased 1- to 3-fold, and IL-4– and IL-10–secreting Th2 cells increased 1- to 3-fold and 1- to 4-fold, respectively, from baseline to last cycle of ECP after the initiation of therapy, as shown in Table 3. Similar proportional changes in cytokine profiles were seen before and after each cycle of ECP and were consistent at initiation of therapy and at 6 and 12 months or last cycle of ECP, as shown in Figure 3.
Modulation of Th cell (Th1/Th2) subpopulations by ECP
| Patient . | Baseline to last cycle of ECP treatment (pre-ECP vs post-ECP-fold difference) . | ||||
|---|---|---|---|---|---|
| CD4 . | CD8 . | IFN-γ . | IL-4 . | IL-10 . | |
| 1 | + 1.4 | − 0.7 | − 2.5 | + 1.7 | + 1.3 |
| 2 | + 1.4 | − 1.3 | − 2.7 | + 1.3 | + 3 |
| 3 | + 1.2 | − 0.9 | − 4 | + 4.2 | + 2.5 |
| 4 | + 1.1 | − 0.6 | − 1.1 | + 3.5 | + 4 |
| 5 | + 3 | − 4 | − 2.8 | + 4.6 | + 2 |
| 6 | + 1.6 | − 1.3 | − 1.3 | + 3.5 | + 3.5 |
| 7 | + 1.2 | − 1.3 | − 2.2 | + 3.2 | + 2.4 |
| 8 | + 2.2 | − 3.5 | − 3 | + 3.5 | + 3 |
| 9 | + 2.1 | − 2.5 | − 1.5 | + 2.4 | + 1.4 |
| 10 | + 1.2 | − 3.5 | − 0.6 | + 0.7 | + 0.3 |
| Patient . | Baseline to last cycle of ECP treatment (pre-ECP vs post-ECP-fold difference) . | ||||
|---|---|---|---|---|---|
| CD4 . | CD8 . | IFN-γ . | IL-4 . | IL-10 . | |
| 1 | + 1.4 | − 0.7 | − 2.5 | + 1.7 | + 1.3 |
| 2 | + 1.4 | − 1.3 | − 2.7 | + 1.3 | + 3 |
| 3 | + 1.2 | − 0.9 | − 4 | + 4.2 | + 2.5 |
| 4 | + 1.1 | − 0.6 | − 1.1 | + 3.5 | + 4 |
| 5 | + 3 | − 4 | − 2.8 | + 4.6 | + 2 |
| 6 | + 1.6 | − 1.3 | − 1.3 | + 3.5 | + 3.5 |
| 7 | + 1.2 | − 1.3 | − 2.2 | + 3.2 | + 2.4 |
| 8 | + 2.2 | − 3.5 | − 3 | + 3.5 | + 3 |
| 9 | + 2.1 | − 2.5 | − 1.5 | + 2.4 | + 1.4 |
| 10 | + 1.2 | − 3.5 | − 0.6 | + 0.7 | + 0.3 |
Th1 and Th2 cells were detected by cytokine secretion. Captured cytokines were analyzed by flow cytometry, and results are shown as -fold difference between baseline and last cycle of ECP (increased [+] or decreased [−]). P < .005.
Changes in Th cell (Th1/Th2) subtypes during ECP treatment.
Dot plots of secreted IFN-γ (Th1); IL-4, IL -10 (Th2) captured by cytokine-catching molecule and detected by FACScan. (A) Th1/Th2 subpopulations of PBMC from patients with cGVHD at first cycle of ECP. (B) Th subpopulations after 6 months of ECP treatment. (C) Th subpopulations after last cycle of ECP treatment.
Changes in Th cell (Th1/Th2) subtypes during ECP treatment.
Dot plots of secreted IFN-γ (Th1); IL-4, IL -10 (Th2) captured by cytokine-catching molecule and detected by FACScan. (A) Th1/Th2 subpopulations of PBMC from patients with cGVHD at first cycle of ECP. (B) Th subpopulations after 6 months of ECP treatment. (C) Th subpopulations after last cycle of ECP treatment.
Discussion
The pathogenesis of cGVHD is controversial and may be related to an extension of the acute alloreactivity seen in aGVHD or to a manifestation of dysfunctional immune reconstitution with generation of tissue autoreactive T-cell clones and dysregulation of CD4+CD25+ immune modulating T cells. In murine GVHD models, donor CD4-enriched cells of Th2 phenotype have been shown to prevent GVHD without affecting engraftment, suggesting that they play a role in down-regulating the TH1 response.12 Similarly, in chronic GVHD, the up-regulation of TH1 cytokines IFN-γ and IL-12 in peripheral blood mononuclear cells implicates in part a TH1-driven mechanism.13 14
Dendritic cells are capable of presenting antigens to helper T cells, including naive T cells, and are likely important for inducing T-cell responses to foreign (allogeneic) MHC molecules in tissue allografts. The MLR is a useful in vitro model of direct T-cell recognition of allogeneic MHC gene products and is used as a predictive test of cell-mediated graft rejection. Alloreactive CD4+ and CD8+ T cells are stimulated during allogeneic MLR. CD8+ T cells differentiate into CTLs and are indistinguishable from self-class MHC I-restricted CTLs specific for foreign protein antigens. The CD4+ T cells differentiate into Th1 and Th2 cells.
Donor and recipient APCs are likely to be involved in the process of graft rejection. The most important APCs stimulating an antigraft response are likely to be DCs, either of donor origin and resident in the interstitium of the graft or of recipient origin and entering the graft through the blood supply. These APCs may stimulate recipient T cells within the graft. It is also possible that donor APCs migrate from the graft into draining lymph nodes, where they activate naive alloreactive T cells by the direct pathway.
We demonstrate here that ECP alters host (autologous) lymphocyte proliferation in MLR by modulating DC antigen presentation and differentiation of DC subtypes, favoring a decrease in DC1 and an increase in DC2 cells (Figure 4). The CD123+ lymphoid DC1 dendritic cells produce large amounts of IFN-α.15 Although these cells might also process and present antigen, they are postulated to enter lymph nodes through the high endothelial venule.16 Previous studies with low-dose cutaneous ultraviolet B radiation in patients with skin manifestations of cGVHD have demonstrated an effect on intradermal antigen-presenting Langerhans cells to preferentially activate CD4+ Th2 cells.17 UVB irradiation has been shown to down-regulate MHC class II expression on APCs and to eliminate costimulatory signals involving cytokine secretion (IL-2 and IL-6) required for signal transmission from APCs to T cells. UVB-irradiated APCs included in the donor bone marrow may present host antigen by the alternative pathway to donor lymphocytes.18
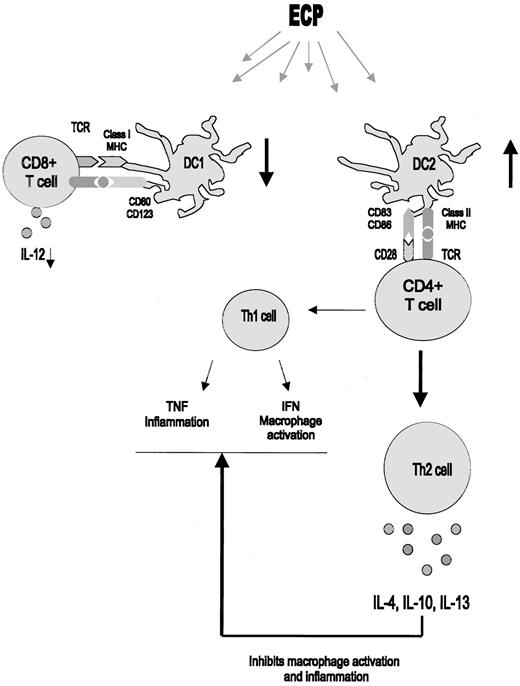
Modulation of DC function by ECP.
ECP modulates DC populations, resulting in an increase in CD83+, CD86+ plasmacytoid DC2 cells with a concordant decrease in CD80+, CD123+ monocytoid DC1 cells. These stimulate Th2 T-helper cells to secrete Th2 cytokines, which indirectly inhibit Th1-mediated alloreactivity.
Modulation of DC function by ECP.
ECP modulates DC populations, resulting in an increase in CD83+, CD86+ plasmacytoid DC2 cells with a concordant decrease in CD80+, CD123+ monocytoid DC1 cells. These stimulate Th2 T-helper cells to secrete Th2 cytokines, which indirectly inhibit Th1-mediated alloreactivity.
Although the paradigm of GVHD is based on alloreactive donor T cells recognizing foreign histocompatibility antigens of the host, there is now substantial experimental and clinical evidence to implicate a dysregulation of cytokine networks as a primary cause for the induction and maintenance of GVHD. The balance between Th1 cytokines (IL-2, IFN-γ) and Th 2 cytokines (IL-4, IL-10) governs the extent to which a cell-mediated immune response or a systemic inflammatory response develops. Because Th2 cytokines can inhibit the production of the proinflammatory cytokines IL-1 and TNF-alpha, a type 1 to type 2 shift in the initial response of donor T cells to host alloantigens may interrupt the cytokine cascade after allogeneic BMT and may offer a new approach to the prevention and treatment of aGVHD.19
To elucidate ECP effects on helper T-cell subsets in cGVHD, we evaluated the helper T-cell subsets by their specific cytokine profile. We observed that Th1 cells are decreased by ECP whereas Th2 cells are increased over the 12-month treatment. Current models of alloreactivity in acute GVHD implicate Th1 cells in the afferent arm and cytokine production by monocytes and macrophages in the efferent arm.20,21 In addition, Th2 cells have been used experimentally to prevent GVHD in a murine model.22,23 Th1 cells induce the activation of macrophages, resulting in delayed-type hypersensitivity responses and the killing of intracellular parasites. In contrast, Th2 cells control humoral responses, including the production of IgE-associated eosinophilia.23 An important feature of Th1 and Th2 cells is the ability of one subset to regulate the activity of the other. This occurs at the level of the effector cells triggered by these subsets, as indicated by the inhibitory effects of IFN-γ–IL-4–induced B-cell activation or those of IL-4 on IL-2–induced T- and B-lymphocyte proliferation. It also occurs directly at the level of these subsets because the products of one subset can antagonize the activation of the other: IFN-γ inhibits proliferation of Th2 cells,24,25 whereas IL-4 inhibits cytokine production by Th1 cells.26
Antigen-specific T-cell responses are characterized by distinct profiles of secreted cytokines. Rissoan et al27 have demonstrated that polarization of the T-cell response into Th1 or Th2 depends on the type of APC. DC1 induces Th1 responses whereas DC2 induces Th2 responses.9 It has been proposed that DC2 might be responsible for maintaining peripheral T-cell tolerance to self-antigens. Therefore, inducing the proliferation of antigen-specific T cells might be obligatory for DC1 but not for DC2.28 During inflammatory or immune responses initiated by macrophages and DC1, however, the secretion of TNF-α and the expression of CD40 ligand induce full activation of DC2. Under these circumstances, DC2 cells initiate the proliferation of antigen-specific T cells, leading to the expansion of Th2 clones and the secretion of IL-4 and IL-10. These cytokines produce a negative feedback on Th1 differentiation and terminate the immune and inflammatory responses.29 In several experimental models, donor Th2 cells have a decreased potential to induce aGVHD.19,21,30,31 It is also possible that donor DC2 contribute to the pathogenesis of GVHD after transfer to the recipient through the indirect presentation of host antigen to donor T cells and the induction of Th2 responses. Interestingly, umbilical cord blood, another source of allogeneic stem cells for transplantation associated with relatively low incidence of aGVHD, contains DC2 but not DC1 cells.31
Direct presentation of donor alloantigens by resident host DC, however, is likely to exert a dominant effect on the activation of donor T cells, resulting in aGVHD and cGVHD.32 Donor DC engraftment in recipients of allogeneic transplants has been associated with prolonged organ transplant survival.33,34 Donor DC1 would activate allogeneic host T cells to produce IFN-γ and generate cytotoxic responses against the graft, favoring rejection. Donor DC2 may induce host T cells to produce IL-4 and IL-10, which suppress Th1 and cytotoxic responses, favoring engrafment.35,36 Thus, the manipulation of DC subsets may be useful in stem cell therapy for the induction of tolerance to hematopoietic cells or solid organ allografts. It has been reported that DC2 might capture alloantigen and undergo maturation after transfer into the host. These DC2 might present alloantigen to donor T cells and induce them to undergo Th2 differentiation, which would attenuate the Th1-driven mechanisms inducing aGVHD.10 37
The modulation of DC subpopulations correlated with clinical response. Of 9 patients who demonstrated a decrease in DC1 and an increase in DC2 cells, 8 had a response to ECP in at least one target organ. Of 2 patients with lung involvement, neither demonstrated a response in the lung. One had early relapse of lymphoma and underwent DLI, whereas the other had a response in skin GHVD but no change in pulmonary function. The patient who had no response in any target organ (patient 10) demonstrated a decrease in DC1 and DC2 populations and, therefore, demonstrated no selective effect of ECP. Interestingly, this patient demonstrated similar changes in T-cell cytokine profile with attenuation of Th1 activity. These results suggest that selective changes in DC subsets might predict for response to ECP, but these observations must be confirmed in larger numbers of patients.
In summary, our data suggest that ECP has a direct effect to modulate APC function in patients with cGVHD. The net inhibitory effect of ECP on activated T cells and APCs favors attenuation of ongoing Th1-mediated events, thereby removing the inhibition to Th2 cytokine secretion and restoring balance between Th1 and Th2 cytokines. We have recently initiated a clinical trial to longitudinally examine the biomodulatory effects of ECP on lymphocyte and DC function in patients with cGVHD randomized to either ECP or to standard immunosuppressive therapy.
Prepublished online as Blood First Edition Paper, April 30, 2002; DOI 10.1182/blood-2002-01-0068.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Francine M. Foss, Departments of Hematology Oncology and Experimental Therapeutics, Tufts New England Medical Center, 750 Washington St, Boston, MA 02111; e-mail:ffoss@lifespan.org.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal