Abstract
Hematopoietic progenitor kinase 1 (HPK1) is a member of germinal center kinases that is predominantly expressed in hematopoietic cells and transiently activated by T-cell receptor (TCR) triggering. We show here that HPK1 supports apoptosis of T cells. When HPK1 was overexpressed in murine CD4+ T cells, a substantial increase was observed in spontaneous and TCR/CD3-mediated apoptosis as well as in Fas ligand (FasL) expression. In H2O2-treated EL-4 thymoma cells, which show an increase in reactive oxygen species (ROS) and apoptosis, overexpression of HPK1 enhanced ROS-mediated apoptosis, whereas expression of HPK1 antisense (AS) RNA impaired apoptosis. HPK1 expression also led to a sustained increase in c-Jun N-terminal kinase (JNK) activity, suggesting that JNK activation contributes to the HPK1-mediated apoptosis in H2O2-treated EL-4 cells. Under the same conditions, a rapid cleavage of HPK1 was observed, and overexpression of N- and C-terminal cleavage products in CD4+ T cells resulted in, similar to full-length HPK1, an increase in apoptosis. In agreement with published data, we show that the C-terminal portion of HPK1 suppresses IκBα degradation, thereby inhibiting nuclear factor (NF)–κB activation. These findings suggest that by inhibiting the antiapoptotic action of NF-κB and inducing the proapoptotic activity of JNK, OHPK1 supports apoptosis in T cells.
Introduction
Activation of peripheral T-effector cells by presented antigens can result in diverging effects for the affected cell. Depending on the “strength” of interaction between the antigen/major histocompatibility complex (MHC) and the T-cell receptor (TCR) complex and the involvement of coreceptors, a resting T cell can be stimulated to produce lymphokines and to proliferate, to become anergic, or to die by apoptosis. Activation Induced Cell Death (AICD) is a particular form of apoptosis that is of pivotal importance for the termination of the immune response and, therefore, homeostasis of the immune system. One important pathway of AICD in lymphocytes is the activation of caspase cascade through so-called death receptors, that is, a subgroup of the tumor necrosis factor (TNF)/nerve growth factor (NGF)–receptor family that comprise Fas, TNF-receptor I, TNF-related apoptosis-inducing ligand (TRAIL), and further receptors.1 Their stimulation leads to the activation of caspase cascade and, in turn, to cleavage of death substrates and, finally, to apoptosis of T cells.
However, the activation of caspase cascade through death receptors is not the only mechanism by which AICD of lymphocytes is executed. Thus, superantigen (SAg) injection into triple-mutant mice deficient in Fas and TNF-receptor I + II expression led to rapid AICD of Vβ8+ T cells, as rapid as those from control animals. This demonstrates that neither Fas nor TNF-mediated signaling pathways are involved in SAg-mediated AICD.2 Instead, SAg-activated T cells showed a distinct rise in reactive oxygen species (ROS), which are known to mediate apoptosis by damaging mitochondrial enzymes, disintegrating mitochondrial membranes, and releasing cytochromec from mitochondria. The activation of caspase cascade by cytochrome c is regulated by a large number of antiapoptotic and proapoptotic molecules, which belong to the family of Bcl-2–like proteins.3 For the apoptosis of lymphocytes, the activity of Bcl-2 and Bim appears to be of particular importance. Bim belongs to the class of “BH-3 only” proteins and binds to Bcl-2, thereby blocking its antiapoptotic activity.4Either overexpression of Bcl-2, such as in human follicular lymphomas bearing the t(14;18) translocation or in transgenic mice,3or inactivation of Bim in mice5 resulted in a drastic increase in the number of lymphocytes due to severe defects in apoptosis.
In spite of numerous studies on apoptosis of lymphocytes, relatively little is known about lymphoid-specific signaling molecules and pathways involved in AICD induced by TCR triggering or other mechanisms. The observation that TCR/CD3 stimulation of Jurkat T cells, which induces AICD, also induces activity of HPK1, whereas CD28 stimulation, which provides a survival signal for T cells, did not show any effect on HPK1 activity6,7 prompted us to investigate whether HPK1 regulates apoptosis of T cells. We show here that overexpression of HPK1 accelerates both spontaneous and αCD3-mediated apoptosis and up-regulates FasL expression on peripheral CD4+ T cells. Moreover, overexpression of HPK1 in EL-4 cells promoted ROS-mediated apoptosis, whereas expression of HPK1 AS-RNA, which impairs HPK1 expression, reduced ROS-mediated apoptosis. When overexpressed in primary T cells, both the N- and C-terminal portions of HPK1, which accumulate after caspase cleavage, were found to promote AICD. Since the released C-terminal HPK1 peptide suppresses nuclear factor (NF)–κB activity8one may conclude that HPK1 supports apoptosis by 2 mechanisms, that is, by inducing c-Jun N-terminal kinase (JNK) activation and NF-κB inhibition.
Materials and methods
Cells, DNA transfections, reporter gene assays, and retroviral infections
Murine EL-4 thymoma cells were grown in RPMI medium containing 5% fetal calf serum (FCS) and human 293T embryonic kidney (HEK) cells in Dulbecco modified Eagle medium (DMEM) containing 10% FCS. Human 293 cells were transfected with DNA using SuperFect according to Qiagen's protocol. For the determination of NF-κB activity, a luciferase reporter gene driven by 3 κB sites from the c-myb intronic enhancer9 was cotransfected with HPK1 expression vectors into EL-4 cells using a conventional diethylaminoethyl (DEAE)-dextran transfection protocol. After 42 hours, the cells were either left untreated or induced with 500 μM H2O2 for 6 hours. Luciferase assays were performed 48 hours after transfection, as described previously.10
EL-4 cells stably transduced with recombinant retroviruses were selected with zeocin (250 nM) for 7 days. HPK1 expression was tested in Western blots, and enhanced green fluorescent protein (EGFP) fluorescence was checked by a fluorescence-activated cell-sorter scanner (FACS). DO11.10 TCR transgenic (tg) BALB/c mice11 were killed between 6 and 8 weeks of age. CD4+ T cells from lymph nodes (LNs) were isolated by passing them through CD4 T-cell recovery columns (Cedarlane, Hornby, Canada) according to the manufacturer's protocol. The cells were cultured at 5 × 105/mL in X-VIVO15 (Bio Whittaker) supplemented with 5% FCS, Gln, nonessential amino acids, pyruvate (all 2 mM), antibiotics (penicillin, streptomycin), and 5 × 10-5 M 2-mercaptoethanol. Dendritic cells were prepared from irradiated spleen and used as antigen-presenting cells (APCs). CD4+ T cells were stimulated by the cognate ovalbumin peptide (OVA)–peptide (323-339) presented on APCs and cultured in the presence of interleukin (IL)-2 as described.12 After 24 hours, they were infected with retroviruses as described previously.13
Induction and determination of apoptosis
TCR-mediated apoptosis of primary T cells infected with retroviruses was induced 4 or 5 days after primary stimulation by incubating cells on petri dishes coated with 5 μg/mL mAb (145-2C11, PharMingen) specific for CD3ε. After 8 hours, cells were stained with Annexin V–PE (PharMingen) according to the manufacturer's protocol. The effect of HPK1 on apoptosis was determined by FACS dividing the Annexin V–PE positive and infected cells by all infected cells (× 100). Apoptosis of EL-4 cells was induced, after careful washing, by incubation of 5 × 105cells per milliliter fresh medium containing 1 mM H2O2. Apoptosis was determined by FACS either using Annexin V-PE staining or a slightly modified SubG1technique.14
Retroviral constructs
The bicistronic retroviral vector pEGZ-HA is based on the vector pczCFG2 hCD8 EYZ in which the cytomegalovirus (CMV) enhancer replaces the U3 region of the 5′ long terminal repeat (LTR) of murine leukemia virus (for further details, see Berberich-Siebelt et al13). An oligonucleotide was cloned into itsEcoRI site, creating a start codon and HA-Flag epitope followed by ClaI, AscI, BsiWI,RsrlI, HpaI, and SacII sites, which facilitate “in frame” cloning of cDNAs. The retroviral vector expressing wild-type (wt) HPK1, pHA-HPK1 wt contains the cDNA (amino acids 2-833) of human HPK1,15 which was amplified by polymerase chain reaction (PCR) and cloned between theClaI/HpaI sites of pEGZ-HA. A mutated version of HPK1, pHA-HPK1 M(46) containing a K46 to M46 substitution15 was used to test a kinase-inactive version of HPK1. The pHA-HPK1 wt N-terminus (encoding amino acid [aa] 2-385) and C-terminus constructs (aa 386-833) were created by cloning PCR products into pEGZ-HA. In addition, a kinase-inactive (K46 to M46) version of pHA-HPK1 wt N-terminus was also constructed. The retroviral AS vector, pHA-HPK1 AS, bears the sequences 386-2 between its ClaI/HpaI sites. All DNA modifying enzymes were purchased from MBI Fermentas (St Leon-Rot, Germany).
Antibodies and Western blot assays
Antibodies (Abs) raised against HPK1 (N-19; sc-6231), phospho-Tyr (PY99; sc-7020), IκBα (C-21; sc-371), Erk1 (K23; sc-94), JNK1 (C-17; sc-474), p-JNK (G-7; sc-6254), and p38 (C-20; sc-535) were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Abs detecting phospho-Erk1/2 (Thr202/Tyr204) or phospho-p38 (Thr180/Tyr182) were purchased from Cell Signaling Technology (Beverly, MA), a mAb against β-actin (AC-15) was obtained from Sigma-Aldrich (Taufkirchen, Germany). A biotin-conjugated mAb against murine FasL was obtained from Alexis Corporation (Grünberg, Germany), and PE-conjugated streptavidin from PharMingen (Heidelberg, Germany). The mAb HA.11 raised against HA tag was purchased from BAbCO (Richmond, VA).
In Western blots, an indicated amount of whole cellular protein lysate was fractionated by 12.5% sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) and electroblotted onto nitrocellulose membrane. For detection of proteins, appropriate peroxidase-coupled secondary Abs were used with a standard enhanced chemiluminescence system (Amersham, Freiburg, Germany).
Protein kinase assays
For in vitro phosphorylation with immunoprecipitated protein kinases, 500 μg of whole cell protein extract from 293 HEK cells transfected with retroviral vectors was incubated with the HA.11 mAb. Immune complexes were collected onto protein G–sepharose beads and, after washing with immunoprecipitation (IP) buffer, used to phosphorylate 10 μg GST/ c-Jun5-89 in 30 μL kinase buffer containing 20 μM adenosine 5-triphosphate (ATP) and 5 μCi (1.8 × 105 Bq) of 32P-γ-ATP.16 After 20 minutes at 37°C, proteins were fractionated by 12.5% SDS-PAGE followed by autoradiography.
Results
HPK1 enhances αCD3-mediated apoptosis of primary T lymphocytes
Stimulation of primary T lymphocytes from DO 10.11 tg mice (which have initially been activated with the cognate OVA peptide) by Abs raised against CD3 (αCD3Abs), a proapoptotic stimulus, for 3 hours leads to a more than 3-fold increase in HPK1 protein levels (Figure1A). This finding and the observation that αCD3+CD28Abs but not αCD28Ab stimulation alone enhanced HPK1 activity7 prompted us to investigate whether HPK1 plays a physiological role in T-cell apoptosis. To this end, we transduced primary murine CD4+ T cells with retroviruses expressing wt HPK1 (pHA-HPK1 wt) or a kinase-inactive version of HPK1 bearing a K46 to M46 substitution (pHA-HPK1 M[46]).15 As shown in Figure 1B, the viral vectors encoding HPK1 protein bear a fusion gene consisting of an N-terminally tagged HA epitope and HPK1 cDNA, which is linked via an internal ribosomal entry site (IRES) to EGFP and zeocin gene (Zeo). Therefore, HPK1, the EGFP fluorescence marker and zeocin resistance genes are coordinately expressed. In control experiments, transfection of 293T cells with constructs encoding the wt but not the kinase-inactive HPK1 mutant led in in vitro kinase assays to the autophosphorylation of HPK1 and phosphorylation of GST-cJun5-89, which was added as an exogenous substrate (Figure 1C).
HPK1 facilitates spontaneous and αCD3-mediated apoptosis in primary murine CD4+ T cells.
(A) Restimulation of primary murine CD4+ T cells with αCD3 mAb for 3 hours results in an increase of HPK1 levels. Naive primary CD4+ T cells from LNs of DO 11.10 TCR tg mice were stimulated with OVA peptide for 24 hours. After 4 days of expansion in the presence of IL-2, the cells were either left untreated (–) or restimulated with plate-bound αCD3ε mAb for 3 hours (+). Using Abs specific for HPK1 or β-actin, 80 μg whole cellular proteins was immunblotted. Representative blots from 3 independent experiments are shown. (B) Schematic structure of HPK1 and HPK1-encoding retroviral vectors. Four proline-rich motifs (PR 1-4) within potential SH3 binding sites, the caspase cleavage motif DDVD, and the position of kinase-death mutation K to M at position 46 are indicated. (C) HPK1 expressed from the retroviral construct pHA-HPK1 wt is catalytically active. The indicated retroviral constructs were transfected into 293T cells. After 48 hours, the cells were lysed, proteins were precipitated with an αHA mAb, and in vitro kinase assays were performed using GST-cJun5-89 as an exogenous substrate. HPK1 expression in precipitates is shown below. Phosphorylation was visualized by autoradiography (top and middle panels). Representative blots from 4 independent experiments are shown. (D) CD4+ T cells from LNs of DO 11.10 TCR tg mice were stimulated with OVA peptide and infected. After 4 days of expansion in the presence of IL-2, the cells were either left untreated (upper panel) or restimulated with plate-bound αCD3ε mAb for 8 hours (lower panel), stained with Annexin V–PE, and analyzed by FACS. Representative data from 3 independent experiments are shown.
HPK1 facilitates spontaneous and αCD3-mediated apoptosis in primary murine CD4+ T cells.
(A) Restimulation of primary murine CD4+ T cells with αCD3 mAb for 3 hours results in an increase of HPK1 levels. Naive primary CD4+ T cells from LNs of DO 11.10 TCR tg mice were stimulated with OVA peptide for 24 hours. After 4 days of expansion in the presence of IL-2, the cells were either left untreated (–) or restimulated with plate-bound αCD3ε mAb for 3 hours (+). Using Abs specific for HPK1 or β-actin, 80 μg whole cellular proteins was immunblotted. Representative blots from 3 independent experiments are shown. (B) Schematic structure of HPK1 and HPK1-encoding retroviral vectors. Four proline-rich motifs (PR 1-4) within potential SH3 binding sites, the caspase cleavage motif DDVD, and the position of kinase-death mutation K to M at position 46 are indicated. (C) HPK1 expressed from the retroviral construct pHA-HPK1 wt is catalytically active. The indicated retroviral constructs were transfected into 293T cells. After 48 hours, the cells were lysed, proteins were precipitated with an αHA mAb, and in vitro kinase assays were performed using GST-cJun5-89 as an exogenous substrate. HPK1 expression in precipitates is shown below. Phosphorylation was visualized by autoradiography (top and middle panels). Representative blots from 4 independent experiments are shown. (D) CD4+ T cells from LNs of DO 11.10 TCR tg mice were stimulated with OVA peptide and infected. After 4 days of expansion in the presence of IL-2, the cells were either left untreated (upper panel) or restimulated with plate-bound αCD3ε mAb for 8 hours (lower panel), stained with Annexin V–PE, and analyzed by FACS. Representative data from 3 independent experiments are shown.
CD4+ T cells from DO 11.10 TCR tg mice were infected with HPK1-expressing retroviruses 1 day after primary stimulation, and 4 days later spontaneous and αCD3-mediated apoptosis of T cells was measured by Annexin V staining. Expression of HPK1 kinase accelerated spontaneous as well as αCD3-mediated apoptosis. As shown in Figure 1D (upper panels), spontaneous apoptosis (induced mainly by the viral infection procedure; data not shown) increased from approximately 43% in cells infected with pEGZ-HA control virus to 82% in cells infected with pHA-HPK1 wt virus. A more moderate increase to 56% was observed for cells infected with virus expressing the kinase-inactive version of HPK1. In contrast, in noninfected cells apoptosis remained constant, indicating that apoptosis induction was an intrinsic property of infected and, therefore, HPK1-expressing cells. Additionally, αCD3-mediated apoptosis increased from 75.5% in cells infected with control virus to more than 93% in cells infected with HPK1 virus (Figure 1D, lower panels; note again that the percentage of apoptotic cells remained almost constant in noninfected cells). These data indicate that HPK1 overexpression promotes spontaneous apoptosis and enhances CD3-mediated apoptosis of peripheral T cells.
HPK1 enhances apoptosis induction of EL-4 thymoma cells by ROS
In order to elucidate molecular mechanisms of HPK1-mediated apoptosis, we established lines of retrovirally infected EL-4 thymoma cells overexpressing different versions of HPK1 protein. These were treated with H2O2, which enhances the levels of free ROS that are important mediators of apoptosis in T cells.2 Treatment of EL-4 cells with 1 mM H2O2 for 5 to 120 minutes increased the level of endogenous HPK1 (Figure 2A), whereas those of other protein kinases, such as of Erk, JNK and p38 mitogen-activated protein (MAP) kinases, remained constant (Figure3). In addition, H2O2 treatment led to an increase in level of N-terminal HPK1 peptide containing the HPK1 kinase domain (Figure 1B). This released N-terminal peptide was shown to exhibit an enhanced kinase activity compared to full-size HPK1.17
HPK1 enhances apoptosis of EL-4 cells by ROS.
(A) HPK1 is up-regulated and cleaved upon induction of H2O2-mediated apoptosis in EL-4 cells. EL-4 cells were stimulated with 1 mM H2O2 for 0 to 120 minutes. Whole cellular proteins (100 μg) were resolved by SDS-PAGE, immunoblotted, and detected with Abs specific for HPK1 (which indicates full-length HPK1, above, or the N-terminal cleavage product) or β-actin. Representative blots from 3 independent experiments are shown. (B) HPK1 promotes H2O2-mediated apoptosis of EL-4 cells. EL-4 cell lines transduced with pEGZ-HA, pHA-HPK1 wt, or pHA-HPK1 AS viruses were left either untreated or treated with 1 mM H2O2 for 2 to 8 hours. Cell-cycle profiles were determined using propidium iodide staining by FACS analysis. SubG1 phase cells were identified as apoptotic cells. The graph shows the fold up-regulation of apoptosis of cells expressing HPK1 (or HPK1 AS) compared to that of control cells transduced with pEGZ-HA. The results presented are the mean values of 5 independent experiments. In the inset (above, left), an immunoblot is shown, which demonstrates the suppressive effect of human HPK1-AS RNA on the expression of endogenous murine HPK1. Whole protein extracts (80 μg) were fractionated from EL-4 cells stably infected with control pEGZ-HA (lane 1), pHA-HPK1 wt (lane 2), or pHA-HPK1 AS viruses (lane 3). Below, the expression of β-actin is shown as loading control. (C) HPK1-AS RNA strongly reduces HPK1 expression. Human 293T cells were cotransfected with retroviral DNAs. After 48 hours, cells were lysed, 50 μg proteins was resolved by SDS-PAGE and immunoblotted with Abs specific for HPK1 or β-actin. Representative blots from 3 independent experiments are shown.
HPK1 enhances apoptosis of EL-4 cells by ROS.
(A) HPK1 is up-regulated and cleaved upon induction of H2O2-mediated apoptosis in EL-4 cells. EL-4 cells were stimulated with 1 mM H2O2 for 0 to 120 minutes. Whole cellular proteins (100 μg) were resolved by SDS-PAGE, immunoblotted, and detected with Abs specific for HPK1 (which indicates full-length HPK1, above, or the N-terminal cleavage product) or β-actin. Representative blots from 3 independent experiments are shown. (B) HPK1 promotes H2O2-mediated apoptosis of EL-4 cells. EL-4 cell lines transduced with pEGZ-HA, pHA-HPK1 wt, or pHA-HPK1 AS viruses were left either untreated or treated with 1 mM H2O2 for 2 to 8 hours. Cell-cycle profiles were determined using propidium iodide staining by FACS analysis. SubG1 phase cells were identified as apoptotic cells. The graph shows the fold up-regulation of apoptosis of cells expressing HPK1 (or HPK1 AS) compared to that of control cells transduced with pEGZ-HA. The results presented are the mean values of 5 independent experiments. In the inset (above, left), an immunoblot is shown, which demonstrates the suppressive effect of human HPK1-AS RNA on the expression of endogenous murine HPK1. Whole protein extracts (80 μg) were fractionated from EL-4 cells stably infected with control pEGZ-HA (lane 1), pHA-HPK1 wt (lane 2), or pHA-HPK1 AS viruses (lane 3). Below, the expression of β-actin is shown as loading control. (C) HPK1-AS RNA strongly reduces HPK1 expression. Human 293T cells were cotransfected with retroviral DNAs. After 48 hours, cells were lysed, 50 μg proteins was resolved by SDS-PAGE and immunoblotted with Abs specific for HPK1 or β-actin. Representative blots from 3 independent experiments are shown.
HPK1 enhances JNK and, to a lesser extent, ERK and p38 MAP kinase activation in H2O2-treated EL-4 cells.
EL-4 cell lines expressing wt HPK1 (pHA-HPK1 wt), a kinase-inactive K46M mutant (pHA-HPK1 M[46]), AS-HPK1 (pHA-HPK1 AS), or control vector RNA (pEGZ-HA) were treated with 1 mmol/L H2O2 for 0 to 120 minutes. 100 μg cellular proteins were resolved by SDS-PAGE, blotted, and detected with the indicated Abs. Representative blots from 4 independent experiments are shown.
HPK1 enhances JNK and, to a lesser extent, ERK and p38 MAP kinase activation in H2O2-treated EL-4 cells.
EL-4 cell lines expressing wt HPK1 (pHA-HPK1 wt), a kinase-inactive K46M mutant (pHA-HPK1 M[46]), AS-HPK1 (pHA-HPK1 AS), or control vector RNA (pEGZ-HA) were treated with 1 mmol/L H2O2 for 0 to 120 minutes. 100 μg cellular proteins were resolved by SDS-PAGE, blotted, and detected with the indicated Abs. Representative blots from 4 independent experiments are shown.
When EL-4 cells overexpressing HPK1 were treated with 1 mM H2O2, a 40% increase in apoptosis was detected after 8 hours compared to cells infected with the pEGZ-HA control vector (Figure 2B). While cells overexpressing the kinase-inactive HPK1 M(46) showed a moderate increase in apoptosis (of approximately 15%, data not shown), cells expressing HPK1 AS-RNA were markedly more resistant against H2O2-mediated apoptosis than control cells (Figure 2B). Since expression of HPK1 AS-RNA suppressed HPK1 levels (Figure 2C) one may conclude that a lower HPK1 level results in a decrease in H2O2-mediated apoptosis. Taken together, these results demonstrate that HPK1 expression and activity significantly influence ROS-mediated apoptosis of EL-4 cells, which represents an important pathway of AICD in T cells.2
HPK1 increases JNK kinase activation in H2O2-treated EL-4 cells and αCD3Ab-stimulated primary CD4+ T cells
HPK1 activity has been described to induce JNK and NF-κB activity in various cell types.8,15,18 19 To elucidate which signaling pathways might be affected by HPK1 in EL-4 cells and primary CD4+ T cells, we studied first Tyr protein phosphorylation and found that expression of wt HPK1 strongly enhanced Tyr phosphorylation of numerous proteins in EL-4 cells treated with H2O2 (results not shown). In addition, H2O2 exerted a strong stimulatory effect on JNK activation.
H2O2 treatment leads to a weak but measurable activation of JNK1, p38, and Erk1/Erk2 MAP kinases in EL-4 cells transduced with the control pEGZ-HA virus (Figure 3, panel pEGZ-HA). As expected, HPK1 expression exerted a strong and persistent enhancement of JNK1 activity (Figure 3, panel pHA-HPK1 wt). Albeit expression of mutant HPK1 M(46) kinase also enhanced JNK1 activity, that of wt HPK1 resulted in a pronounced longer-lasting JNK1 activation. This indicates that at least a part of JNK activation was due to HPK1 kinase activity and not to its adaptor function. Compared to JNK1 activation, a more moderate increase in p38 and Erk activation was observed in cells expressing HPK1. However, almost the same inducible effect was detected in cells expressing HPK1 M(46), suggesting that HPK1 activity plays a minor role for p38 and Erk induction. The increased activity of all 3 MAP kinases but not their expression could be suppressed by HPK1 AS-RNA (Figure 3, panel pHA-HPK1 AS), indicating that overexpression of HPK1 stimulates all 3 MAP kinase signaling cascades, although to a very different extent.
A marked increase in JNK activation by HPK1 was also observed in primary CD4+ T cells infected with a retrovirus expressing HPK1. This is shown in Figure 4, where a distinct, stronger JNK1 phosphorylation can be seen in untreated cells and cells stimulated with αCD3Abs for 30 minutes. In contrast, a subtle or no increase in phosphorylation was detected for p38 and Erk1/2 kinases (Figure 4).
HPK1 enhances JNK activation in primary CD4+T lymphocytes.
CD4+ T cells from LNs of DO 11.10 TCR tg mice were stimulated with OVA peptide for 24 hours and infected with the indicated retroviruses. After 2 days of expansion, the cells were sorted for infected EGFP-positive cells. One day later the cells were either left untreated (–) or restimulated with plate-bound αCD3ε mAb for 30 minutes (+). Whole cell protein extracts were prepared from 3 × 105 cells each and immunoassayed using the indicated Abs.
HPK1 enhances JNK activation in primary CD4+T lymphocytes.
CD4+ T cells from LNs of DO 11.10 TCR tg mice were stimulated with OVA peptide for 24 hours and infected with the indicated retroviruses. After 2 days of expansion, the cells were sorted for infected EGFP-positive cells. One day later the cells were either left untreated (–) or restimulated with plate-bound αCD3ε mAb for 30 minutes (+). Whole cell protein extracts were prepared from 3 × 105 cells each and immunoassayed using the indicated Abs.
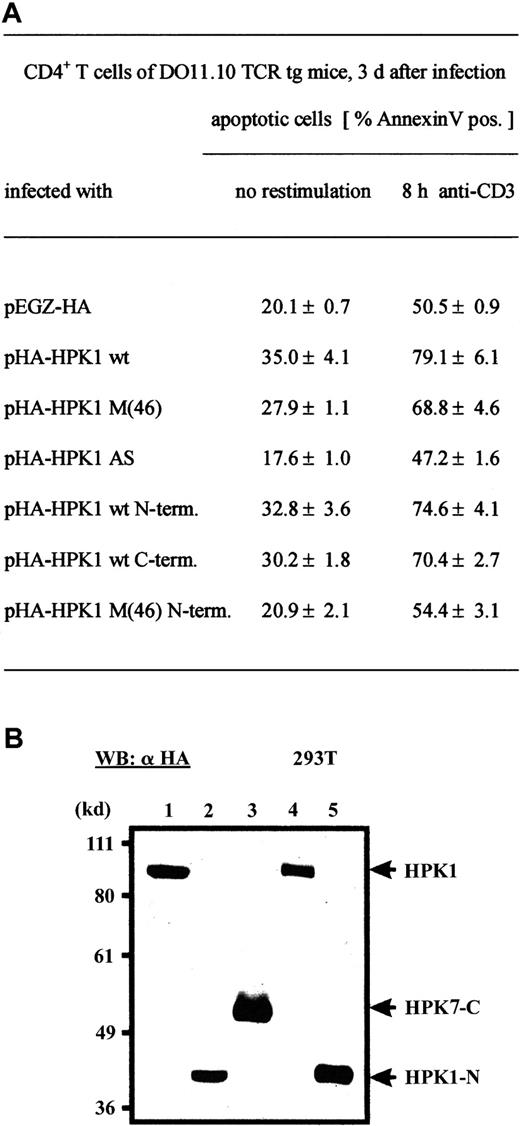
Both N- and C-terminal HPK1 peptides promote apoptosis of CD4+ T cells
The rapid cleavage of HPK1 after H2O2treatment in EL-4 cells (Figure 2A, middle panel) prompted us to investigate whether the cleavage products of HPK1 (presumably generated by caspase 38 17) support apoptosis of primary T cells. To this end, we constructed viruses expressing either the N-terminal cleavage product comprising aa 2-385 or the C-terminal product comprising aa 386-833 (Figure 5B) and infected CD4+ T cells after primary stimulation. As summarized in Figure 5A, similar to cells infected with HA-HPK1 wt virus, infection with viruses expressing one of the two cleavage products resulted in an increase of spontaneous apoptosis from approximately 20% to 30-33% and of αCD3-mediated apoptosis from 50.5% to 70-75% 3 days after primary stimulation. Interestingly, expressing a kinase-inactive version of N-terminal cleavage product (pH-HPK1 M(46) N-terminus) was without effect on apoptosis. In addition, infection with virus-expressing HPK1 AS-RNA impaired slightly spontaneous and αCD3-mediated apoptosis (Figure 5A). These data demonstrate that in HPK1-promoted apoptosis, both the N- and C-terminal portions of HPK1 are involved.
HPK1 activity enhances spontaneous and TCR-mediated apoptosis in primary CD4+ T cells.
(A) CD4+ T cells from LNs of DO 11.10 TCR tg mice were stimulated with OVA peptide for 24 hours and infected with the indicated retroviruses. After 3 days of expansion, the cells were either left untreated or restimulated with plate-bound αCD3ε mAb for 8 hours and analyzed by FACS. Mean values from 5 independent experiments are shown. (B) Expression of HPK1 proteins after transfection of retroviral vectors expressing pHA-HPK1 wt (lane1), pHA-HPK1 wt N-terminus (lane 2), pHA-HPK1 wt C-terminus (lane 3), pHA-HPK1 M(46) (lane 4), or pHA-HPK1 M(46) N-terminus (lane 5) into 293T cells. Forty-eight hours after transfection, 40 μg cellular proteins was electroblotted and assayed using an αHA mAb.
HPK1 activity enhances spontaneous and TCR-mediated apoptosis in primary CD4+ T cells.
(A) CD4+ T cells from LNs of DO 11.10 TCR tg mice were stimulated with OVA peptide for 24 hours and infected with the indicated retroviruses. After 3 days of expansion, the cells were either left untreated or restimulated with plate-bound αCD3ε mAb for 8 hours and analyzed by FACS. Mean values from 5 independent experiments are shown. (B) Expression of HPK1 proteins after transfection of retroviral vectors expressing pHA-HPK1 wt (lane1), pHA-HPK1 wt N-terminus (lane 2), pHA-HPK1 wt C-terminus (lane 3), pHA-HPK1 M(46) (lane 4), or pHA-HPK1 M(46) N-terminus (lane 5) into 293T cells. Forty-eight hours after transfection, 40 μg cellular proteins was electroblotted and assayed using an αHA mAb.
HPK1 modulates NF-κB activation
In order to show whether HPK1 influences NF-κB activation under the conditions of apoptosis induction in EL-4 and primary T cells, we cotransfected first EL-4 cells with a luciferase reporter gene driven by multiples of NF-κB binding sites from the c-myb promoter, with vectors expressing HPK1 wild-type or HPK1 C-terminal peptide. Treatment of HPK1 wt–transfected cells with 500 μM H2O2 led to a 3- to 4-fold increase in NF-κB–driven luciferase activity compared to cells transfected with a control “empty” vector. In contrast, a 2- to 3-fold decrease in NF-κB activity was observed for cells transfected with a HPK1 C-terminus–expressing construct (Figure6A). This shows that HPK1 wt protein exerts a stimulatory and HPK1 C-terminus peptide a suppressive effect on NF-κB activation.
HPK1 affects NF-κB induction in murine T cells.
(A) HPK1 wt supports and the HPK1 C-terminal peptide suppresses NF-κB induction. EL-4 cells were cotransfected with expression vectors encoding HPK1 wt or HPK C-terminus and a luciferase reporter gene driven by 3 copies of a κB site from the c-myb enhancer.9 Forty-two hours after transfection, cells were either left uninduced (–) or induced with 500 μM H2O2 for 6 hours as indicated. The data show mean values of 4 transfections. (B) HPK1 C-terminus impairs IκBα degradation. CD4+ T cells from LNs of DO 11.10 TCR tg mice were stimulated with OVA peptide and infected with the indicated retroviruses. After 2 days of expansion, the cells were sorted for infected EGFP-positive cells. One day later the cells were either left untreated (–) or restimulated with plate-bound αCD3ε mAb for 30 minutes (+). Whole cell protein extracts were prepared from 3 × 105 cells each and immunoassayed using the indicated Abs.
HPK1 affects NF-κB induction in murine T cells.
(A) HPK1 wt supports and the HPK1 C-terminal peptide suppresses NF-κB induction. EL-4 cells were cotransfected with expression vectors encoding HPK1 wt or HPK C-terminus and a luciferase reporter gene driven by 3 copies of a κB site from the c-myb enhancer.9 Forty-two hours after transfection, cells were either left uninduced (–) or induced with 500 μM H2O2 for 6 hours as indicated. The data show mean values of 4 transfections. (B) HPK1 C-terminus impairs IκBα degradation. CD4+ T cells from LNs of DO 11.10 TCR tg mice were stimulated with OVA peptide and infected with the indicated retroviruses. After 2 days of expansion, the cells were sorted for infected EGFP-positive cells. One day later the cells were either left untreated (–) or restimulated with plate-bound αCD3ε mAb for 30 minutes (+). Whole cell protein extracts were prepared from 3 × 105 cells each and immunoassayed using the indicated Abs.
A suppressive effect of HPK1 C-terminus peptide on NF-κB activation was also detected in primary CD4+ T cells. In Western blots studying the degradation of cytosolic NF-κB–inhibitor IκBα after αCD3 stimulation for 30 minutes, we observed that IκBα is partially degraded in primary T cells infected with a control “empty” virus EGZ-HA or HPK1 wt–expressing virus but not with a virus expressing the C-terminal portion of HPK1 (Figure 6B).
HPK1 enhances FasL expression of primary T lymphocytes
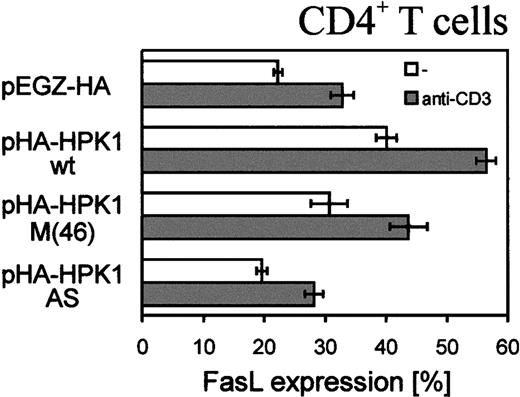
FasL is a key molecule in AICD of peripheral T cells. Therefore, we also investigated if HPK1 can modulate the expression level of FasL. After 3 days of infection with pEGZ-HA control virus, approximately 20% of primary T cells expressed FasL, whereas approximately 40% of cells infected with HPK1 virus were found to express FasL (Figure7). Induction of both cell types through CD3 led to an increase in number of FasL-expressing cells to approximately 40% and 60%, respectively. HPK1 AS-RNA, on the other hand, only slightly affected FasL expression. These findings show that HPK1 can enhance FasL expression, but mechanisms in addition to the FasL/Fas signaling cascade are involved in HPK1-mediated apoptosis of primary T cells.
HPK1 enhances FasL expression on primary CD4+ T cells.
CD4+ T cells from LNs of DO 11.10 TCR tg mice were stimulated with OVA peptide and infected. After 3 days of expansion, the cells were either left untreated or restimulated with plate-bound αCD3ε mAb for 3 hours, stained with biotin-conjugated αFasL mAb, washed, incubated with PE-conjugated streptavidin, and analyzed by FACS. Mean values from 3 independent experiments are shown.
HPK1 enhances FasL expression on primary CD4+ T cells.
CD4+ T cells from LNs of DO 11.10 TCR tg mice were stimulated with OVA peptide and infected. After 3 days of expansion, the cells were either left untreated or restimulated with plate-bound αCD3ε mAb for 3 hours, stained with biotin-conjugated αFasL mAb, washed, incubated with PE-conjugated streptavidin, and analyzed by FACS. Mean values from 3 independent experiments are shown.
Discussion
HPK1 is a member of the family of germinal center kinases that is predominantly expressed in adult hematopoietic tissues. It has originally been described as an upstream Ser/Thr protein kinase of the JNK signaling cascade.15,18 Recently, it was shown that TCR stimulation leads to a rapid but transient activation of HPK1 and its binding to the linker of activated T cells (LAT) and the adaptor proteins Nck, Crk, and Gads. HPK1, which also binds to the signaling molecules SLP-76, Grb2, Grap, and CrkL is localized in lipid rafts and needs lck and ZAP70 for its activation.6,7 20 These data suggested an involvement of HPK1 in the signaling transfer from the TCR to downstream signaling cascades. However, the role for HPK1 in T-cell physiology remained to be shown.
We show here that one of the functions of HPK1 activation upon T-cell stimulation is to control the termination of immune response by supporting apoptosis of T cells. This is demonstrated by overexpressing wt HPK1, which led to a substantial increase in spontaneous and αCD3-mediated apoptosis, as well as to FasL expression on CD4+ T cells (Figures 1, 5, and 7). In contrast, expression of HPK1 AS-RNA exerted a slight, albeit measurable, suppressive effect on both apoptosis and FasL expression. Apoptosis suppression by overexpressing HPK1 AS-RNA was much more pronounced in H2O2-treated EL-4 cells in which HPK1 overexpression stimulated cell death induced by ROS (Figure 2), indicating that HPK1 indeed controls apoptosis in T cells.
Two prominent downstream signaling cascades, the JNK and NF-κB pathways, both of which modulate apoptosis, were shown to be stimulated by HPK1.8,15,18,19 (Figures 3, 4, and 6). Whereas NF-κB factors, in particular RelA/p65, exert a strong antiapoptotic activity,21 the 2 JNK kinases, JNK1 and 2, are potent inducers of apoptosis of thymocytes and peripheral T cells.22-24 It is likely that the substantial increase in AICD that we observed for primary T cells after infection with virus-expressing HPK1 (Figure 1) is due to the modulation of these 2 signaling pathways. However, inhibition of JNK activity in primary T cells by the JNK inhibitor SP600125 (Calbiochem, Bad Soden, Germany) did not lead to a complete suppression of HPK1-enhanced apoptosis induction (data not shown), and in addition to JNK activation, H2O2 treatment of HPK1-infected EL-4 cells led also to a moderate increase of ERK activity, in particular in pHA-HPK1 M(46)–infected cells (see Ling et al6 and Figure 3). This indicates that signaling events others than JNK and NF-κB activation are involved in HPK1-mediated apoptosis induction. One of them might be the scaffolding function of HPK1 through its Proline-rich motifs by binding the SLP-76–related adaptor Clnk25 or other signaling molecules.
H2O2 treatment exerted a strong and, in particular, sustained JNK1 activation in EL-4 cells infected with HPK1-expressing virus (Figure 3). In primary murine embryo fibroblasts deficient for JNK1 and 2, the defect in inducible apoptosis was localized to the mitochondria.26 Therefore, one may assume that effector molecules involved in mitochondrial apoptosis resistance, such as Bcl-2, are the targets of enhanced JNK activity in EL-4 cells. Both Bcl-2 and Bcl-XL, a further member of antiapoptotic group of Bcl-2–like proteins, were described to be phosphorylated and inactivated by JNKs in vivo.27,28 In spite of disagreement with other studies, the Bcl-2–like proteins, including the BH3-only protein Bim and its relatives, appear to be potential targets of JNK in regulating mitochondrial apoptosis induction (see Davis29 for a discussion).
Independent of JNK activation, HPK1 is able to induce NF-κB activation in hematopoietic cells.8,19 Contrary to JNK activation, which is mediated by the kinase activity of HPK1, NF-κB activation requires the full-length HPK1 molecule8 (Figure6A). This suggests that, in addition to kinase activity, binding of signaling molecules to the SH3-like motifs within the C-terminal half of HPK1 is important for NF-κB activation. Since a kinase-deficient version of IκB kinase β was able to abrogate HPK1-mediated NF-κB activation,8 one may conclude that HPK1 acts through the IKK complex to activate NF-κB. Interestingly, overexpression of the C-terminal half of HPK1, which, along with the N-terminal half, is released upon apoptosis induction,19 inhibited NF-κB activation (Figure 6). This converts HPK1 from an activator to an inhibitor of NF-κB.8 The inhibition of NF-κB by the C-terminal portion of HPK1 appears to contribute to the apoptosis observed in EL-4 and primary T cells following HPK1 overexpression. This conclusion is supported by our observation that, similar to the expression of full-length HPK1 or its N-terminal half, expression of the C-terminal half increased both spontaneous and αCD3-mediated apoptosis in CD4+ T cells (Figure 5A).
Taken together, these results lead to a model in which, during the initial phase of T-cell activation, the rapid and transient HPK1 induction provides both proapoptotic and antiapoptotic signals by activating JNK and NF-κB signaling pathways, respectively. At a later stage, due to the accumulation of the C-terminal HPK1 cleavage product, NF-κB activation is suppressed, and the balance between survival and apoptotic signals is shifted, favoring apoptosis. However, signaling events in addition to the stimulation of JNK and inhibition of NF-κB signaling cascades might also be involved in the HPK1-mediated control of T-cell apoptosis. The nature of these events remains to be elucidated.
We are indebted to Christa Kraus, Doris Michel, Ilona Pietrowski, and Olga Reimer for excellent technical assistance. We thank Drs Ingolf Berberich, Stefan M. Feller, and Friedemann Kiefer for gifts of materials and support; and Rajesh Kumar Singh and Stefan Klein-Hessling for critically reading the manuscript.
Prepublished online as Blood First Edition Paper, April 30, 2002; DOI 10.1182/blood-2002-01-0089.
Supported by the W.-Sander-Stiftung (E.S.), theÖsterreichische Akademie der Wissenschaften (providing a PhD fellowship to B.S.-N.), and the Deutsche Forschungsgemeinschaft through SFB 465 (Würzburg), project B5; SFB 466 (Erlangen), project B3; the FOR 303, projects A2 (E.S.) and C1 (A.A.); and the graduate college (GK) “Cell growth” (A.S. and E.S.).
A.A. and E.S. share senior authorship of this manuscript.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Edgar Serfling, Dept of Molecular Pathology, Institute of Pathology, Josef-Schneider-St 2, D-97080 Würzburg, Germany; e-mail: path015@mail.uni-wuerzburg.de.



![Fig. 3. HPK1 enhances JNK and, to a lesser extent, ERK and p38 MAP kinase activation in H2O2-treated EL-4 cells. / EL-4 cell lines expressing wt HPK1 (pHA-HPK1 wt), a kinase-inactive K46M mutant (pHA-HPK1 M[46]), AS-HPK1 (pHA-HPK1 AS), or control vector RNA (pEGZ-HA) were treated with 1 mmol/L H2O2 for 0 to 120 minutes. 100 μg cellular proteins were resolved by SDS-PAGE, blotted, and detected with the indicated Abs. Representative blots from 4 independent experiments are shown.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/100/3/10.1182_blood-2002-01-0089/5/m_h81522889003.jpeg?Expires=1765926471&Signature=N9YX1cffl9qubh-pJwiNGDjoGionADuqicb5jpehaf6YHe3dPfi348Qsehpvz36bc-2xzJCHRJ-Z-NoMaZAJ9dTa4uLIh0ZWzP8YBXZ4GMEXDdXw2o97RJzds6e55VFkAkNz75LnZlN6s-skqLXgJIeq96hEjaMOIsFjIUw3JW5TWcpXzlEewxXIgVkcfELTK4uF2tvZ7vNvRvcRWzFbiex3ZRH69~bFOvM3U0G~3U6lh34VEFJZRvskNc1b3ytc-mdhBZ9wh5uDv69PQYsjsqCFpTkmAnfTVM-KP0xy936zKPw~jDs29rvStN79o7t~jIjR11l14ZAqkF4VLaX8tA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal